Abstract
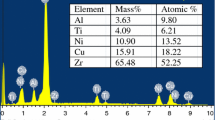
The non-isothermal and isothermal crystallization kinetics of Zr72.5Al10Fe17.5 glassy alloy was investigated using differential scanning calorimeter (DSC). Under non-isothermal heating condition, the primary phase in the initial crystallization is Zr6Al2Fe phase and the final crystallized products consist of Zr6Al2Fe, Zr2Fe and a-Zr phases. The apparent activation energy for crystallization estimated using the Kissinger method is 342.1 ± 8.1 kJ/mol. The local activation energy decreased with the increase in the crystallization volume fraction during nonisothermal crystallization. Under isothermal heating condition, the average Avrami exponent of about 2.76 implies a mainly diffusion-controlled three-dimensional growth with an increasing nucleation rate. The local activation energy for isothermal crystallization shows a different variation trend from that for nonisothermal crystallization, indicating different nucleation-and-growth mechanisms for the two crystallizaiton conditions.
Similar content being viewed by others
References
Wang W H, Dong C, Shek C H. Bulk Metallic Glasses[J]. Mater. Sci. Eng. R, 2004, 44(2-3): 45–89
Inoue A, Zhang T. Fabrication of Bulk Glassy Zr55Al10Ni5Cu30 Alloy of 30 mm in Diameter by a Suction Casting Method[J]. Mater. Trans. JIM., 1996, 37(2): 185–187
Lou H B, Wang X D, Xu F, et al. 73 mm-diameter Bulk Metallic Glass Rod by Copper Mould Casting[J]. Appl. Phys. Lett., 2011, 99(5): 051910
Hua N B, Li R, Wang H, et al. Formation and Mechanical Properties of Ni-free Zr-based Bulk Metallic Glasses[J]. J. Alloys Compd., 2011, 509(S1): S175–178
Hua N B, Pang S J, Li Y, et al. Ni-and Cu-free Zr-Al-Co-Ag Bulk Metallic Glasses with Superior Glass-forming Ability[J]. J. Mater. Res., 2011, 26(4): 539–546
Köster U, Meinhsrdt J, Roos S, et al. Formation of Quasicrystals in Bulk Glass Forming Zr-Cu-Ni-Al Alloys[J]. Appl. Phys. Lett., 1996, 69(2): 179–181
Kim Y H, Inoue A, Masumoto T. Ultrahigh Tensile Strengths of Al88Y2Ni9M1 (M=Mn or Fe) Amorphous Alloys Containing Finely Dispersed Fcc-Al Particles[J]. Mater. Trans. JIM., 1990, 31(8): 747–749
Wang J F, Li R, Hua N B, et al. Ternary Fe-P-C Bulk Metallic Glass with Good Soft-magnetic and Mechanical Properties[J]. Scripta Mater., 2011, 65(6): 536–539
Zhuang Y X, Duan T F, Shi H Y. Calorimetric Study of Non-isothermal Crystallization Kinetics of Zr60Cu20Al10Ni10 Bulk Metallic Glass[J]. J. Alloys Compd., 2011, 509(37): 9 019–9 025
An W K, Cai A H, Li J H, et al. Glass Formation and Non-isothermal Crystallization of Zr62.5Al12.1Cu7.95Ni17.45 Bulk Metallic Glass[J]. J. Non-Cryst. Solids, 2009, 355(34-36): 1 703–1 706
Patel A T, Shevde H R, Pratap A. Thermodynamics of Zr52.5Cu17.9Ni14.6Al10Ti5 Bulk Metallic Glass Forming Alloy[J]. J. Therm. Anal. Calorim., 2012, 107(1): 167–170
Savalia R T, Lad K N, Pratap A, et al. Study of Formation of Nanoquasicrystals and Crystallization Kinetics of Zr-Al-Ni-Cu Metallic Glass[J]. J. Therm. Anal. Calorim., 2004, 78(3): 745–751
Yokoyama Y, Fujita K, Yavari A R, et al. Malleable Hypoeutectic Zr-Ni-Cu-Al Bulk Glassy Alloys with Tensile Plastic Elongation at Room Temperature[J]. Philos. Mag. Lett., 2009, 89(5): 322–334
Hui X, Liu S N, Pang S J, et al. High-zirconium-based Bulk Metallic Glasses with Large Plasticity[J]. Scripta Mater., 2010, 63(2): 239–242
Hua N B, Li R, Wang J F, et al. Biocompatible Zr-Al-Fe Bulk Metallic Glasses with Large Plasticity[J]. Sci. China-Phys. Mech. Astron., 2012, 55(9): 1 664–1 669
Hua N B, Huang L, He W, et al. A Ni-free High-zirconium-based Bulk Metallic Glass with Enhanced Plasticity and Biocompatibility[J]. J Non-Cryst. Solids, 2013, 376: 133–138
Raghavan V. Al-Fe-Zr (Aluminum-Iron-Zirconium)[J]. J. Phase Equilib. Diff., 2006, 27(3): 284–287
Kissinger H E. Reaction Kinetics in Differential Thermal Analysis[J]. Anal. Chem.; 1957, 29(11): 1 702–1 706
Johnson W A, Mehl R F. Reaction Kinetics in Processes of Nucleation and Growth[J]. Trans. Am. Inst. Min. Metall. Pet. Eng., 1939, 135(54): 416–462
Ranganathan S, Heimendahi M V. The Three Activation Energies with Isothermal Transformations: Applications to Metallic Glasses[J]. J. Mater. Sci., 1981, 16(9): 2 401–2 404
Liu L, Wu Z F, Zhang J. Crystallization Kinetics of Zr55Cu30Al10Ni5 Bulk Amorphous Alloy[J]. J. Alloys Compd., 2002, 339(1-2): 90–95
Author information
Authors and Affiliations
Corresponding author
Additional information
Funded by the National Natural Science Foundation of China (No. 51401053), the China Postdoctoral Science Foundation (No.2015T80676), the Natural Science Foundation of Fujian Province (No.2014J05053), and the Postdoctoral Scientific Research Foundation of Fuzhou University (No.0180-601017)
Rights and permissions
About this article
Cite this article
Hua, N., Chen, W., Liu, X. et al. Crystallization kinetics of a high-zirconium-based glassy alloy: A DSC study. J. Wuhan Univ. Technol.-Mat. Sci. Edit. 31, 191–196 (2016). https://doi.org/10.1007/s11595-016-1351-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11595-016-1351-6