Summary
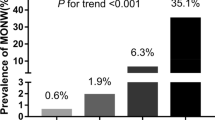
This study investigated the variation of serum monocyte chemoattractant protein-1 (MCP-1) in patients with both diabetes mellitus (DM) and metabolic syndrome (MS). Based on the International Diabetes Federation (IDF) diagnostic criteria, 93 patients enrolled in this study were divided into four groups: normal control (NC), simple DM, simple MS, and DM plus MS (DM-MS) groups. The main measures included height, weight, waist circumference (WC), hip circumference, blood pressure, fasting blood glucose, insulin resistance index (HOMA-IR), serum triglyceride (TG), HDL-ch, LDL-ch, and MCP-1. The results showed that the serum levels of MCP-1 in the DM-MS group were significantly increased as compared with those in the DM and MS groups (P<0.05), and the increase in the MCP-1 level in the DM group was much higher than in the MS group (P<0.05). The DM-MS group had the highest HOMA-IR levels, followed by MS, DM and NC groups (P<0.05). Correlation tests showed that the association of MCP-1 with age, HDL-ch, or LDL-ch was insignificant, whereas that of MCP-1 with body mass index (BMI), waist hip rate (WHR), WC, systolic blood pressure (SBP), diastolic blood pressure (DBP), TG, and HOMA-IR was significantly positive. It was concluded that circulating MCP-1 was substantially increased in patients with both DM and MS as compared with that in the patients with DM or MS alone, and the central obese state may contribute to a more vicious proinflammatory condition and insulin resistance in patients with diabetes.
Similar content being viewed by others
References
Isomaa B, Almgren P, Tuomi T, et al. Cardiovascular morbidity and mortality associated with the metabolic syndrome. Diabetes Care, 2001, 24(4):683–689
Klein BE, Klein R, Lee KE. Components of the metabolic syndrome and risk of cardiovascular disease and diabetes in Beaver Dam. Diabetes Care, 2002, 25(10):1790–1794
Alberti KG, Zimmet P, Shaw J. Metabolic syndrome—a new world-wide definition. A Consensus Statement from the International Diabetes Federation. Diabet Med, 2006, 23(5):469–480
Balkau B, Charles MA. Comment on the provisional report from the WHO consultation. European Group for the Study of Insulin Resistance (EGIR). Diabet Med, 1999, 16(5):442–443
Alberti KG, Zimmet PZ. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabet Med, 1998, 15(7):539–553
Executive summary of the third report of the national cholesterol education program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (adult treatment panel III), JAMA, 2001, 285(19): 2486–2497
Alberti KG, Zimmet P, Shaw J. The metabolic syndrome—a new worldwide definition. Lancet, 2005, 366(9491):1059–1062
Tsubakimoto A, Saito I, Mannami T, et al. Impact of metabolic syndrome on brachial-ankle pulse wave velocity in Japanese. Hypertens Res, 2006, 29(1):29–37
Miyaki K, Hara A, Naito M, et al. Two new criteria of the metabolic syndrome: prevalence and the association with brachial-ankle pulse wave velocity in Japanese male workers. J Occup Health, 2006, 48(2):134–140
Haffner SM, Lehto S, Ronnemaa T, et al. Mortality from coronary heart disease in subjects with type 2 diabetes and in nondiabetic subjects with and without prior myocardial infarction. N Engl J Med, 1998, 339(4):229–234
Vaccaro O, Eberly LE, Neaton JD, et al. Impact of diabetes and previous myocardial infarction on long-term survival: 25-year mortality follow-up of primary screenees of the Multiple Risk Factor Intervention Trial. Arch Intern Med, 2004, 164(13):1438–1443
Evans JM, Wang J, Morris AD. Comparison of cardiovascular risk between patients with type 2 diabetes and those who had had a myocardial infarction: cross sectional and cohort studies. BMJ, 2002, 324(7343):939–942
Alexander CM, Landsman PB, Teutsch SM, et al. NCEP-defined metabolic syndrome, diabetes, and prevalence of coronary heart disease among NHANES III participants age 50 years and older. Diabetes, 2003, 52(5): 1210–1214
Wallace TM, Matthews DR. The assessment of insulin resistance in man. Diabet Med, 2002, 19(7):527–534
Day C. Metabolic syndrome, or what you will: definitions and epidemiology. Diab Vasc Dis Res, 2007, 4(1):32–38
Cai D, Yuan M, Frantz DF, et al. Local and systemic insulin resistance resulting from hepatic activation of IKK-beta and NF-kappaB. Nat Med, 2005, 11(2):183–190
Bugianesi E, McCullough AJ, Marchesini G. Insulin resistance: a metabolic pathway to chronic liver disease. Hepatology, 2005, 42(5):987–1000
Hotamisligil GS, Arner P, Caro JF, et al. Spiegelman, Increased adipose tissue expression of tumor necrosis factor-alpha in human obesity and insulin resistance. J Clin Invest, 1995, 95(5):2409–2415
Arkan MC, Hevener AL, Greten FR, et al. IKK-beta links inflammation to obesity-induced insulin resistance. Nat Med, 2005, 11(2):191–198
Weisberg SP, Hunter D, Huber R, et al. CCR2 modulates inflammatory and metabolic effects of high-fat feeding. J Clin Invest, 2006, 116(1):115–124
Kanda H, Tateya S, Tamori Y, et al. MCP-1 contributes to macrophage infiltration into adipose tissue, insulin resistance, and hepatic steatosis in obesity. J Clin Invest, 2006, 116(6):1494–1505
Weisberg SP, McCann D, Desai M, et al. Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest, 2003, 112(12):1796–1808
de Luca C, Olefsky JM. Inflammation and insulin resistance. FEBS Lett, 2008, 582(1):97–105
Boring L, Gosling J, Chensue SW, et al. Impaired monocyte migration and reduced type 1 (Th1) cytokine responses in C-C chemokine receptor 2 knockout mice. J Clin Invest, 1997, 100(10):2552–2561
Gosling J, Slaymaker S, Gu L, et al. MCP-1 deficiency reduces susceptibility to atherosclerosis in mice that overexpress human apolipoprotein B. J Clin Invest, 1999, 103(6):773–778
McDermott DH, Yang Q, Kathiresan S, et al. CCL2 polymorphisms are associated with serum monocyte chemoattractant protein-1 levels and myocardial infarction in the Framingham Heart Study. Circulation, 2005, 112(8):1113–1120
Kamei N, Tobe K, Suzuki R, et al. Overexpression of monocyte chemoattractant protein-1 in adipose tissues causes macrophage recruitment and insulin resistance. J Biol Chem, 2006, 281(36):26 602–26 614
Simeoni E, Hoffmann MM, Winkelmann BR, et al. Association between the A-2518G polymorphism in the monocyte chemoattractant protein-1 gene and insulin resistance and Type 2 diabetes mellitus. Diabetologia, 2004, 47(9):1574–1580
Kaur S, Panicker SR, James T, et al. Association of monocyte chemoattractant protein-1 -2518 polymorphism with metabolic syndrome in a South Indian cohort, Metab Syndr Relat Disord, 2009, 7(3):193–198
Eckel RH, Grundy SM, Zimmet PZ. The metabolic syndrome. Lancet, 2005, 365(9468):1415–1428
Ezenwaka CE, Nwagbara E, Seales D, et al. Insulin resistance, leptin and monocyte chemotactic protein-1 levels in diabetic and non-diabetic Afro-Caribbean subjects, Arch Physiol Biochem, 2009, 115(1):22–27
Blaha V, Andrys C, Smahelova A, et al. Effect of atorvastatin on soluble CD14, CD40 Ligand, sE- and sP-selectins and MCP-1 in patients with type 2 diabetes mellitus: relationship to cholesterol turnover. Pharmacol Res, 2006, 54(6):421–428
Mine S, Okada Y, Tanikawa T, et al. Increased expression levels of monocyte CCR2 and monocyte chemoattractant protein-1 in patients with diabetes mellitus. Biochem Biophys Res Commun, 2006, 344(3):780–785
Matthews DR, Hosker JP, Rudenski AS, et al. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia, 1985, 28(7):412–419
Kouyama K, Miyake K, Zenibayashi M, et al. Association of serum MCP-1 concentration and MCP-1 polymorphism with insulin resistance in Japanese individuals with obese type 2 diabetes. Kobe J Med Sci, 2008, 53(6):345–354
Nakamura K, Yamagishi S, Adachi H, et al. Circulating advanced glycation end products (AGEs) and soluble form of receptor for AGEs (sRAGE) are independent determinants of serum monocyte chemoattractant protein-1 (MCP-1) levels in patients with type 2 diabetes. Diabetes Metab Res Rev, 2008, 24(2):109–114
Sakallioglu EE, Ayas B, Lutfioglu M, et al. Gingival levels of monocyte chemoattractant protein-1 (MCP-1) in diabetes mellitus and periodontitis: an experimental study in rats. Clin Oral Investig, 2008, 12(1):83–89
Wang QY, Chen FQ. Clinical significance and different levels of urinary monocyte chemoattractant protein-1 in type 2 diabetes mellitus. Diabetes Res Clin Pract, 2009, 83(2):215–219
Sell H, Dietze-Schroeder D, Kaiser U, et al. Monocyte chemotactic protein-1 is a potential player in the negative cross-talk between adipose tissue and skeletal muscle. Endocrinology, 2006, 147(5):2458–2467
Bruun JM, Lihn AS, Verdich C, et al. Regulation of adiponectin by adipose tissue-derived cytokines: in vivo and in vitro investigations in humans. Am J Physiol Endocrinol Metab, 2003, 285(3):E527–533
Christiansen T, Richelsen B, Bruun JM. Monocyte chemoattractant protein-1 is produced in isolated adipocytes, associated with adiposity and reduced after weight loss in morbid obese subjects. Int J Obes (Lond), 2005, 29(1):146–150
Bruun JM, Lihn AS, Pedersen SB, et al. Monocyte chemoattractant protein-1 release is higher in visceral than subcutaneous human adipose tissue (AT): implication of macrophages resident in the AT. J Clin Endocrinol Metab, 2005, 90(4):2282–2289
Sartipy P, Loskutoff DJ. Monocyte chemoattractant protein 1 in obesity and insulin resistance. Proc Natl Acad Sci U S A, 2003, 100(12):7265–7270
Trøseid M, Lappegard KT, Claudi T, et al. Exercise reduces plasma levels of the chemokines MCP-1 and IL-8 in subjects with the metabolic syndrome. Eur Heart J, 2004, 25(4):349–355
Cornier MA, Dabelea D, Hernandez TL, et al. The metabolic syndrome. Endocr Rev, 2008, 29(7):777–822
Pickup JC. Inflammation and activated innate immunity in the pathogenesis of type 2 diabetes. Diabetes Care, 2004, 27(3):813–823
Hotamisligil GS. Inflammatory pathways and insulin action. Int J Obes Relat Metab Disord, 2003, 27(Suppl 3):S53–55
Wisse BE. The inflammatory syndrome: the role of adipose tissue cytokines in metabolic disorders linked to obesity. J Am Soc Nephrol, 2004, 15(11):2792–2800
Kim CS, Park HS, Kawada T, et al. Circulating levels of MCP-1 and IL-8 are elevated in human obese subjects and associated with obesity-related parameters. Int J Obes (Lond), 2006, 30(9):1347–1355
Author information
Authors and Affiliations
Corresponding author
Additional information
The study was supported by grants from the National Natural Sciences Foundation of China (No. 30800531, 30800523) and the Natural Sciences Foundation of Hubei Province of China (No. 2007AA302B05).
Rights and permissions
About this article
Cite this article
Li, H., Deng, X., Li, Z. et al. Variation of serum monocyte chemoattractant protein-1 in patients with diabetes and metabolic syndrome. J. Huazhong Univ. Sci. Technol. [Med. Sci.] 31, 312–316 (2011). https://doi.org/10.1007/s11596-011-0373-8
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11596-011-0373-8