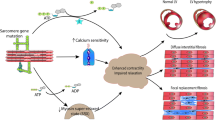
Summary
β-myosin heavy chain mutations are the most frequently identified basis for hypertrophic cardiomyopathy (HCM). A transgenic mouse model (αMHC403) has been extensively used to study various mechanistic aspects of HCM. There is general skepticism whether mouse and human disease features are similar. Herein we compare morphologic and functional characteristics, and disease evolution, in a transgenic mouse and a single family with a MHC mutation. Ten male αMHC403 transgenic mice (at t-5 weeks, −12 weeks, and −24 weeks) and 10 HCM patients from the same family with a β-myosin heavy chain mutation were enrolled. Morphometric, conventional echocardiographic, tissue Doppler and strain analytic characteristics of transgenic mice and HCM patients were assessed. Ten male transgenic mice (αMHC403) were examined at ages −5 weeks, −12 weeks, and −24 weeks. In the transgenic mice, aging was associated with a significant increase in septal (0.59±0.06 vs. 0.64±0.05 vs. 0.69±0.11 mm, P<0.01) and anterior wall thickness (0.58±0.1 vs. 0.62±0.07 vs. 0.80±0.16 mm, P<0.001), which was coincident with a significant decrease in circumferential strain (−22%±4% vs. −20%±3% vs. −19%±3%, P=0.03), global longitudinal strain (−19%±3% vs. −17%±2% vs. −16%±3%, P=0.001) and E/A ratio (1.9±0.3 vs. 1.7±0.3 vs. 1.4±0.3, P=0.01). The HCM patients were classified into 1st generation (n=6; mean age 53±6 years), and 2nd generation (n=4; mean age 32±8 years). Septal thickness (2.2±0.9 vs. 1.4±0.1 cm, P<0.05), left atrial (LA) volume (62±16 vs. 41±5 mL, P=0.03), E/A ratio (0.77±0.21 vs. 1.1±0.1, P=0.01), E/e’ ratio (25±10 vs. 12±2, P=0.03), global left ventricular (LV) strain (−14%±3% vs. −20%±3%, P=0.01) and global LV early diastolic strain rate (0.76±0.17 s−1 vs. 1.3±0.2 s-1, P=0.01) were significantly worse in the older generation. In β-myosin heavy chain mutations, transgenic mice and humans have similar progression in morphologic and functional abnormalities. The αMHC403 transgenic mouse model closely recapitulates human disease.
Similar content being viewed by others
References
Seidman CE, Seidman JG. Molecular genetic studies of familial hypertrophic cardiomyopathy. Basic Res Cardiol, 1998,93(Suppl 3):13–16
Maron BJ. Hypertrophic cardiomyopathy: a systematic review. JAMA, 2002,287(10):1308–1320
Hernandez OM, Housmans PR, Potter JD. Invited review: pathophysiology of cardiac muscle contraction and relaxation as a result of alterations in thin filament regulation. J Appl Physiol, 2001,90(3):1125–1136
Marian AJ, Roberts R. The molecular genetic basis for hypertrophic cardiomyopathy. J Mol Cell Cardiol, 2001,33(4):655–670
Morimoto S. Sarcomeric proteins and inherited cardiomyopathies. Cardiovasc Res, 2008,77(4):659–666
Tsoutsman T, Bagnall RD, Semsarian C. Impact of multiple gene mutations in determining the severity of cardiomyopathy and heart failure. Clin Exp Pharmacol Physiol, 2008,35(11):1349–1357
Redwood CS, Moolman-Smook JC, Watkins H. Properties of mutant contractile proteins that cause hypertrophic cardiomyopathy. Cardiovasc Res, 1999,44(1):20–36
Watkins H, McKenna WJ, Thierfelder L, et al. Mutations in the genes for cardiac troponin T and alpha-tropomyosin in hypertrophic cardiomyopathy. N Engl J Med, 1995,332(16): 1058–1064
Watkins H, Rosenzweig A, Hwang DS, et al. Characteristics and prognostic implications of myosin missense mutations in familial hypertrophic cardiomyopathy. N Engl J Med, 1992,326(17):1108–1114
Geisterfer-Lowrance AA, Christe M, Conner DA, et al. A mouse model of familial hypertrophic cardiomyopathy. Science, 1996,272(5262):731–734
Georgakopoulos D, Christe ME, Giewat M, et al. The pathogenesis of familial hypertrophic cardiomyopathy: early and evolving effects from an alpha-cardiac myosin heavy chain missense mutation. Nat Med, 1999,5(3):327–330
McConnell BK, Fatkin D, Semsarian C, et al. Comparison of two murine models of familial hypertrophic cardiomyopathy. Circ Res, 2001,88(4):383–389
Vikstrom KL, Factor SM, Leinwand LA. Mice expressing mutant myosin heavy chains are a model for familial hypertrophic cardiomyopathy. Mol Med, 1996,2(5):556–567
de Simone G, Wallerson DC, Volpe M, et al. Echocardiographic measurement of left ventricular mass and volume in normotensive and hypertensive rats. Necropsy validation. Am J Hypertens, 1990,3(9):688–696
Nishimura RA, Appleton CP, Redfield MM. Noninvasive Doppler echocardiographic evaluation of left ventricular filling pressures in patients with cardiomyopathies: a simultaneous Doppler echocardiographic and cardiac catheterization study. J Am Coll Cardiol, 1996,28(5):1226–1233
Bauer M, Cheng S, Jain M, et al. Echocardiographic speckle-tracking based strain imaging for rapid cardiovascular phenotyping in mice. Circ Res, 2011,108(8):908–916
Devereux RB, Alonso DR, Lutas EM, et al. Echocardiographic assessment of left ventricular hypertrophy: comparison to necropsy findings. Am J Cardiol, 1986,57(6):450–458
Pavlopoulos H, Nihoyannopoulos P. Strain and strain rate deformation parameters: from tissue Doppler to 2D speckle tracking. Int J Cardiovasc Imaging, 2008,24(5):479–491
Pirat B, McCulloch ML, Zoghbi WA. Evaluation of global and regional right ventricular systolic function in patients with pulmonary hypertension using a novel speckle tracking method. Am J Cardiol, 2006,98(5):699–704
Korinek J, Wang J, Sengupta PP, et al. Two-dimensional strain-a Doppler-independent ultrasound method for quantitation of regional deformation: validation in vitro and in vivo. J Am Soc Echocardiogr, 2005,18(12):1247–1253
Langeland S, D’Hooge J, Wouters PF, et al. Experimental validation of a new ultrasound method for the simultaneous assessment of radial and longitudinal myocardial deformation independent of insonation angle. Circulation, 2005,112(14):2157–2162
Leitman M, Lysyansky P, Sidenko S, et al. Two-dimensional strain-a novel software for real-time quantitative echocardiographic assessment of myocardial function. J Am Soc Echocardiogr, 2004,17(10):1021–1029
Serri K, Reant P, Lafitte M, et al. Global and regional myocardial function quantification by two-dimensional strain: application in hypertrophic cardiomyopathy. J Am Coll Cardiol, 2006,47(6):1175–1181
Wang Q, Moncman CL, Winkelmann DA. Mutations in the motor domain modulate myosin activity and myofibril organization. J Cell Sci, 2003,116(Pt 20):4227–4238
Kim SJ, Iizuka K, Kelly RA, et al. An alpha-cardiac myosin heavy chain gene mutation impairs contraction and relaxation function of cardiac myocytes. Am J Physiol, 1999,276(5 Pt 2):H1780–1787
Tyska MJ, Hayes E, Giewat M, et al. Single-molecule mechanics of R403Q cardiac myosin isolated from the mouse model of familial hypertrophic cardiomyopathy. Circ Res, 2000,86(7):737–744
Seidman JG, Seidman C. The genetic basis for cardiomyopathy: from mutation identification to mechanistic paradigms. Cell, 2001,104(4):557–567
Ferrans VJ, Morrow AG, Roberts WC. Myocardial ultrastructure in idiopathic hypertrophic subaortic stenosis. A study of operatively excised left ventricular outflow tract muscle in 14 patients. Circulation, 1972,45(4):769–792
Spindler M, Saupe KW, Christe ME, et al. Diastolic dysfunction and altered energetics in the alphaMHC403/+ mouse model of familial hypertrophic cardiomyopathy. J Clin Invest, 1998,101(8):1775–1783
Semsarian C, Ahmad I, Giewat M, et al. The L-type calcium channel inhibitor diltiazem prevents cardiomyopathy in a mouse model. J Clin Invest, 2002,109(8):1013–1020
Louie EK, Edwards LC, 3rd. Hypertrophic cardiomyopathy. Prog Cardiovasc Dis, 1994,36(4):275–308
Maron BJ, McKenna WJ, Danielson GK, et al. American College of Cardiology/European Society of Cardiology clinical expert consensus document on hypertrophic cardiomyopathy. A report of the American College of Cardiology Foundation Task Force on Clinical Expert Consensus Documents and the European Society of Cardiology Committee for Practice Guidelines. J Am Coll Cardiol, 2003,42(9):1687–1713
Pinamonti B, Di Lenarda A, Nucifora G, et al. Incremental prognostic value of restrictive filling pattern in hypertrophic cardiomyopathy: a Doppler echocardiographic study. Eur J Echocardiogr, 2008,9(4):466–471
Factor SM, Butany J, Sole MJ, et al. Pathologic fibrosis and matrix connective tissue in the subaortic myocardium of patients with hypertrophic cardiomyopathy. J Am Coll Cardiol, 1991,17(6):1343–1351
Shah PM. Hypertrophic cardiomyopathy and diastolic dysfunction. J Am Coll Cardiol, 2003,42(2):286–297
Gwathmey JK, Warren SE, Briggs GM, et al. Diastolic dysfunction in hypertrophic cardiomyopathy. Effect on active force generation during systole. J Clin Invest, 1991,87(3):1023–1031
Cannon RO, 3rd, Schenke WH, Maron BJ, et al. Differences in coronary flow and myocardial metabolism at rest and during pacing between patients with obstructive and patients with nonobstructive hypertrophic cardiomyopathy. J Am Coll Cardiol, 1987,10(1):53–62
Betocchi S, Hess OM, Losi MA, et al. Regional left ventricular mechanics in hypertrophic cardiomyopathy. Circulation, 1993,88(5 Pt 1):2206–2214
Rajiv C, Vinereanu D, Fraser AG. Tissue Doppler imaging for the evaluation of patients with hypertrophic cardiomyopathy. Curr Opin Cardiol, 2004,19(5):430–436
Koyama J, Ray-Sequin PA, Falk RH. Longitudinal myocardial function assessed by tissue velocity, strain, and strain rate tissue Doppler echocardiography in patients with AL (primary) cardiac amyloidosis. Circulation, 2003,107(19):2446–2452
Bellavia D, Pellikka PA, Abraham TP, et al. Evidence of impaired left ventricular systolic function by Doppler myocardial imaging in patients with systemic amyloidosis and no evidence of cardiac involvement by standard two-dimensional and Doppler echocardiography. Am J Cardiol, 2008,101(7):1039–1045
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Luo, Hc., Pozios, I., Vakrou, S. et al. Age-related changes in familial hypertrophic cardiomyopathy phenotype in transgenic mice and humans. J. Huazhong Univ. Sci. Technol. [Med. Sci.] 34, 634–639 (2014). https://doi.org/10.1007/s11596-014-1329-6
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11596-014-1329-6