Summary
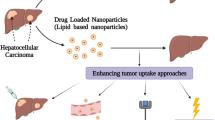
Developing the methodologies that allow for safe and effective delivery of therapeutic drugs to target sites is a very important research area in cancer therapy. In this study, polyethylene glycol (PEG)-coated magnetic polymeric liposome (MPL) nanoparticles (NPs) assembled from octadecyl quaternized carboxymethyl chitosan (OQC), PEGylated OQC, cholesterol, and magnetic NPs, and functionalized with epithelial growth factor receptor (EGFR) peptide, were successfully prepared for in-vivo liver targeting. The two-step liver targeting strategy, based on both magnetic force and EGFR peptide conjugation, was evaluated in a subcutaneous hepatocellular carcinoma model of nude mouse. The results showed that EGFR-conjugated MPLs not only accumulated in the liver by magnetic force, but could also diffuse into tumor cells as a result of EGFR targeting. In addition, paclitaxel (PTX) was incorporated into small EGFR-conjugated MPLs (102.0±0.7 nm), resulting in spherical particles with high drug encapsulation efficiency (>90%). The use of the magnetic targeting for enhancing the transport of PTX-loaded EGFR-conjugated MPLs to the tumor site was further confirmed by detecting PTX levels. In conclusion, PTX-loaded EGFR-conjugated MPLs could potentially be used as an effective drug delivery system for targeted liver cancer therapy.
Similar content being viewed by others
References
Zhong GC, Liu Y, Chen N, et al. Reproductive factors, menopausal hormone therapies and primary liver cancer risk: a systematicreview and dose-response meta-analysis of observational studies. Hum Reprod Update, 2016,23(1):126–138
Ling D, Xia H, Park W, et al. pH-sensitive nanoformulated triptolide as a targeted therapeutic strategy for hepatocellular carcinoma. ACS Nano, 2014,8(8):8027–8039
Wong MCS, Huang JLW, George J, et al. The changing epidemiology of liver diseases in the Asia-Pacific region. Nat Rev Gastroenterol Hepatol, 2019,16(1):57–73
Llovet JM, Zucman-Rossi J, Pikarsky E, et al. Hepatocellular carcinoma. Nat Rev Dis Primers, 2016,2:16018
Pinyol R, Montal R, Bassaganyas L, et al. Molecular predictors of prevention of recurrence in HCC with sorafenib as adjuvant treatment and prognostic factors in the phase 3 STORM trial. Gut, 2019,68(6):1065–1075
Abdelmoneem MA, Mahmoud M, Zaky A, et al. Dual-Targeted Casein Micelles as Green Nanomedicine for Synergistic Phytotherapy of Hepatocellular Carcinoma. J Control Release, 2018,287:78–93
Prieto J, Melero I, Sangro B. Immunological landscape and immunotherapy of hepatocellular carcinoma. Nat Rev Gastroenterol Hepatol, 2015,12(12):681–700
Dhanasekaran R, Venkatesh SK, Torbenson MS, et al. Clinical implications of basic research in hepatocellular carcinoma. J Hepatol, 2016,64(3):736–745
Kwon S, Singh RK, Kim TH, et al. Luminescent mesoporous nanoreservoirs for the effective loading and intracellular delivery of therapeutic drugs. Acta Biomater, 2014,10(3):1431–1442
Ponnappan N, Chugh A. Nanoparticle-Mediated Delivery of Therapeutic Drugs. Pharmaceut Med, 2015,29(3):155–167
Andreu A, Fairweather N, Miller AD. Clostridium Neurotoxin Fragments as Potential Targeting Moieties for Liposomal Gene Delivery to the CNS. Chembiochem, 2008,9(2):219–231
Valent P, Akin C, Metcalfe DD. FIP1L1/PDGFRA is a molecular marker of chronic eosinophilic leukaemia but not for systemic mastocytosis. Eur J Clin Invest, 2007,37(2):153–154
Lukasiewicz S, Szczepanowicz K, Blasiak E, et al. Biocompatible Polymeric Nanoparticles as Promising Candidates for Drug Delivery. Langmuir, 2015,31(23):6415–6425
Shi J, Kantoff PW, Wooster R, et al. Cancer nanomedi cine: progress, challenges and opportunities. Nat Rev Cancer, 2017,17(1):20–37
Khavari A, Orafa Z, Hashem M, et al. Different physical delivery systems: An important approach for delivery of biological molecules in vivo. J Paramed Sci, 2016,7(1):48
Amemiya Y, Tanaka T, Yoza B, et al. Novel detection system for biomolecules using nano-sized bacterial magnetic particles and magnetic force microscopy. J Biotechnol, 2005,120(3):308–314
Wang J, Morabito K, Erkers T, et al. Capture and separation of biomolecules using magnetic beads in a simple microfluidic channel without an external flow device. Analyst, 2013,138(21):6573–6581
Obaid G, Chambrier I, Cook MJ, et al. Cancer targeting with biomolecules: a comparative study of photodynamic therapy efficacy using antibody or lectin conjugated phthalocyanine-PEG gold nanoparticles. Photochem Photobiol Sci, 2015,14(4):737–747
Lu W, Tan YZ, Hu KL, et al. Cationic albumin conjugated pegylated nanoparticle with its transcytosis ability and little toxicity against blood-brain barrier. Int J Pharm, 2005,295(1–2):247–260
Lockman PR, Oyewumi MO, Koziara JM, et al. Brain uptake of thiamine-coated nanoparticles. J Control Release, 2003,93(3):271–282
Turner F, Smith G, Sachse C, et al. Vegetable, fruit and meat consumption and potential risk modifying genes in relation to colorectal cancer. Int J Cancer, 2004,112(2):259–264
Chavanpatil MD, Khdair A, Gerard B, et al. Surfactant-Polymer Nanoparticles Overcome P-Glycoprotein-Mediated Drug Efflux. Mol Pharm, 2007,4(5):730–738
Gibbs PE, Miralem T, Lerner-Marmarosh N, et al. Nanoparticle Delivered Human Biliverdin Reductase-Based Peptide Increases Glucose Uptake by Activating IRK/Akt/GSK3 Axis: The Peptide Is Effective in the Cell and Wild-Type and Diabetic Ob/Ob Mice. J Diabetes Res, 2016,2016(2):4712053
Jafari M, Karunaratne DN, Sweeting CM, et al. Modification of a designed amphipathic cell-penetrating peptide and its effect on solubility, secondary structure, and uptake efficiency. Biochemistry, 2013,52(20):3428–3435
Fan M, Yang D, Liang X, et al. Design and biological activity of epidermal growth factor receptor-targeted peptide doxorubicin conjugate. Biomed Pharmacother, 2015,70:268–273
Cheng L, Huang FZ, Cheng LF, et al. GE11-modified liposomes for non-small cell lung cancer targeting: preparation, ex vitro and in vivo evaluation. Int J Nanomedicine, 2014,9(Issue 1):921–935
Liang X, Shi B, Wang K, et al. Development of self-assembling peptide nanovesicle with bilayers for enhanced EGFR-targeted drug and gene delivery. Biomaterials, 2016,82:194–207
Liang XF, Wang HJ, Jiang XG, et al. Development of monodispersed and functional magnetic polymeric liposomes via simple liposome method. J Nanopart Res, 2010,12(5):1723–1732
Zhao M, Chang J, Fu X, et al. Nano-sized cationic polymeric magnetic liposomes significantly improves drug delivery to the brain in rats. J Drug Target, 2012,20(5):416–421
Gogoi M, Jaiswal MK, Sarma HD, et al. Biocompatibility and therapeutic evaluation of magnetic liposomes designed for self-controlled cancer hyperthermia and chemotherapy. Integr Biol (Camb), 2017,9(6):555–565
Romberg B, Oussoren C, Snel CJ, et al. Pharmacokinetics of poly(hydroxyethyl-l-asparagine)-coated liposomes is superior over that of PEG-coated liposomes at low lipid dose and upon repeated administration. Biochimica et Biophysica Acta, 2007,1768(3):0–743.
Liang X, Sun Y, Duan Y, et al. Synthesis and characterization of PEG-graft-quaternized chitosan and cationic polymeric liposomes for drug delivery. J Appl Polym Sci, 2012,125(2):1302–1309
Maruyama K. Intracellular targeting delivery of liposomal drugs to solid tumors based on EPR effects. Adv Drug Deliv Rev, 2011,63(3):161–169
Mohamed NK, Hamad MA, Hafez MZ, et al. Nanomedicine in management of hepatocellular carcinoma: Challenges and opportunities. Int J Cancer, 2017,140(7):1475–1484
Spangenberg HC, Thimme R, Blum HE. Targeted therapy for hepatocellular carcinoma. Nat Rev Gastroenterol Hepatol, 2009,6(7):423–432
Augusto V, Josep ML. Targeted Therapies for Hepatocellular Carcinoma. Gastroenterology, 2011,140(5):1410–1426
Liang XF, Wang HJ, Luo H, et al. Characterization of novel multifunctional cationic polymeric liposomes formed from octadecyl quaternized carboxymethyl chitosan/cholesterol and drug encapsulation. Langmuir, 2008,24(14):7147–7153
Suk JS, Xu Q, Kim N, et al. PEGylation as a strategy for improving nanoparticle-based drug and gene deliver. Adv Drug Deliv Rev, 2016,99(Pt A):28–51
Gabizon AA, Patil Y, La-Beck NM. New insights and evolving role of pegylated liposomal doxorubicin in cancer therapy. Drug Resist Updat, 2016,29:90–106
Qhattal HS, Hye T, Alali A, et al. Hyaluronan Polymer Length, Grafting Density, and Surface Poly(ethylene glycol) Coating Influence in Vivo Circulation and Tumor Targeting of Hyaluronan-Grafted Liposomes. ACS Nano, 2014,8(6):5423–5440
Liang HF, Chen CT, Chen SC, et al. Paclitaxel-loaded poly(g-glutamic acid)-poly(lactide) nanoparticles as a targeted drug delivery system for the treatment of liver cancer. Biomaterials, 2006,27(9):2051–2059
Ruttala HB, Ramasamy T, Poudal BK, et al. Molecularly targeted co-delivery of a histone deacetylase inhibitor and paclitaxel by lipid-protein hybrid nanoparticles for synergistic combinational chemotherapy. Oncotarget, 2017,8(9):14925–14940
Zhao Y, Wang X, Wang T, et al. Acetylcholinesterase, a key prognostic predictor for hepatocellular carcinoma, suppresses cell growth and induces chemosensitization. Hepatology, 2011,53(2):493–503
Abeylath SC, Ganta S, Iyer AK, et al. Combinatorial-designed multifunctional polymeric nanosystems for tumor-targeted therapeuticdelivery. Acc Chem Res, 2011,44(10):1009–1017
Acknowledgements
We would like to thank the Instrumental Analysis Centre of Shanghai University for providing materials characterization.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Conflict of Interest Statement
The authors have no conflicts of interest to declare.
This research was supported by the Research Program Foundation of the Department of Education of Fujian Province for Young Talents (No. JK2017021) and the Training Program of Department of Health of Fujian Province for Young Talents (No. 2017-ZQN-41).
Rights and permissions
About this article
Cite this article
Lin, Zl., Ding, J., Sun, Gp. et al. Application of Paclitaxel-loaded EGFR Peptide-conjugated Magnetic Polymeric Liposomes for Liver Cancer Therapy. CURR MED SCI 40, 145–154 (2020). https://doi.org/10.1007/s11596-020-2158-4
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11596-020-2158-4