Abstract
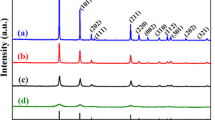
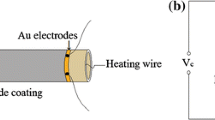
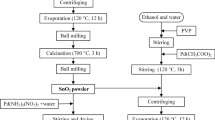
Using organo-tin Sn(OC4H9)4 as precursor, sodium dodecyl sulfonate (SDS) and SDS-gelatin (SDS-G) complex as template, two tin dioxide colloidal particles were prepared by a self-assembly method. Both SnO2 products were respectively labelled SnO2-B particles with SDS and SnO2-C particles with SDS-G, which are applied in fabricating SnO2 gas sensors corresponding to SnO2-B′ and SnO2-C′ sensors. X-ray diffraction (XRD), scanning electron microscopy (SEM), transmission electron microscopy (TEM) and thermogravimetry and different thermal analysis (TG/DTA) were used for characterizations. The experimental results show that SnO2-B colloidal particles are composed of mesoporous piece-like particles, while SnO2-C particles mainly consist of spherical particles. Gas sensing measurements show that SnO2-B′ sensor performs the best sensing response to all target gases, including H2, C2H5OH and liquid petroleum gas (LPG). In particular, the sensing response of SnO2-B′ sensor is achieved at 32 in H2 atmosphere at the concentration of 1000×10−6 M. The gas sensing mechanism was purposely discussed from the electron transfer process and the microstructures of the as-prepared SnO2 products. It is found that serious agglomerations in SnO2-B′ particles facilitate the high gas sensing performance of SnO2-B′ sensor, while mesoporous structures in SnO2-C′ particles decrease the gas sensing response of SnO2-C′ sensor.
Similar content being viewed by others
References
Shen Y B, Yamazaki T, Liu Z F, et al. Porous SnO2 Sputtered Films with High H2 Sensitivity at Low Operation Temperature[J]. Thin Solid Films, 2008, 516: 5 111–5 117
Adamyan A Z, Adamyan Z N, Aroutiounian V M, et al. Sol-gel Derived Thin-film Semiconductor Hydrogen Gas Sensor[J]. International Journal of Hydrogen Energy, 2007, 32: 4 101–4 108
Barsan N, Stetter J R, Findlay M, et al. High-performance Gas Sensing of CO: Comparative Tests for Semiconducting (SnO2-Based) and for Amperometric Gas Sensors[J]. Anal. Chem., 1999, 71: 2 512–2 517
Zhang Y, He X L, Li J P, et al. Fabrication and Ethanol-sensing Properties of Micro Gas Sensor based on Electrospun SnO2 Nanofibers[J]. Sensors and Actuators B, 2008, 132: 67–73
Adamowicz B, Izydorczyk W, Izydorczyk J, et al. Response to Oxygen and Chemical Properties of SnO2 Thin-film Gas Sensors[J]. Vacuum, 2008, 82: 966–970
Wang Y L, Jiang X C, Xia Y N. A Solution-Phase, Precursor Route to Polycrystalline SnO2 Nanowires That Can Be Used for Gas Sensing under Ambient Conditions[J]. J. Am. Chem. Soc., 2003, 125: 16176–16177
Wang B, Zhu L F, Yang Y H, et al. Fabrication of a SnO2 Nanowire Gas Sensor and Sensor Performance for Hydrogen[J]. J. Phys. Chem. C, 2008, 112: 6643–6647
Yu W D, Li X M, Gao X D, et al. Large-Scale Synthesis and Microstructure of SnO2 Nanowires Coated with Quantumsized ZnO Nanocrystals on a Mesh Substrate[J]. J. Phys. Chem. B, 2005, 109: 17078–17081
Duan J H, Yang S G, Liu H W, et al. Single Crystal SnO2 Zigzag Nanobelts[J]. J. Am. Chem. Soc., 2005, 127: 6 180–6 181
Sun S H, Meng G W, Zhang G X, et al. Controlled Growth and Optical Properties of One-dimensional ZnO Nanostructures on SnO2 Nanobelts[J]. Crystal Growth & Design, 2007, 7: 1 988–1 991
Sun J Q, Wang J S, Wu X C, et al. Novel Method for High-yield Synthesis of Rutile SnO2 Nanorods by Oriented Aggregation[J]. Crystal Growth & Design, 2006, 6: 1 584–1 587
Wang Y, Lee J Y, Deivaraj T C. Controlled Synthesis of V-shaped SnO2 Nanorods[J]. J. Phys. Chem. B, 2004, 108: 13 589–13 593
Wang G X, Park J S, Park M S, et al. Synthesis and High Gas Sensitivity of Tin Oxide Nanotubes[J]. Sensors and Actuators B, 2008, 131: 313–317
Hu J Q, Ma X L, Shang N G, et al. Large-scale Rapid Oxidation Synthesis of SnO2 Nanoribbons[J]. J. Phys. Chem. B, 2002, 106: 3823–3826
Vaezi M R. SnO2/ZnO Double-layer Thin Films: A Novel Economical Preparation and Investigation of Sensitivity and Stability of Double-layer Gas sensors[J]. Materials Chemistry and Physics, 2008, 110: 89–94
Wang J X, Sun X W, Xie S S, et al. Preferential Growth of SnO2 Triangular Nanoparticles on ZnO Nanobelts[J]. J. Phys. Chem. C, 2007, 111: 7671–7675
Kovalenko V V, Zhukova A A, Rumyantseva M N, et al. Surface Chemistry of Nanocrystalline SnO2: Effect of Thermal Treatment and Additives[J]. Sensors and Actuators B, 2007, 126: 52–55
Vaishampayan M V, Deshmukh R G, Mulla I S. Influence of Pd Doping on Morphology and LPG Response of SnO2[J]. Sensors and Actuators B, 2008, 131: 665–672
Huo Q S, Margolese D I, Ciesla U, et al. Organization of Organic Molecules with Inorganic Molecular Species Into Nanocomposite Biphase Arrays[J]. Chem. Mater., 1994, 6: 1 176–1 191
Galo J A A S, Clément S, Bénédicte L, et al. Chemical Strategies to Design Textured Materials: From Microporous and Mesoporous Oxides to Nanonetworks and Hierarchical Structures[J]. Chem. Rev., 2002, 102: 4 093–4 138
Liu X H, Kan C, Wang X, et al. Self-assembled Nanodisks with Targetlike Multirings Aggregated at The Air-water Interface[J]. J. Am. Chem. Soc., 2006, 128: 430–431
Ji H J, Liu X H, Wang X, et al. Effect of Proteins on The Selfassembly of Multiring Structural ZrO2 Nanodisks[J]. Colloids and Surfaces A: Physicochem. Eng. Aspects, 2009, 346: 1–4
Ji H J, Liu X H, Wang X, et al. Dimeric Sodium Naphthalene-1-sulfonate Aggregates Guided Self-assembly of TiO2/naphthylene Hybrid Nanocomposite Film[J]. Journal of Molecular Structure, 2009, 935: 8–12
Kan C, Liu X H, Wang X, et al. Effects of Different Anionic Surfactant Templates (H+A−, Na+A−) on Titanium Selfassembly: In The Air-water Interfacial Flms[J]. Colloids Surfaces A: Physicochem. Eng. Aspects, 2007, 311: 93–98
Author information
Authors and Affiliations
Corresponding author
Additional information
Founded by the National Natural Science Foundation of China (No. 50772048), the Natural Science Foundation of China and China Academy Engineering Physics (No. 10776014) and the innovation fund from the Graduate School of Nanjing University of Science and Technology
Rights and permissions
About this article
Cite this article
Ji, H., Liu, X., Wang, X. et al. Self-assembled SnO2 colloidal particles and their gas sensing performance to H2, C2H5OH and LPG. J. Wuhan Univ. Technol.-Mat. Sci. Edit. 26, 661–667 (2011). https://doi.org/10.1007/s11595-011-0287-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11595-011-0287-0