Abstract
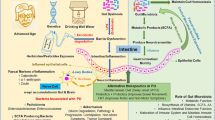
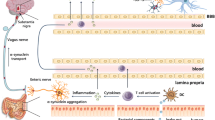
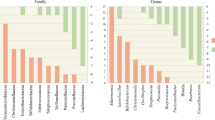
The composition of the gut microbiota, including Akkermansia muciniphila (A. muciniphila), is altered in many neurological diseases and may be involved in the pathophysiological processes of Parkinson’s disease (PD). A. muciniphila, a mucin-degrading bacterium, is a potential next-generation microbe that has anti-inflammatory properties and is responsible for keeping the body healthy. As the role of A. muciniphila in PD has become increasingly apparent, we discuss the potential link between A. muciniphila and various neurological diseases (including PD) in the current review.
Similar content being viewed by others
References
Travagli RA, Browning KN, Camilleri M. Parkinson disease and the gut: new insights into pathogenesis and clinical relevance. Nat Rev Gastroenterol Hepatol, 2020,17(11):673–685
Fang P, Kazmi SA, Jameson KG, et al. The Microbiome as a Modifier of Neurodegenerative Disease Risk. Cell Host Microbe, 2020,28(2):201–222
Fang X. Potential role of gut microbiota and tissue barriers in Parkinson’s disease and amyotrophic lateral sclerosis. Int J Neurosci, 2016,126(9):771–776
Sundman MH, Chen NK, Subbian V, et al. The bidirectional gut-brain-microbiota axis as a potential nexus between traumatic brain injury, inflammation, and disease. Brain Behav Immun, 2017,66:31–44
Perez-Pardo P, Dodiya HB, Broersen LM, et al. Gut-brain and brain-gut axis in Parkinson’s disease models: Effects of a uridine and fish oil diet. Nutr Neurosci, 2018,21(6):391–402
Sampson TR, Debelius JW, Thron T, et al. Gut Microbiota Regulate Motor Deficits and Neuroinflammation in a Model of Parkinson’s Disease. Cell, 2016,167(6):1469–1480.e12
Derrien M, Vaughan EE, Plugge CM, et al. Akkermansia muciniphila gen. nov., sp. nov., a human intestinal mucin-degrading bacterium. Int J Syst Evol Microbiol, 2004,54(Pt 5):1469–1476
O’Toole PW, Marchesi JR, Hill C. Next-generation probiotics: the spectrum from probiotics to live biotherapeutics. Nat Microbiol, 2017,2:17057
Zhai R, Xue X, Zhang L, et al. Strain-Specific Anti-inflammatory Properties of Two Akkermansia muciniphila Strains on Chronic Colitis in Mice. Front Cell Infect Microbiol, 2019,9:239
Li J, Lin S, Vanhoutte PM, et al. Akkermansia Muciniphila Protects Against Atherosclerosis by Preventing Metabolic Endotoxemia-Induced Inflammation in Apoe-/- Mice. Circulation, 2016,133(24):2434–2446
Depommier C, Everard A, Druart C, et al. Supplementation with Akkermansia muciniphila in overweight and obese human volunteers: a proof-of-concept exploratory study. Nat Med, 2019,25(7):1096–1103
Shin NR, Lee JC, Lee HY, et al. An increase in the Akkermansia spp. population induced by metformin treatment improves glucose homeostasis in diet-induced obese mice. Gut, 2014,63(5):727–735
Barcena C, Valdes-Mas R, Mayoral P, et al. Healthspan and lifespan extension by fecal microbiota transplantation into progeroid mice. Nat Med, 2019, 25(8):1234–1242
Heinzel S, Aho VTE, Suenkel U, et al. Gut Microbiome Signatures of Risk and Prodromal Markers of Parkinson Disease. Ann Neurol, 2020,88(2):320–331
Keshavarzian A, Green SJ, Engen PA, et al. Colonic bacterial composition in Parkinson’s disease. Mov Disord, 2015,30(10):1351–1360
Hill-Burns EM, Debelius JW, Morton JT, et al. Parkinson’s disease and Parkinson’s disease medications have distinct signatures of the gut microbiome. Mov Disord, 2017,32(5):739–749
Heintz-Buschart A, Pandey U, Wicke T, et al. The nasal and gut microbiome in Parkinson’s disease and idiopathic rapid eye movement sleep behavior disorder. Mov Disord, 2018,33(1):88–98
Nishiwaki H, Ito M, Ishida T, et al. Meta-Analysis of Gut Dysbiosis in Parkinson’s Disease. Mov Disord, 2020,35(9):1626–1635
Cirstea MS, Yu AC, Golz E, et al. Microbiota Composition and Metabolism Are Associated With Gut Function in Parkinson’s Disease. Mov Disord, 2020, 35(7):1208–1217
Bedarf JR, Hildebrand F, Coelho LP, et al. Functional implications of microbial and viral gut metagenome changes in early stage L-DOPA-naive Parkinson’s disease patients. Genome Med, 2017,9(1):39
Unger MM, Spiegel J, Dillmann KU, et al. Short chain fatty acids and gut microbiota differ between patients with Parkinson’s disease and age-matched controls. Parkinsonism Relat Disord, 2016,32:66–72
Hertel J, Harms AC, Heinken A, et al. Integrated Analyses of Microbiome and Longitudinal Metabolome Data Reveal Microbial-Host Interactions on Sulfur Metabolism in Parkinson’s Disease. Cell Rep, 2019,29(7):1767–1777.e8
Vandeputte D, Kathagen G, D’Hoe K, et al. Quantitative microbiome profiling links gut community variation to microbial load. Nature, 2017,551(7681):507–511
Tettamanti Boshier FA, Srinivasan S, Lopez A, et al. Complementing 16S rRNA Gene Amplicon Sequencing with Total Bacterial Load To Infer Absolute Species Concentrations in the Vaginal Microbiome. mSystems, 2020,5(2):e00777–19
Miller PG, Bonn MB, Franklin CL, et al. TNFR2 Deficiency Acts in Concert with Gut Microbiota To Precipitate Spontaneous Sex-Biased Central Nervous System Demyelinating Autoimmune Disease. J Immunol, 2015,195(10):4668–4684
Cekanaviciute E, Yoo BB, Runia TF, et al. Gut bacteria from multiple sclerosis patients modulate human T cells and exacerbate symptoms in mouse models. Proc Natl Acad Sci USA, 2017,114(40):10 713–10 718
Cekanaviciute E, Probstel AK, Thomann A, et al. Multiple Sclerosis-Associated Changes in the Composition and Immune Functions of Spore-Forming Bacteria. mSystems, 2018,3(6):e00083–18
Liu S, Rezende RM, Moreira TG, et al. Oral Administration of miR-30d from Feces of MS Patients Suppresses MS-like Symptoms in Mice by Expanding Akkermansia muciniphila. Cell Host Microbe, 2019,26(6):779–794.e8
Hogg E, Athreya K, Basile C, et al. High Prevalence of Undiagnosed Insulin Resistance in Non-Diabetic Subjects with Parkinson’s Disease. J Parkinsons Dis, 2018,8(2):259–265
Nam GE, Kim SM, Han K, et al. Metabolic syndrome and risk of Parkinson disease: A nationwide cohort study. PLoS Med, 2018,15(8):e1002640
De Pablo-Fernandez E, Goldacre R, Pakpoor J, et al. Association between diabetes and subsequent Parkinson disease: A record-linkage cohort study. Neurology, 2018,91(2):e139–e142
Charvin D, Medori R, Hauser RA, et al. Therapeutic strategies for Parkinson disease: beyond dopaminergic drugs. Nat Rev Drug Discov, 2018,17(11):804–822
Bosco D, Plastino M, Cristiano D, et al. Dementia is associated with insulin resistance in patients with Parkinson’s disease. J Neurol Sci, 2012,315(1–2):39–43
Kahn SE, Hull RL, Utzschneider KM. Mechanisms linking obesity to insulin resistance and type 2 diabetes. Nature, 2006,444(7121):840–846
Schnurr TM, Jakupovic H, Carrasquilla GD, et al. Obesity, unfavourable lifestyle and genetic risk of type 2 diabetes: a case-cohort study. Diabetologia, 2020,63(7):1324–1332
Sanna S, van Zuydam NR, Mahajan A, et al. Causal relationships among the gut microbiome, short-chain fatty acids and metabolic diseases. Nat Genet, 2019,51(4):600–605
Larsen N, Vogensen FK, van den Berg FW, et al. Gut microbiota in human adults with type 2 diabetes differs from non-diabetic adults. PLoS One, 2010,5(2):e9085
Aron-Wisnewsky J, Prifti E, Belda E, et al. Major microbiota dysbiosis in severe obesity: fate after bariatric surgery. Gut, 2019,68(1):70–82
Cani PD, de Vos WM. Next-Generation Beneficial Microbes: The Case of Akkermansia muciniphila. Front Microbiol, 2017,8:1765
Dao MC, Belda E, Prifti E, et al. Akkermansia muciniphila abundance is lower in severe obesity, but its increased level after bariatric surgery is not associated with metabolic health improvement. Am J Physiol Endocrinol Metab, 2019,317(3):E446–E459
Giannoudaki E, Hernandez-Santana YE, Mulfaul K, et al. Interleukin-36 cytokines alter the intestinal microbiome and can protect against obesity and metabolic dysfunction. Nat Commun, 2019,10(1):4003
Dao MC, Everard A, Aron-Wisnewsky J, et al. Akkermansia muciniphila and improved metabolic health during a dietary intervention in obesity: relationship with gut microbiome richness and ecology. Gut, 2016,65(3):426–436
Schneeberger M, Everard A, Gomez-Valades AG, et al. Akkermansia muciniphila inversely correlates with the onset of inflammation, altered adipose tissue metabolism and metabolic disorders during obesity in mice. Sci Rep, 2015,5:16643
Everard A, Belzer C, Geurts L, et al. Cross-talk between Akkermansia muciniphila and intestinal epithelium controls diet-induced obesity. Proc Natl Acad Sci USA, 2013,110(22):9066–9071
Depommier C, Van Hul M, Everard A, et al. Pasteurized Akkermansia muciniphila increases whole-body energy expenditure and fecal energy excretion in diet-induced obese mice. Gut Microbes, 2020,11(5):1231–1245
Zhang L, Qin Q, Liu M, et al. Akkermansia muciniphila can reduce the damage of gluco/lipotoxicity, oxidative stress and inflammation, and normalize intestine microbiota in streptozotocin-induced diabetic rats. Pathog Dis, 2018,76(4)
Plovier H, Everard A, Druart C, et al. A purified membrane protein from Akkermansia muciniphila or the pasteurized bacterium improves metabolism in obese and diabetic mice. Nat Med, 2017,23(1):107–113
Wu F, Guo X, Zhang M, et al. An Akkermansia muciniphila subtype alleviates high-fat diet-induced metabolic disorders and inhibits the neurodegenerative process in mice. Anaerobe, 2020,61:102138
Greer RL, Dong X, Moraes AC, et al. Akkermansia muciniphila mediates negative effects of IFNγ on glucose metabolism. Nat Commun, 2016,7:13329
Su H, Mo J, Ni J, et al. Andrographolide Exerts Antihyperglycemic Effect through Strengthening Intestinal Barrier Function and Increasing Microbial Composition of Akkermansia muciniphila. Oxid Med Cell Longev, 2020,2020:6538930
Fujisaka S, Usui I, Nawaz A, et al. Bofutsushosan improves gut barrier function with a bloom of Akkermansia muciniphila and improves glucose metabolism in mice with diet-induced obesity. Sci Rep, 2020,10(1):5544
Li N, Wang X, Sun C, et al. Change of intestinal microbiota in cerebral ischemic stroke patients. BMC Microbiol, 2019,19(1):191
Stanley D, Moore RJ, Wong CHY. An insight into intestinal mucosal microbiota disruption after stroke. Sci Rep, 2018,8(1):568
Blacher E, Bashiardes S, Shapiro H, et al. Potential roles of gut microbiome and metabolites in modulating ALS in mice. Nature, 2019,572(7770):474–480
Talbot K, Wang HY, Kazi H, et al. Demonstrated brain insulin resistance in Alzheimer’s disease patients is associated with IGF-1 resistance, IRS-1 dysregulation, and cognitive decline. J Clin Invest, 2012,122(4):1316–1338
de la Monte SM. Insulin resistance and Alzheimer’s disease. BMB Rep, 2009,42(8):475–481
Baglietto-Vargas D, Shi J, Yaeger DM, et al. Diabetes and Alzheimer’s disease crosstalk. Neurosci Biobehav Rev, 2016,64:272–287
Nagpal R, Neth BJ, Wang S, et al. Modified Mediterranean-ketogenic diet modulates gut microbiome and short-chain fatty acids in association with Alzheimer’s disease markers in subjects with mild cognitive impairment. EBioMedicine, 2019,47:529–542.
Ou Z, Deng L, Lu Z, et al. Protective effects of Akkermansia muciniphila on cognitive deficits and amyloid pathology in a mouse model of Alzheimer’s disease. Nutr Diabetes, 2020,10(1):12
Yang Y, Zhong Z, Wang B, et al. Early-life high-fat diet-induced obesity programs hippocampal development and cognitive functions via regulation of gut commensal Akkermansia muciniphila. Neuropsychopharmacology, 2019,44(12):2054–2064
Kuhn P, Kalariya HM, Poulev A, et al. Grape polyphenols reduce gut-localized reactive oxygen species associated with the development of metabolic syndrome in mice. PLoS One, 2018,13(10):e0198716
Roopchand DE, Carmody RN, Kuhn P, et al. Dietary Polyphenols Promote Growth of the Gut Bacterium Akkermansia muciniphila and Attenuate High-Fat Diet-Induced Metabolic Syndrome. Diabetes, 2015,64(8):2847–2858
de la Cuesta-Zuluaga J, Mueller NT, Corrales-Agudelo V, et al. Metformin Is Associated With Higher Relative Abundance of Mucin-Degrading Akkermansia muciniphila and Several Short-Chain Fatty Acid-Producing Microbiota in the Gut. Diabetes Care, 2017, 40(1):54–62
Etxeberria U, Hijona E, Aguirre L, et al. Pterostilbene-induced changes in gut microbiota composition in relation to obesity. Mol Nutr Food Res, 2017,61(1)
Hansen CH, Krych L, Nielsen DS, et al. Early life treatment with vancomycin propagates Akkermansia muciniphila and reduces diabetes incidence in the NOD mouse. Diabetologia, 2012,55(8):2285–2294
Guevara-Cruz M, Flores-Lopez AG, Aguilar-Lopez M, et al. Improvement of Lipoprotein Profile and Metabolic Endotoxemia by a Lifestyle Intervention That Modifies the Gut Microbiota in Subjects With Metabolic Syndrome. J Am Heart Assoc, 2019,8(17):e012401
Ma D, Wang AC, Parikh I, et al. Ketogenic diet enhances neurovascular function with altered gut microbiome in young healthy mice. Sci Rep, 2018,8(1):6670
Sela U, Euler CW, Correa da Rosa J, et al. Strains of bacterial species induce a greatly varied acute adaptive immune response: The contribution of the accessory genome. PLoS Pathog, 2018,14(1):e1006726
Leventhal GE, Boix C, Kuechler U, et al. Strain-level diversity drives alternative community types in millimetre-scale granular biofilms. Nat Microbiol, 2018,3(11):1295–1303
Guo X, Li S, Zhang J, et al. Genome sequencing of 39 Akkermansia muciniphila isolates reveals its population structure, genomic and functional diverisity, and global distribution in mammalian gut microbiotas. BMC Genomics, 2017,18(1):800
Yan Y, Nguyen LH, Franzosa EA, et al. Strain-level epidemiology of microbial communities and the human microbiome. Genome Med, 2020,12(1):71
Guo X, Zhang J, Wu F, et al. Different subtype strains of Akkermansia muciniphila abundantly colonize in southern China. J Appl Microbiol, 2016,120(2):452–459
Ansaldo E, Slayden LC, Ching KL, et al. Akkermansia muciniphila induces intestinal adaptive immune responses during homeostasis. Science, 2019,364(6446): 1179–1184
Fujio-Vejar S, Vasquez Y, Morales P, et al. The Gut Microbiota of Healthy Chilean Subjects Reveals a High Abundance of the Phylum Verrucomicrobia. Front Microbiol, 2017,8:1221
Xiao Y, Angulo MT, Friedman J, et al. Mapping the ecological networks of microbial communities. Nat Commun, 2017,8(1):2042
Bashan A, Gibson TE, Friedman J, et al. Universality of human microbial dynamics. Nature, 2016,534(7606):259–262
Bartolomaeus TUP, Birkner T, Bartolomaeus H, et al. Quantifying Technical Confounders in Microbiome Studies. Cardiovasc Res, 2021,117(3):863–875
Tang Q, Jin G, Wang G, et al. Current Sampling Methods for Gut Microbiota: A Call for More Precise Devices. Front Cell Infect Microbiol, 2020,10:151
Panek M, Cipcic Paljetak H, Baresic A, et al. Methodology challenges in studying human gut microbiota — effects of collection, storage, DNA extraction and next generation sequencing technologies. Sci Rep, 2018, 8(1):5143
Louca S, Polz MF, Mazel F, et al. Function and functional redundancy in microbial systems. Nat Ecol Evol, 2018,2(6):936–943
Xiao Y, Angulo MT, Lao S, et al. An ecological framework to understand the efficacy of fecal microbiota transplantation. Nat Commun, 2020,11(1):3329
Author information
Authors and Affiliations
Corresponding author
Additional information
Conflict of Interest Statement
The authors declare that there are no conflicts of interest related to this work.
This work was supported by grants from Double thousand talents program of Jiangxi province (No. jxsq2019101021), the National Natural Science Foundation of China (No. 82060222), the Natural Science Foundation of Jiangxi Province (No. 20181BAB205030), the Key R & D Plan of Jiangxi Science and Technology Agency-General Project (No. 20192BBG70031), and Administration of Traditional Chinese Medicine of Jiangxi Province (No. 2021B101).
Rights and permissions
About this article
Cite this article
Fang, X., Li, Fj. & Hong, Dj. Potential Role of Akkermansia muciniphila in Parkinson’s Disease and Other Neurological/Autoimmune Diseases. CURR MED SCI 41, 1172–1177 (2021). https://doi.org/10.1007/s11596-021-2464-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11596-021-2464-5