Summary
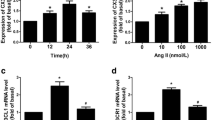
This study examined the effect of astilbin on the proliferation of rat aortic smooth muscle cells (RASMCs) induced by angiotensin II (AngII) and explored the possible mechanisms. Cell proliferation model of RASMCs was induced by treatmente with AngII. Cells were randomly divided to 8 groups. Normally cultured VSMCs serves as blank control group; in AngII model group, cells were treated with AngII at 10−7 mol/L; in three astilbin groups, cells were treated with 10, 15, 30 mg/L of astilbin; in three AngII+astilbin groups, cells were treated with AngII (at 10−7 mol/L) and astilbin at 10, 15, 30 mg/L. Cell proliferation ability was detected by MTT method and the cell cycles and proliferation index were flow cytometrically determined. The expression of c-myc mRNA was assessed by using reverse transcription polymerase chain reaction (RT-PCR), and the expression of NF-κB in RASMCs was immunocytochemically observed. Our results showed that MTT metabolism in RASMCs in the basic and AngII stimulated situation was inhibited by astilbin, and the cells numbers of G0/G1 phase were increased and that of G2/S phase were decreased markedly. Not only highly expression of c-myc gene stimulated by AngII could be inhibited by Astilbin significantly, but also the expression of NF-κB protein can be down regulated by Astilbin. We are led to conclude that astilbin astilbin can inhibit the AngII-mediated proliferation of RASMCs by blocking the transition of RASMCs from G0/G1 phase to S phase and by down-regulating the expression of NF-κB, c-myc gene.
Similar content being viewed by others
References
Schmauss D, Weis M. Cardiac allograft vasculopathy: recent developments. Circulation, 2008,117(16):2131–2141
Martínez-Dolz L, Almenar L, Reganon E, et al. Follow-up study on the utility of von Willebrand factor levels in the diagnosis of cardiac allograft vasculopathy. J Heart Lung Transplant, 2008,27(7):760–766
Yan R, Xu Q. Astilbin selectively facilitates the apoptosis of interleukin-2-dependent phytohemagglutinin-activated jurkat cells. Pharmacol Res, 2001,44(2):135–139
Gao SH, Li P, Pan TC, et al. Astilbin induces apoptosis of activated T cells of mouse heart transplantation model with acute rejection in vitro. Chin J Exp Surg (Chinese), 2004,21(4): 502
Han LK, Ninomiya H, Tangiguchi M, et al. Norepinephrine-augmenting lipolytic effectors from Astilbe thunbergii rhizomes. J Nat Prod, 1998,61(8):1006–1011
Gao SH, Pan TC. Protective effects of Astilbin on rat isolated heart reperfusion injury. Chin J Pract Chin Mod Med (Chinese), 2003,3(16):1693–1694
Zhao J, Li P, Zhang Y, et al. The inhibitory effect of Astilbin on the arteriosclerosis of murine thoracic aorta transplantat. J Huazhong Univ Sci Technolog [Med Sci], 2009,29(2):212–214
Moien-Afshari F, McManus BM, Laher I. Immunosuppression and transplant vascular disease: benefits and adverse effects. Pharmacol Ther, 2003,100(2):141–156
Gao SH, Chen T, Pan TC. Effect of Astilbin on expression of Bax,Bcl-2 and caspase-3 in allograft reactive T cells. Chin J Pract Chin Mod Med (Chinese), 2003,3(16): 2110–2111
Gao SH, Li P, Pan TC. Effect of astilbin on expression of Fas, FasL, TNF-R1 and TNF-R2 in allograft reactive T cells. Chin J Pract Chin Mod Med (Chinese), 2004,4(17): 217–218
Cai Y, Chen T, Xu Q, et al. Astilbin suppresses collagen-induced arthritis via the dysfunction of lymphocytes. Inflamm Res, 2003,52(8):334–340
Cai Y, Chen T, Xu Q, et al. Astilbin suppresses delayed-type hypersensitivity by inhibiting lymphocyte migration. J Pharm Pharmacol, 2003,55(5):691–696
Robbiani DF, Bothmer A, Callen E, et al. AID is required for the chromosomal breaks in c-myc that lead to c-myc/IgH translocations. Cell, 2008,135(6):1028–1038
Kumar N, Patowary A, Sivasubbu S, et al. Silencing c-MYC expression by targeting quadruplex in P1 promoter using locked nucleic acid trap. Biochemistry, 2008,47(50):13179–88
Nahmias C, Strosberg D. The angiotensin AT recep tor: searching for signal transduction pathways and physiological function. Trends Pharmacol Sci, 1995,16:223–225
Cotterman R, Jin VX, Krig SR, et al. SN-Myc regulates a widespread euchromatic program in the human genome partially independent of its role as a classical transcription factor. Cancer Res, 2008,68(23):9654–9662
Kang OH, Jang HJ, Chae HS, et al. Anti-inflammatory mechanisms of resveratrol in activated HMC-1 cells: pivotal roles of NF-kappaB and MAPK. Pharmacol Res, 2009,59(5):330–337
Author information
Authors and Affiliations
Corresponding author
Additional information
This work was supported by a grant from the National Natural Science Foundation of China (No. 30500656).
Rights and permissions
About this article
Cite this article
Li, P., Gao, S., Jie, W. et al. Astilbin inhibits proliferation of rat aortic smooth muscle cells induced by angiotensin II and down-regulates expression of protooncogene. J. Huazhong Univ. Sci. Technol. [Med. Sci.] 32, 181–185 (2012). https://doi.org/10.1007/s11596-012-0032-8
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11596-012-0032-8