Summary
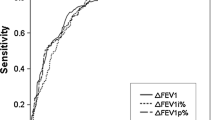
Changes of maximum expiratory flow at 25% and 50% of vital capacity (MEF25 and MEF50, respectively), and predominant parameters indicating small airways function in asthmatics before and after bronchodilator (BD) reversibility test have been less interpreted. Our study aimed to investigate the clinical role of changes of MEF25 and MEF50 before and after BD reversibility test in diagnosing asthma. Forced expiratory volume in the first second (FEV1), MEF25, and MEF50 were measured before and after BD reversibility test in 207 asthmatic patients using standard process. Forty healthy individuals were enrolled as controls. Receiver operating characteristic (ROC) curve was used to assess the diagnostic accuracy of reversibility of MEF25 and MEF50 before and after BD reversibility test (ΔMEF25% and ΔMEF50%, respectively) in diagnosing asthma. Among these functional criteria, ΔMEF25% and ΔMEF50% ≥ 25% performed the best diagnostic performance. The sensitivity, specificity, and accuracy of ΔMEF25% ≥ 25% as an objective diagnostic test for asthma were 63.29%, 87.50%, and 67.21%, and of ΔMEF50% ≥ 25% were 79.23%, 85.00%, and 80.16%, respectively. The area under the ROC curve of the indicators was 0.8203 and 0.9104, respectively. By contrast, an increase in FEV1 ≥ 12% and 200 mL demonstrated a sensitivity of 62.32%, specificity of 82.50%, and accuracy of 65.59% in diagnosing asthma. The changes of MEF25 and MEF50 before and after BD reversibility test may be of additional value in the clinical diagnosis of asthma, with cutoff values of 25% being the most.
Similar content being viewed by others
References
Global Initiative for Asthma (GINA). Global strategy for asthma management and prevention, http://ginasthma.org/; 2016 [accessed 20 March 2016].
Miller MR, Hankinson J, Brusasco V, et al. Standardisation of spirometry. Eur Respir J, 2005,26(2):319–338
Tavares ECA, Matos P, Tavares B, et al. Alternative functional criteria to assess airflow-limitation reversibility in asthma. Rev Port Pneumol, 2015,21(02):69–75
Hyatt RE, Scanlon PD, Nakamura M. Interpretation of Pulmonary Function Tests: A Practical Guide. 4th edition. Philadelphia: Wolters Kluwer Healt, 2014
Hamid Q. Pathogenesis of small airways in asthma. Respiration, 2012,84(1):4–11
Finkas LK, Martin R. Role of small airways in asthma. Immunol Allergy Clin North Am, 2016,36(3):473–482
Burgel, PR. The role of small airways in obstructive airway diseases. Eur Respir Rev, 2011,20(119):23–33
American Thoracic Society. Guidelines for methacholine and exercise challenge testing-1999. Am J Respir Crit Care Med, 2000,161:309–329
Vergnon JM, Costes F, Bayon MC, et al. Efficacy of tracheal and bronchial stent placement on respiratory functional tests. Chest, 1995,107(3):741–746
Wu JZ, Ma LJ, Zhao LM, et al. Significance of fractional exhaled nitric oxide combined with serum procalcitonin and C-reactive protein in evaluation of elderly asthma. J Huazhong Univ Sci Technolog Med Sci, 2013,33(2):185–188
van den Berge M, Ten Hacken NHT, Cohen J, et al. Small airway disease in asthma and COPD: Clinical implications. Chest, 2011,139(2):412–423
Bonini M, Usmani OS. The role of the small airways in the pathophysiology of asthma and chronic obstructive pulmonary disease. Ther Adv Respir Dis, 2015,9(6):281–293
Takishima T. Flow-volume curve and small airway diseases. Kokyu to Junkan, 1977,25(1):19–26
Gelb AF, Macanally BJ. Early detection of obstructive lung disease by analysis of maximal expiratory flow-volume curves. Chest, 1973,64(6):749–753
Guo XX, Deng NS, Chen QH, et al. Application of Inflammatory Markers in Induced Sputum in Stable Chronic Obstructive Pulmonary Disease Patients With Positive Bronchodilation Tests. Curr Med Sci, 2019,39(4):560–567
Corvol H, Burchard EG. Pharmacogenetic response to albuterol among asthmatics. Pharmacogenomics, 2008,9(5):505–510
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that there is no conflict of interest with any financial organization or corporation or individual that can inappropriately influence this work.
Additional information
This project was supported by the National Natural Science Foundation of China (No. 81970024) and partly by Scientific Research Project of Wuhan Health Committee (No. WX16C45).
Rights and permissions
About this article
Cite this article
Guo, Xx., Liu, Xf., Wang, Al. et al. The Clinical Role of Changes of Maximum Expiratory Flow at 25% and 50% of Vital Capacity before and after Bronchodilator Reversibility Test in Diagnosing Asthma. CURR MED SCI 40, 677–682 (2020). https://doi.org/10.1007/s11596-020-2237-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11596-020-2237-6