Summary
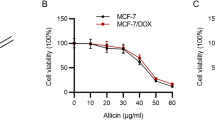
Ac-Phe-Lys-PABC-DOX (PDOX) is a smart doxorubicin (DOX) prodrug designed to decrease toxicities while maintaining the potent anticancer effects of DOX. This study was aimed at elucidating the effectiveness and toxicities of DOX and PDOX in patient-derived MCF-7 breast cancer cells in vitro. The MCF-7 cells were exposed to both PDOX and DOX, and cytotoxicities, cell cycle and P53/P21 signaling alterations were studied. Abundant cathepsin B was found in the MCF-7 cells, and treatment with PDOX and DOX triggered dose- and time-dependent cytotoxicity and resulted in a significant reduction in cell viability. The IC50 of PDOX and DOX was 3.91 and 0.94 μmol/L, respectively. Both PDOX and DOX caused an up-regulation of the P53/P21-related signal pathway, and PDOX significantly increased expression of P53 and caspase 3, and arrested the cell cycle at the G1/G2 phase. As compared with DOX, PDOX reduced toxicities, and it may have different action mechanisms on breast cancer cells.
Similar content being viewed by others
References
Sleeman J, Steeg PS. Cancer metastasis as a therapeutic target. Euro J Cancer, 2010,46(7):1177–1180
Jemal A, Bray F, Center MM, et al. Global cancer statistics. Ca Cancer J Clin, 2011 (61):69–90
Groner B, Weiss A. Targeting survivin in cancer: novel drug development approaches. Bio Drugs, 2014,28(1):27–39
Ferreira AL, Matsubara LS, Matsubara BB. Anthracyclineinduced cardiotoxicity. Cardiovasc Hematol Agents Med Chem, 2008,6(4):278–281
Sawyer DB. Anthracyclines and heart failure. N Engl J Med, 2013,368(12):1154–1156
Turk V, Stoka V, Vasiljeva O, et al. Cysteine cathepsins: from structure, function and regulation to new frontiers. Biochim Biophys Acta, 2012,1824(1):68–88
Rothberg JM, Bailey KM, Wojtkowiak JW, et al. Acidmediated tumor proteolysis: contribution of cysteine cathepsins. Neoplasia, 2013,15(10):1125–1137
Raghav N, Singh M. Design, synthesis and docking studies of bischalcones based quinazoline-2(1H)-ones and quinazoline-2(1H)-thiones derivatives as novel inhibitors of cathepsin B and cathepsin H. Eur J Pharm Sci, 2014,54(1):28–39
Dubowchik GM, Firestone RA. Cathepsin B-sensitive dipeptide prodrugs. 1. A model study of structural requrements for efficient release of doxorubicin. Bioorg Med Chem, 1998,8(23):3341–3346
Dubowchik GM, Mosure K, Knipe JO, et al. Cathepsin B-sensitve dipeptide prodrugs. 2. Models of anticancer drugs paclitaxel (taxol), mitomycin c and doxorubicin. Bioorgan Med Chem, 1998,8(23):3347–3352
Dubowchik GM, Firestone RA, Padilla L, et al. Cathepsin B-labile dipeptide linkers for lysosomal release of doxorubicin from internalizing immunoconjugates: model studies of enzymatic drug release and antigen-specific in vitro anticancer activity. Bioconjug Chem, 2002,13(4):855–869
Shao LH, Liu SP, Hou JX, et al. Cathepsin B cleavable novel prodrug Ac-Phe-Lys-PABC-ADM enhances efficacy at reduced toxicity in treating gastric cancer peritoneal carcinomatosis: an experimental study. Cancer, 2012,118(11):2986–2996
Wang Q, Zhong YJ, Yuan JP, et al. Targeting therapy of hepatocellular carcinoma with doxorubicin prodrug PDOX increases anti-metastatic effect and reduces toxicity: a preclinical study. J Transl Med, 2013,11(1):192
Peng C, Liu XL, Chen C, et al. Patterns of cancer invasion revealed by QDs-based quantitative multiplexed imaging of tumor microenvironment. Biomaterials, 2011,32(11):2907–2917
Meyer HJ, Wilke H. Treatment strategies in gastric cancer. Dtsch Arztebl Int, 2011,108(41):698–705
Bang YJ, Van Cutsem E, Feyerislova A, et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet, 2010,376(9742):687–697
Scott AM, Lee FT, Jones R, et al. A phase I trial of humanized monoclonal antibody A33 in patients with colorectal carcinoma: biodistribution, pharmacokinetics, and quantitative tumor uptake. Clin Cancer Res, 2005,11(13): 4810–4817
Hanaki H, Yamamoto H, Sakane H, et al. An anti-Wnt5a antibody suppresses metastasis of gastric cancer cells in vivo by inhibiting receptor-mediated endocytosis. Mol Cancer Ther, 2012,11(2):298–307
Schmid B, Chung DE, Warnecke A, et al. Albuminbinding prodrugs of camptothecin and doxorubicin with an Ala-Leu-Ala-Leu-linker that are cleaved by cathepsin B: synthesis and antitumor efficacy. Bioconjug Chem, 2007,18(3):702–716
Kawajiri H, Yashiro M, Shinto O, et al. A novel transforming growth factor beta receptor kinase inhibitor, A-77, prevents the peritoneal dissemination of scirrhous gastric carcinoma. Clin Cancer Res, 2008,14(9):2850–2860
Huynh H, Chow PK, Soo KC. AZD6244 and doxorubicin induce growth suppression and apoptosis in mouse models of hepatocellular carcinoma. Mol Cancer Ther, 2007,6(9):2468–2476
Yang S, Nqo VC, Lew GB, et al. AZD6244 (ARRY-142886) enhances the therapeutic efficacy of sorafenib in mouse models of gastric cancer. Mol Cancer Ther, 2009,8(9):2537–2545
Lebrecht D, Geist A, Ketelsen UP, et al. The 6-maleimidocaproyl hydrazone derivative of doxorubicin (DOXO-EMCH) is superior to free doxorubicin with respect to cardiotoxicity and mitochondrial damage. Int J Cancer, 2007,120(4):927–934
Kratz F, Fichtner I, Graeser R. Combination therapy with the albumin-binding prodrug of doxorubicin (INNO-206) and doxorubicin achieves complete remissions and improves tolerability in an ovarian A2780 xenograft model. Invest New Drugs, 2012,30(4):1743–1749
Unger C, Haring B, Medinger M, et al. Phase I and pharmacokinetic study of the (6-maleimidocaproyl) hydrazone derivative of doxorubicin. Clin Cancer Res, 2007,13(16):4858–4866
Chen Q, Fei J, Wu L, et al. Detection of cathepsin B, cathepsin L, cystatin C, urokinase plasminogen activator and urokinase plasminogen activator receptor in the sera of lung cancer patients. Oncol Lett, 2011,2(4):693–699
Abu Ajaj K, Graeser R, Fichtner I, et al. In vitro and in vivo study of an albumin-binding prodrug of doxorubicin that is cleaved by cathepsin B. Cancer Chemother Pharmacol, 2009,64(2):413–418
Schally AV, Naqy A. Cancer chemotherapy based on targeting of cytotoxic peptide. Eur J Endocrinol, 1999,141(1):1–14
Cummings J, McArdle CS. Studies on the in vivo disposition of adriamycin in human tumours which exhibit different responses to the drug. Br J Cancer, 1986,53(6): 835–838
Shukla A, Hillegass JM, MacPherson MB, et al. Blocking of ERK1 and ERK2 sensitizes human mesothelioma cells to doxorubicin. Mol Cancer, 2010,9:314
Cagnol S, Chambard JC. ERK and cell death: mechanisms of ERK-induced cell death—apoptosis, autophagy and senescence. FEBS J, 2010,277(1):2–21
Okumura T. Mechanisms by which thiazolidinediones induce anti-cancer effects in cancers in digestive organs. J Gastroenterol, 2010,45(11):1097–1102
Pritchard AL, Hayward NK. Molecular pathways: mitogenactivated protein kinase pathway mutations and drug resistance. Clin Cancer Res, 2013,19(9):2301–2319
Zhong Y, Liu SP, Firestone RA, et al. Anticancer effects of Ac-Phe-Lys-PABC-doxorubicin via mitochondria-centered apoptosis involving reactive oxidative stress and the ERK1/2 signaling pathway in MGC-803 cells. Oncol Rep, 2013,30(4):1681–1686
Author information
Authors and Affiliations
Corresponding author
Additional information
These authors contributed equally to this work.
This project was supported by the grants from the Science Fund for Doctorate Mentors by Ministry of Education of China (No. 20120141110042), New Strategies to Treat Peritoneal Carcinomatosis from Hubei Sciences and Technology Bureau, China (No. 2008BCC011, and No. 2060402-542), and the Fundamental Research Fund for the Central Universities of China (No. 2012303020208).
Rights and permissions
About this article
Cite this article
Zhang, J., He, L., Geng, Xf. et al. Anti-cancer effects of novel doxorubicin prodrug PDOX in MCF-7 breast cancer cells. J. Huazhong Univ. Sci. Technol. [Med. Sci.] 34, 521–528 (2014). https://doi.org/10.1007/s11596-014-1309-x
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11596-014-1309-x