Abstract
The present work of review is focused on the recent advancements regarding the exploration of the ionic liquids, ionic liquids with the incorporation of nanoparticles of several materials, and ionic liquid-grafted nanoparticles operating as liquid electrodes in electrochemical cells and capacitors. The ionic liquids are generally synthesized at room temperature and by adding a solution, which can be an acid, a base, or a salt in water, and are composed of organic cations and a great number of charge-delocalized organic/inorganic anions. The electrochemical features such as the electrical conductivity and capacitance of the promising ionic liquids and their hybrids are addressed thoroughly, together with their influencing factors like the nature, concentration, and functionalization of the nanoparticles, type of base fluids, working temperature, and addition of surfactants. Moreover, this overview identifies and discusses the main applications of ionic liquids and their hybrids with nanoparticles in various possible electrochemical device configurations, along with a brief evaluation of the associated feasibility issues. Additionally, this survey of the published scientific papers on the subject enabled the listing and evaluation of the beneficial features related to the usage of these fluids including enhanced electrical conductivity and improved capacitance in comparison with the commonly employed solvents and electrolytes. Finally, it addresses the main problems associated with such types of fluids and outlines the primary prospects for further research and use of ionic liquids and their nanocomposites in different electrochemical technological applications.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The ionic liquids are low-melting salts typically consisting of organic cations and organic or inorganic anions. The ionic liquids exhibit many useful features such as negligible vapor pressure, improved thermal stability, high ionic conductivity, broad electrochemical potential windows, and enhanced solubility [1]. The ionic liquids generally have asymmetric ions with delocalized electrostatic charges [2]. The combination of strong Coulombic interactions and weak directional interactions such as hydrogen bonding, cation-π, and Van der Waals inductive and dispersion interactions induces the production of nano-scaled structures in the ionic liquids and in ionic liquid/molecular solvent or ionic liquid-solute mixtures [3]. For those ionic liquids with cations incorporating long alkyl side chains, the alkyl chains can segregate to form nonpolar domains, while other parts of the ionic liquid form polar domains [4]. With their highly hydrogen-bonded networks and nanodomains, ionic liquids can facilitate the dissolution of various substances, where the solutes tend to localize into the nano-scale domains for which they have higher affinity [5]. The ionic liquids are also used as reaction media acting as solvent and/or template [6]. The polymerized ionic liquids or polyionic liquids have been applied as reaction media [7] and in the areas of energy conversion and storage, catalysis, and separations because of the combining properties of polymers and ionic liquids [8]. Based on properties that include high ionic conductivity, non-flammability, and electrochemical and thermal stability, ionic liquids have a great potential to act as electrolytes in electrochemical devices like rechargeable metal batteries [9], fuel cells [10], dye-sensitized solar cells [11], and sensors [12]. Also, the ionic liquids and nanoparticles can form together various hybrid structures, depending on a balance of intra- and intermolecular interactions between them. For example, the ionic liquids serve as solvent media for colloidal dispersions [13], facilitating the dispersion of metal nanoparticles [14], nanostructured inorganic nanoparticles [15], and graphene [16] and carbon nanotubes [17], assisting the dissolution of cellulose [18] and dispersing the as-obtained nanocellulose [19]. Some colloidal nanoparticles can be stably suspended in ionic liquids without the need to add classical stabilizers such as surfactants and/or polymers [13]. Ionic liquids, contributing electrostatic forces on the surface of nanoparticles, can also be utilized as colloidal stabilizers for nanoparticles synthesized in aqueous solution [20, 21]. By joining nanoparticles with ionic liquids, the high electron conductivity of nanoparticles combines with the good proton-transfer property of ionic liquids, with additional benefits to electrochemical applications. Novel functional materials comprising nanoparticle and ionic liquid composites can be generated with improved features, sharing properties of both ionic liquids and nanoscale materials [22]. Figure 1 summarizes the main properties of the ionic liquids. Figure 2 summarizes the main applications of the ionic liquids.
Notable synergistic effects have been observed in materials that combine ionic liquids and nanoparticles or the so-called ionic liquid hybrids. The nanoparticle properties such as thermal stability, catalytic efficiency, adsorption efficiency, and electrical and electrochemical response can be enhanced via physical/chemical surface modification with ionic liquids [23]. On the other hand, due to the interactions between ionic liquids and nanoparticles, the physicochemical and/or electrochemical properties of the ionic liquid and nanoparticle hybrid can be superior to those of the ionic liquid alone [24]. For instance, by dispersing titania nanotubes into an ionic liquid-impregnated polymer matrix, the nanocomposite electrolytes exhibited a favorable ionic conductivity of 10−3 S/cm at 120 °C, high thermal stability of more than 250 °C, and better morphology (a decrease in the fraction of the crystalline domains and increase in the amorphous phase), delivering great potential for various device applications [25]. Also, the diffusion coefficient and electrical conductivity of 1-butyl-3-methylimidazolium hexafluorophosphate [Bmim][PF6] were enhanced by 35% and 65%, respectively, with the addition of 0.08% wt. copper nanoparticles encapsulated within carbon shell Cu@C [24]. The ionic liquid and nanoparticle hybrid structures are thermally and chemically stable even under extreme conditions. The unique characteristics of ionic liquid and nanoparticle hybrids show promise in various applications, including catalysis, electrochemistry, and separations. This review addresses emerging applications of ionic liquid and nanoparticle hybrid materials from the perspective of the relationship between material nanostructure and function. The current review provides an updated overview on select applications of ionic liquids and nanoparticle hybrid materials with a focus on their structure-based properties. It will discuss several types of ionic liquid and nanoparticle hybrid materials, including colloidal dispersions, colloidal gels and glasses, and ionic liquid-grafted nanoparticles. Also, there were addressed the better investigated recent applications of ionic liquid and nanoparticle hybrid materials in the fields of electrochemistry. The catalytic properties of nanoparticles as well as the ion and electron conductivity of both ionic liquid and nanoparticle components benefit the electrochemical processes. The intermolecular interactions in ionic liquid and nanoparticle hybrid systems act in tandem to organize materials with various structures. Stable colloidal dispersions, colloidal gels, or colloidal glasses can be achieved by suspending nanoparticles into ionic liquids.
The ionic liquids form a protective layer surrounding nanoparticles, improving their chemical and thermal stability. The ionic liquids can bond covalently on nanoparticle surfaces, thus combining the desired properties of the ionic liquid and nanoparticles. Other types of nanoparticle and ionic liquid hybrid materials include nanoparticles incorporated into ionic liquid-based preformed structures and nanoparticle-stabilized ionic liquid-containing emulsions. Ionic liquids and nanoparticles are also fabricated into other materials such as films/membranes to improve their performances. The development of ionic liquids comprises the selection of lithium salts or acids for anions, a design of the counter cations, and an assessment of the resulting combinations. Among various anions, which are commercially available in the form of lithium salts, bis(trifluoromethanesulfonyl)imide [TFSI] and hexafluorophosphate PF6 anions are suitable for battery applications. In the form of ionic liquids, the former has the potential to enhance high ionic conductivity at room temperature, and the latter possesses attractive electrochemical stability. The TFSI anions have been commonly employed to prepare ionic liquids owing to their tendency to widen the temperature range, in which the ionic liquids retain the liquid state. In addition to these, FSI anions have also been increasingly recognized as a potential anion for electrolytes. The ionic liquids consisting of FSI anions have low viscosity and high ionic conductivity, although FSI anions have a small ion size and high charge density. This is because the negative charge on FSI anions is delocalized by the fluorine atoms [26]. Moreover, the ionic liquids containing FSI anions are known to form solid electrolyte interphases, which allow a sufficient charge transfer of lithium and protect both the electrolyte itself and electrodes from decompositions [27]. Nevertheless, most of the ionic liquids containing FSI anions crystallize at sub-zero temperatures and are not sufficiently ion conductive below these temperatures [28, 29]. To suppress the crystallizing tendency of FSI anions, the anions should be combined with the cations with a delocalized charge or flexible substituents. Referring to cation structures, aliphatic cations such as pyrrolidinium, piperidinium, and phosphonium are preferred for battery applications because they are thermally and electrochemically stable due to the absence of unsaturated bonds [30]. Nonetheless, these cations tend to increase the melting point of the ionic liquids compared to imidazolium cations when they have the same alkyl chains and counter anions. This is due to the presence of p electrons in imidazolium cations which contributes to diminishing the melting temperature [31].
To prevent the crystallization of ionic liquids without the use of unsaturated bonds, the functionalization of the aliphatic cations by using alkoxy groups has been found to be an effective methodology [32]. Taking these facts into account, N-ethoxyethyl-N-methylpiperidinium FSI [P1,2O2][FSI] and N-ethoxyethyl-N-methylmorpholinium FSI [M1,2O2][FSI] were newly synthesized, and their combinations of ions were assessed in terms of thermal and electrochemical properties. These ionic liquids were mixed with LiFSI, and their properties were compared in relation to the presence and position of the ether oxygens. Figure 3 summarizes the fundamental applications of the ionic liquids with embedded nanoparticles.
Ionic liquids as electrolytes
In an energy storage-generation electrochemical device such as battery, fuel cell, or solar cell, the electrolyte serving as the medium of the transfer of charges defines how fast the energy can be released and undergoes no net chemical changes during the operation of the device [33]. To properly function, the electrolyte should be electrochemically stable (resistant to electrochemical reduction and oxidation), which requires a wide electrochemical window to avoid degradation within the range of the working potentials [34]. In addition, the electrolyte should have good ionic conductivity and electronic insulation. With a lower total cost and less environmental impact than disposable batteries, the demand for rechargeable batteries with improved energy storage capacity and increased charge/discharge cycling stability has increased in recent years. Nonetheless, following repeated charge and discharge cycles, metal dendrites would grow into the electrolyte and bridge the inter-electrode space, resulting in short-circuits and even fires. Classic electrolytes usually consist of salts dissolved in aqueous or organic solvents with high dielectric constants [35]. Polymer electrolytes have become an important class of electrolytes because they are inherently safer than classic electrolytes that are based on flammable organic solvents. Nonetheless, a major part of the polymers exhibiting high dielectric constants have poor ion transport properties [35]. The ionic liquids are being employed as electrolytes due to their inherent ionic conductivity and wide electrochemical windows. An electrochemical stability window of around 4.5 V and a conductivity in the range of 0.1–18 mS/cm has been reported for common ionic liquids electrolytes [36]. However, the high viscosity of ionic liquids hinders their ionic conductivity. Composite electrolytes containing a significant volume fraction of well-dispersed submicron particles in an insoluble second phase have been developed with an ionic conductivity that is improved compared to that of a single-phase electrolyte [37]. Through these interactions, ionic liquid and nanoparticle hybrids have been reported to increase the performance of an electrolyte with greater diffusion coefficient, higher electrical conductivity, and better thermal and electrochemical stability, which enables them to work well as electrolytes in cells and batteries.
For this application, many structures of ionic liquid and nanoparticle hybrids have been synthesized in cooperation with the electrolytes or electrodes, among which nanoparticle dispersions in ionic liquids and ionic liquid-grafted nanoparticles are the most common. The dispersions of nanoparticles in ionic liquids can be directly used as electrolytes. For instance, with the addition of 0.5% wt. of gold nanoparticles in [Emim][EtSO4], the capacitance of the resulting electrolyte was enhanced by 190% and the ionic conductivity was also significantly increased. The observed electrochemical enhancements have been linked to the contribution of the interaction energy between the ionic constituents and the nanoparticles, resulting from the attraction of [EtSO4]− anions to the gold nanoparticle surface, that subsequently cause a change in charge distribution and in ionic structure [38]. Moreover, the authors Chen et al. [39] employed the ionic liquid [Bmim][PF6] with 0.08% wt. copper nanoparticles encapsulated within a carbon shell Cu@C and demonstrated that this solution was an improved electrolyte for dye-sensitized solar cells, exhibiting a 35% larger coefficient of diffusion and 65% greater electrical conductivity compared to those obtained with the ionic liquid alone. The higher electrical conductivity of this ionic liquid electrolyte was attributed by the authors to the interactions between the Cu@C nanoparticles and the [Bmim]+ cations. The dispersion of Cu@C nanoparticles in ionic liquids might cause the ionic bonding energy between the [Bmim]+ cations and the [PF6]− anions of the ionic liquid to decrease, leading to an increase in the diffusion coefficient of the [Bmim]+ cations and a reduction of the viscosity of the ionic liquid. Also, researchers Neo and Ouyang [40] incorporated 0.1% wt. of multi-walled carbon nanotubes into 1-propyl-3-methylimidazolium iodide [Pmim]I and found that the formation of hydrogen bonds between the [Pmim]+ cation, and the carboxylic groups of the nanotubes reduced the coulombic attractions between the [Pmim]+ cations and the I− anions and, thus, consequently decreased the ionic liquid viscosity and improved the thermal stability. In addition to the enhancement of electrochemical and thermal properties of the ionic liquid electrolytes by the dispersion of nanoparticles, ionic liquids can improve the chemical stability of the electrolyte by forming protective ionic double-layers to prevent the nanoparticles from being corroded. A plasmonically enhanced thin dye-sensitized solar cell was reported to exhibit good stability with an electrolyte consisting of silver nanoparticles dispersed in an imidazolium-dicyanamide-based ionic liquid [41].
In the absence of added ionic liquid, the acetonitrile-based electrolyte, which is volatile and contains redox compounds that corroded the silver nanoparticles, would damage the long-term stability of the device and, consequently, degrade the device’s efficiency. However, the nonvolatile ionic liquid-based electrolyte protected the surface of silver nanoparticles from directly contacting the redox compounds by forming an ionic double-layer surrounding the surface of the silver nanoparticles, thus reducing the occurrence of corrosion. The ionic liquid-functionalized nanoparticles designated by NIMs have been explored as additives to increase the electrochemical stability of electrolytes. For example, the brush-like structure of the ionic liquid tethered to the nanoparticles assisted the dispersion of silica nanoparticles in a conventional propylene carbonate-LiTFSI-based electrolyte by providing steric as well as double-layer interactions [42]. The impermeable nanometer-sized particles formed a mechanically strong and highly tortuous porous mesh to prevent the formation of dendrites that could eventually bridge the cathode and anode and may result in short-circuits and thermal runaway. The NIMs have also been studied as electrolytes for lithium-ion batteries due to their high degree of particle dispersion, surface functionality, and ability to dissolve salts [43]. Also, the authors Moganty et al. [44] developed an ionic liquid electrolyte with enhanced electrochemical and thermal stabilities by covalently tethering one portion of a long-chain imidazolium ionic liquid to zirconia nanoparticle cores. Additionally, the cores of the nanoparticles enhanced the mechanical strength of the electrolyte, which resisted the formation of lithium dendrites. Figure 4 presents the evolution in time of the main formulations of the ionic liquid electrolytes.
In sum, the utilization of ionic liquids as electrolytes brings the benefits of lower oxidation and decomposition of the electrolytes, increased ion transference numbers (a low ion transference number may lead to charge concentration gradients that increase cell polarization, thereby deteriorating the charging-discharging performance), dendrite formation mitigation, and prevention of the formation of uneven solid electrolyte interphase (SEI). Figure 5 exposes the main problems of rechargeable lithium-ion batteries that can be suppressed with the use of ionic liquids as electrolytes.
Figure 6 summarizes the main advantages of using ionic liquid electrolytes in different electrochemical devices. Details of these applications shown in Fig. 6 will be discussed throughout the text. For example, in fuel cells, the use of ionic liquids increases the efficiency of the cells; in batteries, especially lithium batteries, they make them more chemically stable and improve their ionic conductivity, making them safer and with better performance. In dye-sensitized solar cells, they function as a thermal transfer fluid for solar collectors, and finally, in capacitors, they increase the charge storage capacity of the device.
Ionic liquids forms
Pure ionic liquids
A surprising number of devices used daily by everyone, from domestic to industrial levels, employ various types of fluids for heat transfer, distribution, or storage. However, analyzing the thermal performance of these fluids is indispensable for ensuring operability, reducing investment costs, maintaining safety levels, and minimizing environmental impacts. Within this context, certain properties of these fluids become prominent including the thermal and electrical conductivities, specific heat, viscosity, density, boiling, and flash points, among other properties. According to the authors Oster et al. [45], one of these options is the ionic liquids. The ionic liquids are compounds formed from molten organic salts at room temperature, composed of organic cations and many charge-delocalized inorganic and organic anions, with a melting point inferior to 100 °C [46]. Due to their enriched thermophysical properties, according to the authors Asleshirin et al. [47] and Ribeiro et al. [48], the ionic liquids have influenced in recent years the performance of equipment such as reactors, distillation columns, heat exchangers, and units for physical–chemical processing and reactions. The ionic liquids have negligible vapor pressure, improved thermal and electrochemical stabilities, and a broad solubility and affinity to several chemical compounds. All these properties can also be adjusted through the coupling of cations and anions via controlling the Van der Waals interactions and the Lewis acidity or basicity, among other ion functionalities. Based on the cation and anion combinations and their improved biological, physical, chemical, and electrochemical characteristics including the miscibility in aqueous solvents, ionic conductivity, and acidity or basicity degrees, the ionic liquids can be divided/classified into several categories.
Nonetheless, ionic liquids can be generally categorized into three fundamental types as protic, aprotic, and zwitterionic fluids. Besides, it should be stated that not all ionic liquids are in liquid state below 100 °C, and some of them merely exhibit a comparatively low glass transition temperature and slow crystallization kinetics. In the study conducted by researchers Chen et al. [49], it was demonstrated that ionic liquids are very suitable for applications in electrochemical systems designed to enhance the hydrogen evolution reaction. The authors emphasize that these liquids possess a unique combination of specific features including superior electrical conductivity, low vapor pressure, and high electrochemical stability, stemming from a large variety of available functional groups. Such attributes imbue them with superior characteristics compared to traditional liquids. An electrochemical stability window of approximately 4.5 V and an electrical conductivity range between 0.1 and 18 mS.cm−1 has been found for the commonly used ionic liquids. Nonetheless, the considerable viscosity of the ionic liquids may hinder the ionic conductivity of these fluids.
Ionic liquid hybrids
The electrolytes with a significant concentration of nanoparticles in an insoluble second phase have been produced exhibiting an improved ionic conductivity in reference to the one obtained with a single-phase electrolyte. By means of these interactions, the composite ionic liquid and nanoparticle have been found to improve the electrochemical performance of an electrolyte with enhanced electrical conductivity, electrochemical and thermal stabilities, and diffusion coefficient that enabled these hybrid fluids to operate smoothly as liquid electrolytes in cells and batteries. In this scope, different combinations of nanoparticles and ionic liquids have been adopted, making nanoparticle dispersions in ionic liquids one of the most explored. Various types of structured ionic liquid and nanoparticle hybrids have been developed based on a balance of several intermolecular interactions. These hybrids can combine the novel properties of both nanoparticles and ionic liquids, which benefit potential applications. This review addressed the synergistic effects of ionic liquid and nanoparticle hybrids in the context of emerging applications of such hybrids in the fields of separation, catalysis, and electrochemistry. To better support these applications, various structures of ionic liquid and nanoparticle hybrids have been explored. Stable nanoparticle dispersions in ionic liquids can be achieved when repulsive intermolecular interactions are stronger than attractive intermolecular interactions between neighboring nanoparticles [50].
The ionic liquid can form a layer around the nanoparticles to prevent them from aggregation and/or corrosion, which in general improves the working efficiency of devices or increases their performance under extreme operating conditions. For catalytic applications, the nanoparticles, especially metallic ones, usually play important roles as catalysts, while the ionic liquids are used to stabilize and protect the nanoparticles to improve their catalytic efficiency and recycling capability [51]. The rationally designed metal nanoparticle and ionic liquid composites have been applied as highly efficient catalysts in multiphase catalytic reactions [52]. Sharing the good proton-transfer properties of ionic liquids and the high electron conductivity of metal nanoparticles or carbon nanotubes, nanoparticle dispersions in the ionic liquids have been utilized in electrochemical applications such as batteries and electrochemical sensors.
The incorporation of ionic liquids and nanoparticles in electrolytes to be used in batteries has been shown to improve the diffusion coefficient of the cations in the ionic liquid electrolyte [53], electrical conductivity [54], thermal and electrochemical stabilities [55], and reduce the corrosion potential [56]. The incorporation of nanoparticles with catalytic properties can decrease the overpotentials of many electrochemical reactions, thus contributing to highly sensitive electrochemical sensors [57]. With special intermolecular interactions and unique properties of designable structures, nanoparticle dispersions in ionic liquids have been used in separation methods, especially solid-phase extraction. The good thermal stability and low volatility of ionic liquids make them promising alternatives for volatile organic solvents, while the high surface-area-to-volume ratio of nanoparticles contributes to high separation efficiency [58].
Figure 7 schematically represents the ion clusters surrounding the nanoparticles and the formation of a protective electric double layer by the ion clusters. In the diagram presented in the figure, a nanoparticle attracts ions and their clusters to its center through electrostatic forces. As a result of this interaction, a semi-organized ionically protective layer is formed, thus contributing to the stability of the electrolyte used in batteries, for example.
The nanoparticles can be surface-modified by chemical bonding with ionic liquids. The resulting ionic liquid-grafted nanoparticles can combine the properties of nanoparticles and ionic liquids and often exhibit improved properties when compared to those of the nanoparticles themselves and the bulk ionic liquids. The grafted ionic liquid can render nanoparticles stably dispersed in their working media, which increases the lifetime of such materials in applications like reusable catalysts and rechargeable electrolytes. The ionic liquid-grafted nanoparticles combine the advantages of homogenous and heterogeneous catalysts and facilitate the separation of the catalyst following the reaction [59]. The ionic liquid-grafted nanoparticle catalysts with high recoverability and reusability can further improve the reaction rate and yield.
The ionic liquid-functionalized nanoparticles have been applied as additives in electrolytes, whereby the brush-like structure of the ionic liquid-grafted nanoparticles increases the electrochemical stability by providing steric interactions and double-layer interactions [60]. The ionic liquid-grafted nanoparticles promote the conductivity and electron transfer ability of electrochemical sensors or serve as catalysts for electrochemical reactions. The nanoparticle-supported ionic liquids have been applied in chromatographic separations, with the optimal separation efficiency achieved by adjusting the hybrid material composition [61]. Figure 8 schematically represents the network structure in the 1,3-dialkylimidazolium ionic liquids with the addition of metal nanoparticles in the supramolecular ionic liquid network with electrostatic and steric stabilization was indicated by the formation of a suggested primary anion layer around the metal nanoparticles. This effect is like the illustrated in the scheme in Fig. 7, ions from ionic liquids can form an ionic double layer around the nanoparticles, similar to the Debye layer. This results in the emergence of an electrostatic repulsive force between these nanoparticles, preventing the agglomeration phenomenon [62].
In addition to nanoparticle dispersions and surface functionalization, ionic liquids and nanoparticles can be fabricated into other materials as desired to provide their synergistic effects. The colloidal gels are formed when unstable dispersed nanoparticles interconnect to form a three-dimensional network, while the colloidal glasses are obtained when the concentrated dispersed nanoparticles are trapped by the neighboring nanoparticles [63]. Other types of nanoparticle and ionic liquid hybrid structures include nanoparticle and ionic liquid-based liquid crystalline composites and nanoparticle-stabilized ionic liquid emulsions. Figure 9 presents the scheme of a non-structural organization in binary amorphous porous silicon nanoparticles and ionic liquids mixtures.
The ionic liquid and nanoparticle hybrids are often fabricated into membranes or films as coating materials for electrodes [64], as supported catalysts, or for gas separations. Improved performance of the membranes has been achieved following the incorporation of nanoparticles and ionic liquids. The membranes incorporating ionic liquids and nanoparticles have been widely studied to separate gases. The observed better selectivity for gas separations has been attributed to the interactions between the nanoparticles and ionic liquids and the reaction or separation media. An excellent synergistic effect of nanoparticle and ionic liquid film was observed, enhancing the catalytic activity and durability due to the establishment of a more efficient porous contact region between gas and liquid phases within the membrane structure. The film structure allowed for additional electrostatic interactions between the nanoparticles and other charged objects, which lead to enhanced conductivity and good electrochemical and thermal stability [65].
A higher sensibility and enhanced response were observed in the electrochemical sensors with films fabricated with ionic liquids and nanoparticles [66]. An improved understanding of the relationships between interactions, structure, and properties of ionic liquid and nanoparticle hybrids would facilitate the design of these materials for different purposes. For an intended application, an optimized performance under specified operating conditions can be achieved by a careful selection of nanoparticles and ionic liquids. The applications of ionic liquid and nanoparticle hybrids have expanded in recent years, motivated by the improved properties of such materials. Given their promising applications, further research on the structure–property relationships of nanoparticle and ionic liquid hybrids is posed to facilitate the rational design of such novel materials and their translation to practice. The nanoparticles can be synthesized and dispersed stably in ionic liquids without aggregation, because of the stabilization induced by the ionic liquid [67]. The ionic liquids can form cationic-anionic ion layers around the nanoparticles, providing electrostatic forces [68]. The imidazolium-based ionic liquids tend to form semi-organized supramolecular aggregates, with the [(Im)x − n(X)x] n − formula for the anionic aggregate and [(Im)x(X)x − n] n + for the cationic aggregate, where Im represents the imidazolium cation and X the anion [69]. The ionic liquid double layer offers electrostatic interactions that efficiently keep nanoparticles from aggregating through balancing with Van der Waals interactions between nanoparticles, a situation described by the Derjaguin–Landau–Verwey–Overbeek theory [70].
In addition to these forces, alkyl side chains of ionic liquids stretch far away from the surface of the nanoparticles, further contributing a secondary steric stabilization to impede the nanoparticles from approaching each other [71]. The hydrogen bonds within the ionic liquid structure and between ionic liquid and nanoparticle surfaces can also stabilize nanoparticles [72]. In a study on ultrafine of 2.6 ± 0.3 nm of diameter gold nanoparticles synthesized and stabilized in [Bmim][PF6] ionic liquid, the ionic liquid supramolecular aggregates were observed to be loosely coordinated to the nanoparticles of gold [73]. In general, it is hard to stably disperse graphene or other nanocarbons in water without surface functionalization or the incorporation of stabilizers and surfactants. Nonetheless, stable dispersions of reduced graphene oxide with 0.85 nm of thickness were obtained at relatively high concentrations of 7 mg/mL in 1-alkyl-3-methylimidazolium and in N-alkylpyridinium based ionic liquids without using any added surfactants/stabilizers [74]. π-electron-rich nanocarbons such as graphene and carbon nanotubes interact with the ionic liquids via short-range cation–π interactions and long-range dispersion interactions, which can trap neighboring graphene plates in their metastable structure of bilayer graphene without assembling into graphite [75]. Besides, the stabilization provided only by the ionic liquid, polymers, and/or surfactants that are compatible with the ionic liquid can physically adsorb or chemically graft on nanoparticle surfaces to assist the stabilization of nanoparticle dispersions in ionic liquids [76].
The polymer-surfactant layer on the surface of the nanoparticles will generate repulsive steric interactions when put in compression [77]. A magnetic fluid was prepared by dispersing magnetite nanoparticles with 7.4 nm of average diameter coated with oleate into 1-ethyl-3-methylimidazolium ethylsulfate Emim-EtSO4 ionic liquid with the addition of oleic acid as surfactant [78]. The oleic acid tended to form a double layer via hydrophobic interactions and stretched out from the surface of the magnetite nanoparticles, offering steric repulsion to achieve effective stabilization [79]. The poly(ionic liquid)s, sharing the properties of ionic liquid and polymer, are good stabilizers for nanoparticle dispersions and therefore can be applied in nanoparticle synthesis [80]. Through surface coating, nanoparticles can be modified by rationally designed poly(ionic liquid)s to prevent them from agglomeration and to obtain additional properties [80]. A triblock copolymer derived from imidazolium bromide-based ionic liquid was reported to stabilize the dispersion of graphene or multi-walled carbon nanotubes in water [81]. It was reported that gold, silver, and nickel nanoparticles showed to be stable without any agglomeration for periods longer than 75 days in the presence of poly(1-vinyl-3-alkyl imidazolium) type polymer ionic liquids. The polymer ionic liquids electrostatically interact with nanoparticles and tune the particle size, depending on the polymer ionic liquid chain length, which synergistically enhances the stability of the nanoparticles [23].
Figure 10 shows the main interactions involved and the types of polymeric ionic liquids. These solid electrolytes represented in Fig. 10 are alternatives to liquid electrolytes, which are not desirable for flexible projects since they need to be encapsulated [82]. On the other hand, the possibility of solidifying these liquids is interesting because it maintains the original ionic conductivity, whereas organic solvents, for example, do not.
In some cases, the nanoparticles cannot be stably dispersed in a solvent due to interactions and/or high concentration, and solid-like soft materials called colloidal gels and colloidal glasses can form. A colloidal gel can result from the dispersed phase forming an interconnected network structure [83], whereas a colloidal glass is formed when large concentrations of suspended nanoparticles are impeded from moving by neighboring nanoparticles through a cage effect. The colloidal gels that involve a three-dimensional nanoparticle and/or polymer network percolated throughout the ionic liquid can be designated as ionogels [84]. For example, an ionogel can be synthesized by dispersing 3% wt. of silica nanoparticles having 12 nm diameter and hydrophilic silanol Si–OH groups on the surface into 1-ethyl-3-methyl-imidazolium bis(trifluoromethanesulfonyl)amide [Emim][NTf2] ionic liquid, due to the formation of interconnected particulate silica networks in the ionic liquid [85]. For nanoparticles that are surface-modified with grafted polymers, the affinity between the grafted polymers and the ionic liquid solvents impacts the structure of the nanoparticle suspension. In an ionic liquid that is a bad solvent for the grafted polymer, the steric forces between neighboring nanoparticles diminish, and the unstable polymer-grafted nanoparticles attach to each other to form ionogels [86].
Also, the colloidal glasses form when the grafted polymers have an affinity with the ionic liquid they are dispersed in, which provides strong repulsive forces. At elevated volumetric concentrations, depending on their surface tension and size, the nanoparticles can form colloidal glasses [87]. For instance, a dispersion of poly(methyl methacrylate)-grafted silica nanoparticles in [Emim][NTf2] became a colloidal glass above between 0.70 and 0.74% vol. concentrations [88]. Acting as the structuring media for inorganic ionogel formation, the intrinsic organization and physicochemical properties of ionic liquids influence the interconnected network structure of the nanoparticles [89], resulting in ionic liquid colloidal gels that can be applied in batteries because of the improved ionic conductivity, while maintaining a solid-like rheological response [89]. A stimulus-responsive glass-to-gel transition is a noteworthy property that may contribute to applications such as thermo-sensitive detectors [90].
Ionic liquid–grafted nanoparticles
Apart from being physically adsorbed on nanoparticles or acting as dispersion media, the ionic liquids can be chemically bonded onto the surface of the nanoparticles. The ionic liquid layers immobilized onto the surface of desired solid support materials including nanoparticles are referred to as supported ionic liquids [91]. The immobilization of ionic liquid on nanoparticles can result in novel performance while retaining the useful properties of both moieties [92]. The nanoparticles functionalized with ionic liquids have tunable properties, such as better dispersibility and thermostability. For example, carbon nanotubes modified by imidazolium ionic liquids with Cl− or Br− counterions were dispersible in water, while those modified with imidazolium ionic liquids with BF4− or PF6− (hydrophobic) counterions were able to disperse in organic solvents such as CHCl3 [93]. The strong alkyl interactions between the ionic liquids and the graphitic support allowed the ionic liquids to be entrapped on the carbon nanotubes or graphene, which delivered the possibility to tune the nanocarbon surface properties by altering the temperature of synthesis [94]. The supported ionic liquids can also modify the physicochemical properties of the ionic liquids. For instance, the imidazolium-based ionic liquids immobilized onto the surface of SiOx nanoparticles were found to possess lower melting points in comparison to those of the neat ionic liquids [43].
Van der Waals interactions between the nanoparticles and the formation of intermolecular hydrogen bonds between the anions of ionic liquids and the silanol groups on the surface of SiOx nanoparticles decrease the mobility of ionic liquid cations near the interface, which trap the cations at a higher entropy state, and consequently lead to a melting temperature decrease. Another type of grafted nanoparticles is the nanoscale ionic materials comprising a nano-sized particle core and an oppositely charged canopy produced by ionic coupling, which are special types of grafted nanoparticles that have received great attention. The nanoscale ionic materials core can be functionalized with a charged corona that can be an ionic liquid fraction [95]. The dynamics of the ionic links bring up one of the primary characteristics of the nanoscale ionic materials, the similarity of their dynamic behavior and structure to that of the ionic liquids [96]. The nanoscale ionic materials are also referred to as nano-scaled ionic liquids, as they share properties with ionic liquids, including an ionic attraction between the components of the nanoscale ionic materials, a low-to-zero tended vapor pressure, and a high density of ionic groups. Resulting from the fluidization medium provided by the electrostatically grafted organic layer on the surface of the nanoparticles [97]. Also, the nanoscale ionic materials exhibit liquid-like characteristics without solvent in room conditions, which for this reason are also commonly designated by solvent-free nanofluids [98]. For example, by surface functionalizing with a thiol-containing ionic liquid followed by anion exchange of bromide for sulfonate, metal nanoparticles such as gold, palladium, and rhodium, can flow like a liquid at room temperature. The nanoscale ionic materials prepared by surface modification of silica cores through an alkyl silane monolayer paired with an amine-terminated poly(ethylene oxide)-poly(propylene oxide) block copolymer [99] canopy exhibited liquid-like behavior under ambient conditions in the absence of solvent, which was attributed to the rapid exchange of the block copolymer canopy with the ionically modified silica cores.
Also, the nanoscale ionic materials comprised of multi-walled carbon nanotubes exhibited good flowability with viscosities in the range of 20–110 Pa·s at 30 °C, depending on the molecular weight of the organic moiety. By varying the organic modifiers, i.e., polyethylene glycol-substituted tertiary amines with various molecular weights, the core–shell structure of the nanoscale ionic materials can be regulated, and the physical properties, like the rheological response, can be adjusted accordingly [100]. The fluid-like nano-scaled ionic materials can act as functional ionic liquids and as surface-functionalized nanoparticle dispersions. These materials are very useful to be applied in applications like lithium-ion rechargeable batteries, carbon capture materials, and catalysts. A gold nanorod ionic material could flow at room conditions and a significant and fully reversible optical response was verified in the cases where a shear force was applied, attributed to the formation of large gold nanorod clusters affecting the surface plasmon resonance [101]. The authors Luo et al. [102] prepared ionic liquid mixed with poly(methyl methacrylate)-grafted iron oxide nanoparticle aggregates at low particle concentrations which was found to exhibit different dynamics and ionic conductivity than that of pure ionic liquid in our previous studies. It was reported on the quasi-elastic neutron scattering results of ionic liquid containing polymer-grafted iron oxide nanoparticles at higher particle concentrations. The diffusivity of imidazolium HMIM+ cations of 1-hexyl-3-methylimidazolium bis(trifluoromethylsulfonyl)imide HMIM-TFSI in the presence of poly(methyl methacrylate)-grafted iron oxide nanoparticles were discussed through the confinement.
The analysis of the elastic incoherent structure factor revealed that the confinement radius decreased with the addition of grafted particles in the HMIM-TFSI-solvent mixture. It was proposed that the confinement promoted by the high concentration of grafted particles shrinks the HMIM-TFSI restricted volume. The authors further hypothesized that this enhanced diffusivity occurs because of the local ordering of cations within aggregates of poly(methyl methacrylate)-grafted iron oxide nanoparticles. Also, researchers Li et al. [103] prepared poly(methyl methacrylate)-b-poly(styrene sulfonate) PMMA-b-PSS copolymer-grafted iron oxide nanoparticles with different sulfonation levels from around 4.9 to 10.9% mol SS which were synthesized, and their concentration-dependent ionic conductivities were reported in acetonitrile and HMIm–TFSI-acetonitrile mixtures. It was found that conductivity enhancement with the particle concentration in acetonitrile derived from the aggregation of grafted nanoparticles resulted in sulfonic domain connectivity.
The ionic conductivity was found to be related to the effective hopping transfer within ionic channels. On the contrary, the conductivity decreased or remained constant with increasing particle concentration in HMIm–TFSI-acetonitrile. The result was attributed by the researchers to the ion coupling between ionic liquids and copolymer domains. A series of PMMA-b-PSS copolymer-grafted iron oxide nanoparticles at varying sulfonation levels were prepared to study the macroscopic ionic conductivities of particles solvated in HMIm–TFSI. It is known that pure HMIm–TFSI conducts ions in a vehicular mode. This work aims to understand how copolymer-grafted nanoparticles interfere with the conductivity of HMIm–TFSI. The SEM–EDS elemental mapping demonstrates the PMMA–TFSI and PSS-HMIm+ affinities. It was shown that the network of polymer-grafted particles establishes ion channels where ions hop through the percolating sulfonated polymer in acetonitrile, which significantly enhances the ionic conductivity with concentration. The equivalent circuit analysis of EIS data also supports that the percolating structures guide ion transport through hopping rather than free diffusion. Particles in HMIM–TFSI exhibited an opposite trend where conductivity decreased with grafted polymer concentration. The coupling of each polymer domain with the ionic liquid by ion–dipole interactions led to lower conductivity than that of the neat ionic liquids. The low graft density and polymer interactions with ionic liquids can enhance the dispersion state of the polymer-grafted nanoparticles but lower the transport of ionic liquid species. In conclusion, polymer–ion coupling and organization of sulfonated domains (in an ionic liquid or a solvent) both contribute to the ion transport in grafted-particle-based electrolytes.
Moreover, the NIMs (ionic liquid functionalized nanoparticles) have been incorporated into the electrolytes to enhance their electrochemical stability. For instance, the brush-like structure of the ionic liquid tethered to the nanoparticles promoted the silica nanoparticle suspension in a propylene carbonate-Li-TFSI electrolyte by offering steric and electric double-layer interactions [52]. The nanoparticles created a strong and tortuous porous mesh, preventing the production of dendrites that might bridge the anode and cathode resulting in short-circuits. Figure 11 shows the schematic representation of the ionic liquid EMIM-PF6 ionic liquid immobilized on a silica surface. The ionic liquid immobilized SiOx nanoparticles possess inferior melting points compared to the bulk ionic liquids, as indicated in the schematic.
The conductivity of the ionic liquids decreases markedly at low temperatures because of strong interactions arising between the component ions. Metal–organic frameworks (MOFs) are appropriate microporous host materials that can control the dynamics of the ionic liquids by the nanosizing of the ionic liquids and tunable interactions of the MOFs with the guest ionic liquids. In this direction, the authors Fujie et al. [104] reported on the ionic conductivity of an ionic liquid incorporated within a MOF. The system consisted of EMI-TFSA 1-ethyl-3-methylimidazolium bis-trifluoromethylsulfonyl-amide and ZIF-8 Zn-(MeIM)2, H(MeIM) ¼ 2-methylimidazole as the ionic liquid, and the MOF, respectively. While the ionic conductivity of the bulk EMI-TFSA ionic liquid displayed a strong reduction due to freezing, the EMI-TFSA@ZIF-8 showed no considerable reduction because of the phase transition absence. The ionic conductivity of EMI TFSA@ZIF-8 was greater than the one with the bulk EMI-TFSA at temperature values below 250 K.
Moreover, the researchers Chen et al. [105] introduced an ionic liquid electrolyte (ILE) into a metal–organic framework to synthesize with success an ionogel electrolyte offering a stable interface between the electrode and electrolyte for the electrodeposition of lithium and permits a lithium metal battery to function at very high temperatures. This ILE@MOF electrolyte exhibited an elevated ionic conductivity of 0.99 × 10−3 S.cm−1 at a temperature of 30 °C and very high thermal stability up to 325 °C. The ILE@MOF formed a protective layer of MOF particles on the lithium and prevented the production of lithium dendrites. This material also allowed the lithium to undergo electrochemical stripping and plating at a temperature of 150 °C at a high current density of 0.5 mA.cm2. The LiFePO4-Li cells with this electrolyte exhibited a capacity of 151 mA.h.g−1 at a temperature of 60 °C and a Coulombic efficiency superior to 99%. The Li-LiNi0.33Mn0.33Co0.33O2 full cells with the ILE@MOF electrolyte also demonstrate enhanced performance at temperature values between 60 and 120 °C. Also, the Li-LiNi0.33Mn0.33Co0.33O2, Li-LiNi0.8Mn0.1Co0.1O2, and Li-Li4Ti5O12 cells with the ILE@MOF can be operated even at 150 °C because of the enhanced stability of the electrolyte during extended exposure to harsh settings.
Furthermore, the researchers Bose et al. [106] developed a series of free-standing, porous, P(VdF-HFP) nanocomposite membranes that were prepared by blending the copolymer with imidazolium-based ionic liquid tethered zinc sulfur nanoparticles, the nanoscale hybrid ionic fluid. The corresponding gel polymer electrolytes were obtained by submerging those porous membranes in the lithium salt–dissolved liquid electrolyte. Many characterization methods confirmed that the nanocomposite membrane-based gel polymer electrolytes had superior performance than that using the pure P(VdF-HFP). The 40% wt. of NHIF was reported to be optimum to develop a nanocomposite membrane with 64% porosity and highest (89.0%) electrolyte uptake capacity. The gel polymer electrolyte obtained from the membrane exhibited the highest ionic conductivity of 3.3 × 10−3 S.cm−1 at ambient temperature along with considerable lithium-ion transport. At C/10, the gel polymer electrolyte with 2032 coin cells in combination with LiFePO4-Li electrodes delivered a discharge capacity of around 161 mA.h.g−1 with nearly 89% retention after 50 cycles together with nearly 100% Coulombic efficiency.
Nanoparticles in ionic liquid exhibiting pre-formed structures
The nanoparticles can also be localized into preformed structures, which are based on ionic liquids, including long-range ordered liquid crystalline structures, emulsions, and films. Also, the ionic liquids with long-side alkyl chains present amphiphile-like characteristics because of the combination of a nonpolar alkyl chain part and a polar cation head group. As a result, the long-chain ionic liquids can form long-range ordered liquid crystalline structures at molten states, while they can form lyotropic liquid crystalline phases [107] in the presence of solvents including other ionic liquids [108]. Alternatively, the lyotropic liquid crystalline structures can be formed by the self-assembly in ionic liquids of concentrated amphiphiles such as surfactants, lipids, and block copolymers [109]. The nanoparticles can be synthesized or incorporated in these preformed ionic liquid long-range structures, and form nanoparticle-liquid crystal composites owing to the interplay between the particle–particle excluded volumetric interactions, preferential nanoparticle-molecule interactions, and the enthalpic and stretching interactions present in the polymer molecules [110]. The ionic liquids can be used as water and oil surrogates, and surfactants to obtain emulsions and microemulsions [111]. The nanoparticles can be localized at the interfaces of the ionic liquid–water-oil emulsions and microemulsions [112]. The inner and outer droplets of such emulsions, including oil in ionic liquid, ionic liquid in oil, and three-component emulsion, could be regulated by changing the volumetric fractions of the liquid components and the concentration values of the stabilizing nanoparticles [113].
Moreover, it has been observed that copper nanoparticles in 1-butyl-3-methylimidazolium tetrafluoroborate [Bmim][BF4] ionic liquid-castor oil microemulsion exhibited high physical stability even after its prolonged storage for half-a-year at room conditions that imparts advantageous features in applications like high-performance nanolubricants. Apart from the structures formed by the ionic liquids and nanoparticles under various intermolecular interactions, the ionic liquids and nanoparticles can be incorporated into other materials. For instance, a film can be formed from solid materials functionalized with ionic liquids interacting with nanoparticles [114], or by dispersing nanoparticles in ionic liquids and polymer mixtures [115]. By the immobilization of the ionic liquids onto solid materials, which are referred to as supported ionic liquids, the intended properties of the ionic liquids can be transferred to the solid substrate [116]. The nanoparticles can also be incorporated into aggregates like long-range ordered liquid crystals, spherical and/or wormlike micelles, and microemulsions formed by SAILs, which contain longer alkyl chains and possess non-polar-polar behavior at the same time.
These categories of amphiphiles produce self-assembled liquid crystals in the molten state and the accumulation of selective solvents like alcohol, water, glycerol, and ionic liquids into this inherent complex provides a highly organized lyotropic liquid crystalline structure. The localization of the nanoparticles in the lyotropic liquid crystalline structures developed the nanoparticle-liquid crystal-lyotropic liquid crystalline composites [117]. In this sense, the authors Zhao et al. [118] formed lyotropic liquid crystalline phases by the binary mixture of two ionic liquids [C14MIM][Cl] and EAN, in which [C14MIM][Cl] act as a surfactant. This hexagonal liquid crystal phase was used to disperse multi-walled carbon nanotubes and led to super conductive composite. Generally, the ionic liquids have high viscosities from 10 to 500 cP as compared to that of the water of 0.89 cP, which ultimately reduces the mass transfer rate. Consequently, it is most demanding to use ionic liquids in microemulsion systems either as oil phase, aqueous phase, surfactant, or even two of these components [119]. Microemulsions are thermodynamically stable and facilitate an environmentally benign media for the synthesis of nanoparticles with controllable shape and size.
The authors Kataria et al. [120] stated that the micellar surface areas of the ionic liquids are linked with their alkyl chain length: the larger the chain length is, the more compact will be the micelles. These composites possessed advanced ionic conductivity, less viscosity, and better solubility as compared to pure ionic liquids. Moreover, the authors Pei et al. [121] designed high-temperature microemulsions comprising only ionic liquids whose thermal decomposition temperature was higher than 499 K for the synthesis of porous platinum nanoparticles. It was noticed that resulted nanosized droplets could be maintained up to a high temperature of around 473 K and remain stable for at least 30 months at room conditions which was not possible in the case of conventional microemulsions consisting of water, organic solvent, and surfactant because of their thermal instability. Furthermore, the authors Sun et al. [122] reported an ionic liquid microemulsion for the electrochemical deposition of bimetallic silver-palladium nanoparticles onto the surface of the electrode. These uniform and monodispersed silver-palladium nanoparticles can be potentially preferred toward ethanol oxidation and revealed excellent activity and stability.
Quasi-solid-state ionic liquids
The quasi-solid-state electrolytes are highly desirable in supercapacitor applications to satisfy the growing power need for smart and wearable electronics [123]. The exploitation of diverse ionic liquid (quasi-)solid-state electrolytes is expected to relieve the intrinsic environmental risks associated with liquid leakage, component corrosion, and assembly issues for compact energy storage [124]. The ionogels comprising ionic liquids with polymer matrices, like for instance polyethylene oxide, polyvinyl alcohol, and polymethyl methacrylate provided are of great potential giving the benefits of leak-proofing, mechanical flexibility, high-temperature ion transport, and wide electrochemical stability window [125].
The authors Rana et al. [126] proposed a green cellulose-derived ionogel electrolyte through phosphorylating and mixing a microcrystalline cellulose scaffold in the 1,3-dimethylimidazolium methyl phosphite medium coupled with subsequent polymerization. The as-obtained ionogel electrolyte exhibited a peak toughness of 1.46 MJm−3 and high ion mobility from 2.6 to 22.4 mS.cm−1 in the broad operational temperature range between 30 and 120 °C, endowing the resultant activated carbon-loaded flexible supercapacitor with an excellent specific capacitance of 174 F.g−1 under 2.5 V at 120 °C. Furthermore, the authors Qin and Panzer [127] used a variety of zwitterionic copolymers as solid scaffolds to produce Na+-conducting ionic liquid gel electrolytes. The noncovalent cross-links within such ionogel electrolytes resulted in room-temperature ion conductivities of 1 mScm−1 and elastic modulus values from 0.7 to 11 MPa, which introduced a new family of zwitterionic copolymer matrices for further designing of the ionic liquid gel electrolytes. The polyionic liquids prepared by the in situ polymerization of monomeric ionic liquids are taken as very promising solid-state polyelectrolytes, since weakly bound ions within poly(ionic liquid)s result in an enhanced compatibility with ionic liquids and better ion conductivity compared with common polymer matrices [124].
Moreover, the researchers Li et al. [128] designed a cross-linked poly(ionic liquid)-nanotubes of halloysite composite electrolytes derived from the in situ polymerization of charged halloysite nanotubes and ionic liquid monomers. Benefiting from the poly(ionic liquid) nanocomposite electrolyte with high modulus and mechanical stability up to 26.7 MPa and 4.4 MPa, respectively, the fabricated solid-state supercapacitor device displayed remarkable and temperature-dependent capacitances, as well as competitive cycling-bending stability. This could be the right way for the design and implementation of innovative and robust poly(ionic liquid) electrolytes for durable applications like, for instance, advanced electronics. Furthermore, the authors Li et al. [129] incorporated the ionic liquid N-methyl-N-propyl-pyrrolidinium bis(-fluorosulfonyl)imide Pyr13FSI into a hybrid network to produce a series of gel polymer electrolytes. The electrochemical and physical characteristics of the gel polymer electrolytes were regulated by modifying the network structure and amounts of ionic liquid. The authors attained an ionic conductivity above 1 mS.cm−1 in room conditions. The developed gel polymer electrolytes were flame-retardant and exhibited excellent thermal and electrochemical stabilities together with ultra-stability with the lithium metal anode. Symmetrical lithium cells with the gel polymer electrolytes exhibited stable cycling for more than 6800 h at a current density of 0.1 mA.cm−2 and stable lithium stripping-plating at 1 mA.cm−2, being the highest current density reported until now for an ionic liquid gel polymer electrolyte. Moreover, the lithium-LiFePO4 batteries with the obtained gel polymer electrolyte exhibited improved cycling stability and rate performance over the broad temperature range of between 0 and 90 °C, further suggesting that this innovative hybrid-network-ionic liquid gel polymer electrolyte is a very suitable one to be applied in the next generation liquid–metal batteries.
Additionally, the authors Zhou et al. [130] developed a hierarchical polyionic liquid solid electrolyte by in situ polymerizing 1,4-bis-3–2-acryloyloxyethyl-imidazolium-1-yl]butane bis-(bis-trifluoromethanesulphonyl-imide) C1-4-TFSI monomer in 1-ethyl-3-methylimidazolium bis-trifluoromethanesulfonyl-imide EMI-TFSI electrolyte which is filled in poly(diallyldimethylammonium) bis-trifluoromethanesulfonyl-imide PDDA-TFSI porous membrane. The well-designed hierarchical structure also offered the prepared electrolyte an ionic conductivity above 10−3 S.cm−1 at 25 °C, electrochemical stability, incombustibility, good mechanical strength, and flexibility. More intriguingly, the in situ-assembled LiFePO4-Li and Na0.9[Cu0.22Fe0.30Mn0.48]O2/Na cells using the hierarchical polyionic liquid solid electrolyte exhibited improved cycling performances with increased specific capacities. The HPILSE showed improved safety without combustibility and leakage, elevated ionic conductivity, great electrochemical and thermal stabilities, and good mechanical properties. The in situ synthesis simplified the assembly of the polymer lithium-ion and sodium-ion batteries and ensured an intense interfacial contact between the hierarchical polyionic liquid solid electrolyte and the electrodes against the electrode volumetric changes during the charging and discharging cycles, leading to remarkable electrochemical performances for the Li-LiFePO4 and Li-HPILSE and Na0.9[Cu0.22Fe0.30Mn0.48]O2/Na-HPILSE-Sodium polymer batteries.
Moreover, the researchers Singh et al. [131] prepared an ionic liquid-based solid polymer electrolyte via solution cast employing polymer polyethylene oxide, lithium bis-trifluoromethane sulfonyl-imide lithium-TFSI salt, and the 1-butyl-3 methylimidazolium bis-trifluoromethane sulfonyl-imide BMIM-TFSI ionic liquid. The obtained polymer electrolytes of PEO − 20% wt. Li-TFSI − x% wt. BMIM-TFSI where x = 0, 5, 10, 15, and 20, were characterized by diverse techniques. The ionic conductivity of optimized composition solid polymer electrolyte PEO-20% wt. Li-TFSI-20% wt. BMIM-TFSI was approximately 1.5 × 10−4 S.cm−1 at a temperature of 30 °C and followed an Arrhenius-type thermally activated trend. The prepared solid polymer electrolytes were free-standing and flexible with excellent thermal and mechanical stability. SEM, XRD, and DSC analyses confirmed that the amorphic degree of the solid polymer electrolytes increased on increasing the amount of ionic liquid due to the plasticization effect of this one. The used cell provided electrochemical stability and cationic transference number tLi+ of around 0:27. The capacity of the cell, Li-PEO + 20% wt. Li-TFSI + 20% wt. BMIM-TFSI-LiMn2O4 demonstrated a stable cyclic performance and elevated Coulombic efficiency.
On the other hand, the authors Chen et al. [132] reported the synthesis of ionic liquid immobilized polymer gel electrolytes having strong ion–dipole interactions between imidazolium ionic liquid and the fluorinated copolymer gel for stable and dendrite-free Li+ plating and stripping. The adoption of the ionic liquid leads to the formation of a cross-linked gel framework with tethered anions, offering enhanced mechanical strength, heat resistance, ionic conductivity, and a stable electro chemical window up to 4.5 V. The Li+/Li can satisfy the demand of high-voltage cathodes. The membrane of the ionic liquid-immobilized polymer gel electrolyte enabled the lithium deposition without dendrite production, exhibiting stable cycling durability for 1000 h at 0.5 mA.cm−2, and the functional mechanism was carefully investigated. Through coupling with this gel electrolyte membrane, the LiFePO4-Lithium cell exhibited much superior cycling stability and rate performance. Moreover, the lithium-sulfur batteries assembled with the ionic liquid-immobilized polymer gel electrolyte also show the suppression of polysulfide shuttling and self-discharge, bringing high specific capacity and long cycling life.
Moreover, the researchers Tian et al. [133] prepared a composite self-healing electrolyte for rechargeable lithium metal batteries comprising dicationic polymerized ionic liquids as a backbone filled in an ionic liquid within interspaces among chain segments. The self-healing ability was achieved by the reversible ionic bonds constructed by the anions and cations in the polymer chains and ionic liquids in the interspaces. Also, the polymer backbone incorporated with the ionic liquid formed a sea-island system, which functioned as interlaced channels for lithium-ion transfer, augmenting the ionic conductivity of the electrolytes. In sum, the composite electrolytes exhibit efficient self-healing performance, good flexibility, and elevated ionic conductivity.
Properties of the ionic liquids
Physicochemical properties
During the synthesis stage, the physicochemical and electrochemical properties of the electrolytes can be varied by selecting specific anionic and cationic species to produce wide liquid phases and high ionic conductivities. As such, tweaks in the ionic liquid compositions could be posited to enable operations at elevated temperatures, or at least above room temperature, or other designated environments. When selecting the components, simply mixing multiple inorganic salts with Li+, K+, Rb+, and Cs+ can aid in reducing the melting point of the ionic liquids based on the Gibbs energy of mixing. Accordingly, combinations of M[FSA]-M′ [FSA], and M [TFSA]-M′ [TFSA] binary salts have shown a lower melting point than the one of the comprising single salts. In addition, ternary salt systems have lower eutectic points than binary systems; as has been affirmed by the ternary salt mixture of (Na[FSA])0.4)(K[FSA])0.25(Cs[FSA])0.3 system, which demonstrated a eutectic temperature of 36 °C [134]. Besides, the adoption of inorganic salts presents the added benefits of a high concentration of charge-carrier ions indeed a propitious strategy for even preparing highly concentrated electrolytes in organic electrolytes and aqueous electrolytes. Another common formulation strategy has been the use of sulfonyl-imide anions and organic cationic species with alkyl chains in the development of inorganic–organic hybrid ionic liquid electrolytes. Indeed, the inorganic–organic hybrid ionic liquids consisting of sulfony-limide anions have gained widespread use among energy storage devices due to their wide liquid phase in the low-to-intermediate temperature ranges [135].
Although a computational study [136] suggested fluoro complex anions [PF6]− and [BF4]− could provide excellent electrochemical stability, the anions are less likely to be used as ionic liquid electrolytes for batteries due to their low stabilities against hydrolysis and high viscosities, unlike the conventional organic electrolytes. Also, in line with the physicochemical properties of the ionic liquids, it is worth noting that the ionic conductivity and viscosity of the ionic liquids show temperature dependency but do not strictly follow the Arrhenius-type temperature dependence. This necessitates the use of the Volgel–Tammann–Fulcher equations instead of the Arrhenius fitting. A reduction in the ionic conductivity and an increase in the viscosity are observable with decreasing temperature and increasing salt concentration, which is in accordance with the Volgel–Tammann–Fulcher theory. Additionally, the Walden rule is often used to assess ionicity and the correlations between molar conductivities and viscosities in ionic liquids. The Walden plot provides valuable insights into the general trends of ionic liquid physicochemical properties: the viscosity increases while ionic conductivity decreases when the salt concentration is increased and the temperature is decreased. The KCl line is often used as a baseline for the Walden plot.
The ionicity of the ionic liquids is quantified by the vertical distance to the KCl (△W) line. In most cases, an increase in the alkyl chains results in decreased ionic conductivities and increased viscosities. Viscosity is also an important factor in practical cell-construction processes because highly viscous ionic liquids are difficult to impregnate into porous structures including separators. The ionic conductivity of the ionic liquid (σ), metal ion conductivity σm+, and transference number tm+ are correlated by the following expression in the cases where the metal ion is univalent: σm+ = σ⋅tm+ These three parameters are decisive factors for ion transport in the ionic liquids. The tm+ values are commonly determined by different methods including AC-DC and very low-frequency impedance [137]. In general, σ and tm+ decrease and increase, respectively, with increasing m+ concentration and, consequently, the m+ concentration dependence of the σm+ alters, depending on the ionic species in the ionic liquid. The electrical conductivity of the ionic liquid hybrids can be affected by the free ion or electron and, hence, the availability of nanoparticles and ions provided by the ionic liquids contributed to the improvement of the electrical conductivity of the ionic liquid hybrids. Furthermore, the authors Rodriguez-Palmeiro et al. [138] investigated the electrical conductivity of nanoparticles of silver iodide at distinct concentrations suspended in the [P6,6,6,14]-Cl ionic liquid. The peak electrical conductivity enhancement was reported at 20% wt., but beyond this concentration, the electrical conductivity was found to be reduced.
Additionally, Chereches and Minea [139] determined the electrical conductivity of hybrid ionic liquids composed of alumina nanoparticles dispersed in a mixture of water and [C2mim][CH3SO3] ionic liquid. The authors observed a remarkable electrical conductivity enhancement of about 300% when compared to the one obtained with the ionic liquid base fluid alone. Moreover, the authors confirmed also that the electrical conductivity depended on the temperature and amount of the nanoparticles. It could be also stated that the enhancements in the concentration of the nanoparticles diminished the electrical conductivity of the ionic liquid hybrids, which was ascribed by the authors to the viscosity alteration imposed by the increasing impact of the nanoparticles within the base ionic liquid. Moreover, the researchers He and Alexandridis [71] reviewed the published scientific articles on hybrid ionic liquids, regarding mainly their electrochemical application in batteries, sensors, fuel cells, batteries, and photovoltaic solar cells. The authors stated that the hybrid ionic liquids exhibited remarkable proton-transfer ability and electrochemical stability. The authors verified also that the nanoparticles dispersed in the ionic liquids ameliorated the performance of the electrolyte with a higher diffusion coefficient, which promoted the achievement of a larger electrical conductivity.
Additionally, the researchers Hamm et al. [38] confirmed that the incorporation of gold nanoparticles in the [Emim][EtSO4] ionic liquid augmented the capacitance by approximately 190% and decreased the resistance of the electrolyte by about 70% resulting in a considerable increase in the electrical conductivity. Additionally, Deb et al. [140] evaluated the electrical conductivity of hybrid ionic liquids presenting zinc sulfide dispersed in [Deim][NTf2] at different temperature values and concentrations of nanoparticles. The researchers found that the electrical conductivity of the hybrid ionic liquids increased with a growing number of nanoparticles up to a certain limit and, after that, it sharply decreased. Also, an inverse evolution was reported with the fluctuation of the working temperature.
The properties of the ionic liquids can be measured by different characterization techniques. Figure 12 summarizes the main properties of the ionic liquids and corresponding measuring techniques.
Electrochemical windows
For a long time, it has been generally understood that the electrochemical stability of electrolytes can be determined using the lowest unoccupied molecular orbitals LUMO electronic structure theory [141]. Nonetheless, recent studies have shown that this energy gap is often inaccurate when representing electrolyte stability because it is challenging to compare electrochemical windows established under different conditions since the stability of an electrolyte is dependent on many other parameters including the reactions between the electrolyte and solvent, the concentrations, electrode materials, and electrode surface states [142, 143]. As such, comparing electrochemical window results from different experiments under different conditions is not recommended. Another discrepancy in establishing electrochemical windows comes from the common understanding that cathodic limits are entirely governed by cationic species, whereas anionic species influence anodic limits. Nevertheless, some studies have demonstrated that both the cathodic and anodic limits can be determined by the cationic species. This trend has been reported for imidazolium ionic liquids wherein [C2C1im] and [C4C1im] cations were observed to decompose before anionic species [144].
Additionally, the calculations made to ascertain the anodic stability of ionic liquids with the same TFSA anion [C4C1im][TFSA] and [C3C1pyrr][TFSA], revealed that although the HOMO was dominated by the TFSA anion, the ionic liquids exhibited distinct anodic stabilities. Further, other discrepancies may arise from the differences in ion stabilization and ion pairings, which have also been found to contribute to the overall electrochemical stability of the ionic liquids. Hence, the experimental electrochemical window of electrolytes may not necessarily be like its theoretical stability. Therefore, to determine the electrochemical stability of electrolytes, linear sweep voltammetry and cyclic voltammetry are commonly used under different conditions using diverse electrodes for insight into the electrochemical windows and anodic stability among the ionic liquids. Here, ionic liquids based on the [TFSA]– anion are seen to exhibit superior anodic stabilities in comparison to [C2C1im][OTf], [C2C1im][BF4] and [C2C1im][TFSA] ionic liquids.
The cationic species play a major role in the cathodic stability of the ionic liquids. The cathodic stability tends to be higher for saturated cations with quaternary ammonium structures, whereas the aromatic cations in ionic liquids are less stable due to their lower LUMO levels than saturated cations. The LUMO energy levels of these cations are in the order of [C4pyr]+ < [C2C1im]+ < [N4441]+ in agreement with experimentally determined cathodic stabilities [145]. The length of the alkyl chain has also been found to influence the cathodic stability of the ionic liquids. The increased chain length of aliphatic substituents in organic cations improves the cathodic stability. Many published studies have suggested that quaternary ammonium ions with cyclic structures manifest improved cathodic stability compared to their non-cyclic counterparts [146]. A linear sweep voltammetry analysis on the oxidation stability of electrolytes has also revealed that the temperature increase results in a sharp reduction in the oxidation stability of the electrolyte. At 25 °C, the anodic limits of above 5.5 V (threshold, 0.01 mA.cm–2) were achieved in the 1 mol.dm–3 Na[FSA]− [C3C1pyrr][FSA], Na[PF6]-EC-DMC, and Na [PF6]-PC electrolytes, whereas the 1 mol.dm–3 Na[ClO4]-PC was found to attain 4.7 V. Nonetheless, when temperatures were increased to 45 °C and 60 °C, only the Na[FSA]−[C3C1pyrr][FSA] electrolyte maintained a wide oxidative potential limit, as all organic solvent electrolytes indicated narrow anodic limits characterized by irreversible oxidative decomposition occurring above 4 V. For a comprehensive evaluation model of an actual battery electrode, linear sweep voltammetry experiments were also performed on carbon electrodes along with Na[ClO4]-PC, Na[PF6]-EC-DMC, and Na[FSA]− [C3C1pyrr][FSA] electrolytes. The FEC and VC additives were observed to improve the anodic current of the Na[PF6]−EC-DMC electrolyte across the temperature range. Nonetheless, the energy difference between the highest occupied molecular orbitals HOMO and the Na[FSA]−[C3C1pyrr][FSA] was observed to produce the greatest anodic stability, independently of the changes in temperature.
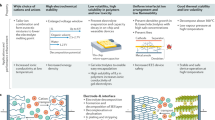
Electrochemical applications of the ionic liquids
The following sub-sections will briefly describe some of the electrochemical applications of the ionic liquids, whose suitability depends mainly on the type of the ionic liquids as it is summarized in Fig. 13.
Rechargeable metal batteries
Lithium batteries
The ionic liquids have emerged as innovative electrolytes, but their ionic conductivity is hindered by their comparatively high viscosity levels, limiting the electrochemical assessment of cells at medium-to-high temperature values. In the overview conducted by researchers Chen et al. [147], the safety concerns that have hindered the development of lithium-ion batteries were addressed. The researchers noted that the incorporation of small quantities of additives or charges to the traditional liquid electrolytes could prevent risky misuses without compromising the electrochemical behavior. The cathode, electrolyte, separator, and anode are the fundamental constituents of a lithium-ion battery, being the electrolyte the eligible primary component in a battery system, given that it facilitates the ion transfer, between the electrodes. In such conditions, the ionic liquids may play the role of surrogates for the commonly used liquid electrolytes, with the aim of improving the safety, electrochemical performance, and efficiency of a lithium-ion battery.
Furthermore, the authors Rana et al. [148] examined the exploration of ionic liquids as electrolytes in lithium-ion batteries. The research team reported that the ionic liquids have emerged as more competitive liquid electrolytes in comparison to the highly flammable volatile organic electrolytes. Apart from this, the ionic liquids, due to their unique characteristics, would not compromise the cyclability and safety-related issues of batteries. Nonetheless, the same researchers highlighted that the ionic liquids do not seem to be completely free from causing environmental problems, specifically when dealing with their degradability and toxicity. Another limitation emphasized by the researchers Ortiz et al. [149] comes associated with the employment of ionic liquids at room temperature. Moreover, the practicality related to the use of fluidic electrolytes based on ionic liquids faces challenges such as their poor cost-effectiveness and, sometimes, their low-rate capacity.
Furthermore, Tsurumaki et al. [150] synthesized ionic liquids containing ether oxygens and FSI anions with the absence of crystallization and enhanced ionic conductivity. There were synthesized the 9:1 molar fraction electrolyte mixture of [P1,2O2][FSI]-LiFSI and [M1,2O2][FSI]-LiFSI. The [P1,2O2][FSI]-LiFSI showed the best ionic conductivity of 2.4 × 103 Scm−1 at room temperature, wide electrochemical stability window, and low interfacial resistance on lithium surfaces, compared to the [M1,2O2][FSI]-LiFSI electrolyte mixture. Because of these advantageous properties, the Li j LiFePO4 cell with the [P1,2O2][FSI]-LiFSI electrolyte exhibited a capacity reaching 150 mAh.g−1 in the cases where it was galvanostatically cycled at C/10. Also, the cell exhibited a flat potential profile even after 50 cycles, this being a considerable benefit to be encountered in electrolytes made of ionic liquids.
Moreover, the authors Nirmale et al. [151] demonstrated that a modification of the imidazolium cation with an adequate anion enhanced the diffusion process, ultimately enhancing the ionic conductivity and cyclability. For this purpose, the research team prepared the ionic liquid [C6(mim)2][TFSI]2 and evaluated its suitability to be used in lithium-ion batteries. The highest ionic conductivity of 1.02 × 10−3 S.cm−1 at 30 °C and electrochemical stability window up to 5.3 V were far superior. A lithium-LiFePO4 cell with a dicationic ionic liquid at a 0.1 C rate showed a capacity of 133 mAhg−1 after 100 cycles with 98.8% coulombic efficiency. Even at a higher current rate, the cell retains the initial discharge capacity, suggesting superior reversibility and stabilization of the system. The cells exhibited discharge capacities of about 113.3 and 73.6 mAh.g−1 at 0.2 and 0.5 C rates, respectively. The dicationic ionic liquid performance at room temperature proves that the mobility of clusters and the formation of aggregates affect Li+-ion coordination and diffusion. Imidazolium-based dicationic ionic liquids were successfully synthesized and characterized and further used as electrolytes for lithium batteries.
The ionic conductivity decreased with the growing alkyl chain, which is due to an increase in viscosity with the alkyl chain of dicationic ionic liquids. The highest conductivity presented by [C6(mim)2][TFSI]2 at 30 °C was around 1 × 10−3 S.cm−1, which increases to 6.58 × 10−3 S.cm−1 at 70 °C. Importantly, the electrochemical stability window up to 5.3 V is far superior to that of other commercial electrolytes and some imidazolium ionic liquids. With such higher anodic stability, the DIL can be used for high-voltage cathode materials, for example, LiCoO2 and LiMn2O4. A lithium-ion cell fabricated with [C6(mim)2][TFSI]2 was run for cycling study at a 0.1 C rate, where the observed capacity after the completion of one hundred cycles was 133 mAh.g−1 with 98.8% coulombic efficiency. After 100 cycles with a higher current rate, the cell retained its starting discharge capacity. This indicates that the dicationic ionic liquid could offer outstanding cyclic performance even at ambient temperature when compared to other monocationic ionic liquids, which show good cycling at an elevated temperature only.
The capacity fading is also slowed down after 50 cycles, suggesting a superior stabilization and reversibility of the system after a few cycles. In conclusion, DIL [C6(mim)2][TFSI]2 proved to be an excellent alternative to flammable liquid electrolytes for lithium-ion batteries and can be further optimized for specific applications such as high-temperature and flexible lithium-ion batteries. It has been observed that the electrochemical and thermal properties of the ionic liquids rely on the structures of cations, anions, and their arrangement. The mobility of clusters and the formation of aggregates vary with the structures of ionic liquids. These structural changes can cause subtle variations in the physicochemical properties and resulting interactions. It is observed that an electric field causes the migration of Li+ ions, while an electrolyte concentration gradient and bulk motion are responsible for ion diffusion and convection. The convection term is not useful for lithium-ion batteries. Hence, the ion Li+ transfer happens through migration and diffusion processes.
The ionic liquids always show a certain amount of ion association. As in the context of the TFSI anion, four anions can coordinate one Li+ ion. A negatively charged cluster hinders the Li+ ion mobility, resulting in poor battery performance. In addition to this, these negatively charged clusters are repelled by electrodes opposing Li+ ion diffusion [152]. Few groups have used organic solvents as diluents to reduce such aggregation [153]. However, these solvents again contain liquid organic flammable components. The finest alternative to these solvents is specially designed ionic liquids, as with proper designing of ionic liquids these clusters can be broken to prepare favorable lithium electrochemistry. Earlier, it was found by molecular dynamics simulation that the addition of LiTFSI into ionic liquids conducted the aggregation of ionic liquid anions around the Li+ ion, which formed an additional rigid structure. Figure 14 summarizes the progress of the lithium-ion rechargeable batteries using ionic liquids as electrolytes.
Additionally, the structure of the dicationic ionic liquid did not change considerably with the inclusion of the ion Li+ in it when compared to a monocationic ionic liquid. Also, in the case of higher alkyl chain dicationic ionic liquids, [C9(mim)2]-[TFSI]2 and [C12(mim)2][TFSI]2, the ionic conductivity of these dicationic ionic liquids was smaller in comparison to the [C6(mim)2][TFSI]2. Consequently, the authors selected the [C6(mim)2][TFSI]2 dicationic ionic liquid for further cell studies. In the case of imidazolium-based ionic liquids, the hydrodynamic radius decreases with alkyl chain length, and the change in the hydrophobic interaction also could play an important role. The ionic conductivity of the dicationic ionic liquids was less affected by the lithium salt addition in comparison to what happened to a monocationic ionic liquid, which was due to the arrangement of the structure during the Li+ ion coordination [154]. The dicationic ionic liquids can form distinct clusters with the Li+ ions such as [Li(TFSI)2]− and [Liy/2(TFSI)y](y/2) − . However, cross-linking of different TFSI anions in the second structure can affect the physical properties.
Other configurations like [Li(TFSI)4]3− are also possible, though they suffer from a short life period. Among all others, the [Li(TFSI)2]− triplet structure involves less crosslinking due to the large size of the cation [154]. Such structural arrangements around the lithium-ion were not noticed in the case of monocationic ionic liquids and, hence, it was observed that the dicationic ionic liquid provided higher lithium-ion ionic conductivity and demonstrated to be a potential alternative to the traditional organic liquid electrolytes. Moreover, the ionic liquids could interact with solvent molecules, which could decrease the interactions between solvents and the water traces, thus avoiding degradation of the electrolyte and increasing the stability of the electrolyte. The ionic liquid crystal molecules (C14-Im-C14)Br preferred to absorb on electrodes proved by the peak shifted to lower potentials in the cyclic voltammetry diagram and consequently could reduce the activation energy of the oxidation–reduction reaction at the electrodes. The ionic liquid crystal could increase the insertion of magnesium ions by adsorbing counterions in the electrolyte solution, thus improving the performance of the magnesium battery with 250 cycles without loss under 300 mA.g−1 [155].
Moreover, the authors Liu et al. [156] studied the impact on the electrochemical performance of locally concentrated ionic liquid electrolytes of the organic cations. The research team analyzed the performance of lithium metal batteries through the direct comparison of two locally concentrated ionic liquids electrolytes employing either the 1 butyl 1-methylpyrrolidinium cation Pyr14+ or 1-ethyl-3-methylimidazolium cation Emim+. It was demonstrated that the structure of the organic cation in the locally concentrated ionic liquid electrolytes had only a little impact on the Li+- bis-fluorosulfonyl-imide anion FSI− coordination. Nonetheless, the coordination of FSI− with the organic cations is different. The less coordination of FSI− to Emim+ than to Pyr14+ results in lower viscosity and faster Li+ ion transport in the Emim+ electrolyte than in the Pyr14+ electrolyte. Additionally, the chemical composition of the solid-electrolyte interphase formed on lithium metal is affected by the organic cations. A more stable solid-electrolyte interphase growing in the presence of Emim + led to greater lithium plating-stripping coulombic efficiency of 99.2%. As a result, Li-EmiBE-LiNi0.8Mn0.1Co0.1O2 cells exhibited a capacity of 185 mAh.g−1 at 1C discharge (2 mA.cm−2), and a capacity retention of 96% after 200 cycles. Under the same conditions, the PyrBE cells exhibited only a capacity of 34 mAhg−1 with 39.6% retention. The structure of the organic cation in LCILEs has only a limited effect on the Li+-FSI− coordination. Nonetheless, the coordination of the organic cations to FSI− is different, affecting the properties of the electrolyte solutions. The less coordination of FSI− to Emim+ than to Pyr14+ participates in lower viscosity and, hence, a quicker Li+ ion transport in the EmiBE than PyrBE. Additionally, the two organic cations contribute differently to the solid electrolyte interface formation on lithium metal. In particular, the high N content of Emim+ with reference to Pyr14+ results in a more stable SEI growing in the presence of the former cation. Overall, the use of Emim+ organic cation is effective in optimizing the Li+ ion transport and the chemical composition of the SEI formed on the lithium anode, conducting to an enhanced cyclability and rate capability electrochemical performance of the lithium-lithium and lithium-NMC811 cells.
Moreover, the researcher Swiderska-Mocek [157] developed an innovative electrolyte for lithium-ion batteries in the form of lithium salt solution in ionic liquid based on imidazolium cation with vinyl group reported. This electrolyte was obtained by dissolution of solid lithium bis(trifluoromethanesulphonyl)imide LiNTf2 in 1-ethyl-3-vinylimidazolium bis(trifluoromethanesulphonyl)imide EVImNTf2. One molar of LiNTf2 in the EVImNTf2 electrolyte exhibited good cathodic stability induced by the presence of the vinyl group and a flash point superior to 220 °C, which makes it practically non-flammable. The olivine-type lithium iron phosphate (LiFePO4, LFP) cathode and the graphite-lithium anode working together with the electrolyte were tested with the use of cyclic voltammetry, galvanostatic charge/discharge cycles and electrochemical impedance spectroscopy. The charge/discharge tests of LiFePO4/1 M LiNTf2 in the EVImNTf2-lithium cell at different C rates exhibited a good specific capacity of 115 mAh.g−1 and 110 mAh.g−1 at 0.5 C and 1 C, respectively. The graphite anode showed good cyclability with 307 mAhg−1 after 40 cycles and a coulombic efficiency of 94%. The efficiency of the full LFP-electrolyte-G cell charging-discharging under study reached 125 mAh.g−1 after 30 cycles.
Sodium batteries
The rechargeable sodium metal batteries having high energy density can be relevant to a wide range of energy harvesting applications. The pursuit of higher energy density should ideally come with increased durability and safety, which is difficult for electrolytes based on organic solvents and somewhat facilitated with the use of ionic liquid electrolytes. In this direction, the researchers Chagas et al. [158] manufactured two groups of pyrrolidinium ionic liquid electrolytes composed by the mixture of bis-fluorosulfonyl-imide NaFSI or sodium bis-trifluoromethanesulfonyl-imide NaTFSI salts with N-methyl-N-propyl-pyrrolidinium bis-fluorosulfonyl-imide Pyr13FSI, N-butyl-N-methylpyrrolidinium bis-fluorosulfonyl-imide Pyr14FSI, and N-butyl-N methyl-pyrrolidinium bis-trifluoromethanesulfonyl-imide Pyr13FSI. The prepared electrolytes were evaluated based on the single anion electrolytes and binary anion mixtures, regarding specifically certain properties such as viscosity, density, conductivity, electrochemical stability, and cycling performance operating in room conditions sodium metal cells were examined by the authors. The authors concluded that the electrolytes with Pyr14FSI were the ones with better performance enabling the layered P2 Na0.6Ni0.22Al0.11Mn0.66O2 cathode to deliver about 140 mAh.g−1 after 200 cycles.
Additionally, the researchers Sun et al. [159] designed an electrolyte for rechargeable sodium metal batteries composed of aluminum chloride-1-methyl-3-ethylimidazolium chloride-sodium chloride ionic liquid with the inclusion of ethyl aluminum dichloride and 1-ethyl-3-methylimidazolium bis(fluor sulfonyl)imide additives. The batteries using the developed electrolyte reached approximately 4 V of voltage, 100% of Coulombic efficiency. Also, the authors reported considerable power and energy densities of around 1766 W.kg−1 and 420 W.h.kg−1, respectively. Moreover, the batteries retained more than 90% of the original capacity after the completion of 700 charge/discharge cycles. Another noteworthy work was the one conducted by Duncan et al. [160] who prepared six ionic liquids from fluoroborate anions for electrolytes of rechargeable sodium metal batteries. The authors reported the design of the room-temperature ionic liquids N-ethyl-N,N,N-tris-2–2-methoxyethoxy-ethyl-ammonium tetrakis-hexafluoroisopropoxy borate N2(2O2O1)3-B(HFIP)4. The best was the N2(2O2O1)3-B(HFIP)4 ionic liquid operating at room conditions, which exhibited 5.3 V of the electrochemical window and passivation of aluminum current-collector foil shown up to 7 V versus Na+/Na. The electrochemical cycling of the sodium battery was achieved with an electrolyte of an equimolar mixture of Na-FSI and N2(2O2O1)3-B(HFIP)4 where high stability was verified after 15 cycles. In addition to the electrochemical properties, the N2(2O2O1)3-B(HFIP)4 was found to exhibit good ionicity, low glass transition point at 73 °C, low viscosity, and non-flammability.
Magnesium batteries
The rechargeable magnesium batteries are most promising for sustainable and cost-effective energy harvesting applications; since magnesium is a highly abundant raw material, it has an increased charge capacity of 2205 Ah.kg−1, taking advantage of its two-electron redox chemistry, and it undergoes less dendritic growth than other metals like zinc, sodium, and lithium. However, the design and implementation of magnesium batteries are still hindered in producing chemically stable magnesium electrolytes. In this scope, the researchers Bi et al. [161] synthesized an ionic liquid electrolyte for magnesium-ion batteries through the mixture of MgCl2, AlCl3, magnesium, and 1-butyl-1-methylpyrrolidinium bis-trifluoromethanesulfonyl-imide Pyr14TFSI ionic liquid. The electrolyte had improved anodic and cathodic electrochemical behaviors. Also, the developed electrolyte delivered an increased magnesium deposition and stripping cycling for 300 h with minimum polarization change in magnesium-magnesium half-cell experiments. Additionally, the highly conductive electrolyte prevented the shuttle effect of the magnesium polysulfides and provided a stable magnesium-sulfur battery using the CMK-sulfur cathode.
Also, the cost-competitive magnesium salt Mg(CF3SO3)2, which is structurally like Mg(TFSI)2, has been proved to have high hydrophobicity, weaker reactivity with the magnesium anodes, while limited self-generated oxidation resistance of the anion and inferior ionic conductivity in aprotic solvents restrain its practical application. In this sense, the authors Zhang et al. [162] employed the PP14TFSI ionic liquid as an additive in Mg(CF3SO3)2 electrolytes and demonstrated comprehensive improvements in performance, including broadened electrochemical window, enhanced ionic conductivity of nearly 6 mS.cm−1, and stable cycling reversibility with low polarization of 0.11 V. The ionic liquid electrolytes also showed compatibility with the Mo6S8 with 66.9 mA.h.g−1 after 100 cycles at 0.1 C and Cu3Se2 with 131.7 mA.h.g−1 after 100 cycles at 200 mA.h.g−1.
Potassium batteries
The rechargeable potassium metal batteries possess great potential for energy harvesting purposes. These batteries are a promising complementary technology to lithium-ion batteries because of the comparative abundance and affordability of potassium. Currently, the most promising potassium-ion batteries electrochemistry is based on potassium manganese hexacyanoferrate. Nonetheless, such batteries face some limitations like the dendritic formation on the surface of the potassium anode that directly affects their longevity and safety. The use of ionic liquids as electrodes may positively answer this challenge.
In this sense, the authors Yamamoto et al. [163] studied the K-FSA-C2C1im-FSA, where FSA is bis-fluorosulfonyl-amide and C2C1im is 1-ethyl-3-methylimidazolium ionic liquids to be applied as electrolytes of potassium-ion batteries. There were identified binary mixtures in a liquid state below 273 K at molar fractions of x(K[FSA]) ≤ 0.20. The ionic conductivity was 10.1 mS.cm−1 at 298 K at x(K[FSA]) = 0.20, which corresponds to 1 M K+ ion concentration. This result was superior to the ones of other ionic liquid electrolytes for potassium batteries and was comparable to those of organic solvent electrolytes for potassium cells.
The electrochemical window of the potassium ionic liquid was around 5.2 V, which was wider than the one of sodium and lithium ionic liquids because of the more negative K+/K potential. Therefore, the conductive K-FSA − C2C1im-FSA was considered by the authors an electrolyte system with great potential for exploration in potassium-ion batteries. Furthermore, the authors Fiore et al. [164] developed a potassium-ion full cell with a KMF cathode, graphite anode, and a KFSI in Pyr13FSI ionic liquid electrolyte. The electrochemical testing of the KFSI in Pyr13FSI ionic liquid electrolyte demonstrated that an intermediate particle size of about 180 nm was the ideal compromise between a high specific capacity of 119 mA.h.g−1 and an extended cycling life with around 87% retention after 100 cycles at 4 V versus K+/K. The authors also reported a very high Coulombic efficiency superior to 99.3%. The authors ascribed this improvement to the stability of the electrolyte at high voltages and suppression of the aluminum current collector corrosion. In the same electrolyte, graphite displayed excellent cyclability retaining 99% of its initial capacity after 400 cycles and rate performance maintaining 88% of its C/20 capacity at 2C.
Finally, it was achieved the extremely high cycle Coulombic efficiency of a KMF-KFSI in Pyr1,3FSI-graphite full cell of 67.7%. The authors demonstrated that the use of an ionic liquid electrolyte in a KMF-graphite potassium cell is a very suitable approach for minimizing the irreversible capacity loss in potassium-ion batteries. On the other hand, the researchers Zhang et al. [165] prepared uniformly dispersed antimony nanodots in carbon frameworks by in situ polymerization of the ionic liquids. The synthesized antimony-ND@C composite was to be applied as anode for potassium-ion batteries and had an increased reversible capacity of 591 mA.h.g−1 at 100 mA.g−1, a rate performance of 320 mA.h.g−1 at 5 A.g−1, and improved cycling stability of 486 mA.h.g−1 after 1000 cycles, achieving almost 90% of capacity retention. The much-improved electrochemical characteristics of the antimony-ND@C are attributed to the homogenous distribution of the antimony nanodots in antimony-ND@C through in situ polymerization that alleviated the volume alteration and suppressed the agglomeration of antimony in the charging and discharging processes.
Moreover, the authors Jeon et al. [166] synthesized a potassium salt-concentrated ionic liquid electrolyte consisting of potassium bis-fluorosulfonyl-imide KFSI and 1-methyl-1-propyl pyrrolidinium bis-fluorosulfonyl-imide Pyr13FSI to be applied in potassium-ion batteries. Compared to conventional carbonate 1 M KPF6EC-PC and dilute potassium ionic liquid electrolytes, the potassium salt-concentrated ionic liquid electrolyte demonstrated favorable features such as high oxidation stability and non-flammability. The prepared electrolyte facilitated the formation of a KF-enriched solid electrolyte interface at the potassium metal anode, effectively suppressing the dendrite production and increasing the cyclability of the potassium anode. With these advantages, the K-KVPO4F cells with 0.8 mA.h.cm−2 areal capacity of KVPO4F incorporating potassium salt-concentrated ionic liquid electrolytes demonstrated remarkable cycle stability with capacity retention after 300 cycles of around 75% at 25 °C and high average cell efficiency of 99.6% at 25 °C after 300 cycles. Moreover, the developed electrolyte had superior rate performance in comparison to that of traditional carbonate and dilute potassium ionic liquid electrolyte, attributed to effective interfacial charge transfer kinetics at the potassium anode. Furthermore, the electrolyte demonstrated outstanding resistance to aluminum corrosion, ensuring improved stability during high-voltage cycling.
Zinc batteries
The safe zinc metal batteries display considerable potential for diverse energy harvesting ends. Nonetheless, the choice of adequate electrolytes for zinc batteries is still challenging because of the passivation and dendritic growth associated with zinc, which limits the longevity of the batteries. Also, the strong reactivity of water in aqueous electrolytes toward metallic zinc, especially at aggressive operating conditions, remains the fundamental obstacle to the commercialization of aqueous zinc metal batteries. In this regard, the ionic liquids are gradually gaining importance as potential electrolytes for zinc batteries.
Moreover, the authors Yu et al. [167] designed a water-immiscible ionic liquid diluent 1-ethyl-3-methylimidazolium bis-fluorosulfonyl-amide Emim-FSI reported that can suppress the water activity of aqueous electrolyte by serving as water pocket, enveloping the active water-dominated Zn2+ solvates and protecting them from side reactions. During the zinc deposition, the cation Emim+ and anion FSI− function respectively in mitigating the tip effect and regulating the solid electrolyte interphase, thereby favoring a smooth zinc deposition layer protected by inorganic species-enriched solid electrolyte interphase with high uniformity and stability. Combined with the enhanced chemical/electrochemical stability endowed by the intrinsic merits of ionic liquid, this ionic liquid-incorporated aqueous electrolyte enables the stable operation of Zn-Zn0.25V2O5·nH2O cells even at a challenging temperature of 60 °C with more than 85% of capacity retention after 400 cycles. At the molecular scale, it was revealed that due to their poor Zn2+ solvating ability of Emim-FSI in the electrolyte, Emim−- and FSI−-containing species largely occupy the interspace of highly active water-dominated Zn2+ solvates and prevent them from involving in avoidable side reactions like the hydrogen evolution and metal corrosion.
Additionally, the researchers De Anastro et al. [168] argued that the nature of the ionic liquids and polyionic liquids facilitate the ionic conductivity in ionogels in reference to the conductivity of the polymer electrolytes and avoids the exuding of the ionic liquids because of the chemical structure similarity. In this scope, they designed different polyionic liquid ionogels to be applied in zinc metal batteries and composed of poly-diallyldimethylammonium bis-trifluoromethanesulfonyl-imide), polyDADMATFSI (the polymer matrix) and 1-ethyl-3-methylimidazolium dicyanamide, emim-DCA ionic liquid. The authors verified that the incorporation of the ZnDCA2 salt in the ionogel increased the ionic liquid amount in the ionogel and, hence, enhanced the ionic conductivity caused by the plasticizer effect of the ionic liquid. The water in the pure emim-DCA favored the mass transport ability of the electrolyte and the inclusion of ceramic nanoparticles of alumina improved the conductivity of the formulation, given that the particles in the ionogel aided on the cation–anion dissociation at their surface. The optimized ionogel possessed increased conductivity values of 1.1 × 102 S.cm−1 at a temperature of 50 °C. The ionogel with 65% wt. of polyDADMATFSI and 35% wt. of ZnDCA2-emim-DCA-H2O-Al2O3 was successfully experimented by the authors in symmetric zinc cells and rechargeable all-solid zinc-ionogel-PEDOT batteries.
Fuel cells
The fuel cells in general, and proton exchange membrane fuel cells in particular, offer a promising alternative for clean power production since they convert chemical energy to electrical power with their improved power-generating potential in the exploration of renewable energy resources. The proton exchange membrane fuel cells have been receiving growing interest for the replacement of rechargeable batteries. They also possess beneficial features including low working temperatures in comparison to other types of fuel cells, fast startup, elevated power density, low to zero emissions depending on the fuel, and solid construction. The polymer Nafion is the most widely used membrane in proton exchange membrane fuel cells. This is due to its very good proton conductivity and chemical and mechanical stability, which results from its fluorinated backbone. Also, this polymer has a sulfonated chain which makes it hydrophilic. The proton conductivity of Nafion membranes is determined by the water content and, hence, sharply decreases when the water evaporates. The protons transfer by hopping mechanisms from one water molecule to another. Consequently, the dehydration of the Nafion membranes causes a drop in proton conductivity and this limits the operation of a proton exchange membrane fuel cell. At ambient pressure, this usually occurs at temperatures nearly 100 °C. and, therefore, the Nafion fuel cells are limited to temperatures lower than 80 °C.
Nonetheless, the ordered alignment of the ionic clusters in Nafion is limited by their defined molecular structure [169]. Therefore, the ionic liquid crystals with adjusted nanostructures are promising electrolytes to enrich ion conductive pathways for proton hopping with high ionic conductivity [169]. Merely 2.8 molecules of water per sulfonic acid group were needed to induce hopping in the ionic liquid crystal electrolytes, and this amount was near to, or even less, than those reported when using organic electrolytes. Nevertheless, the ionic conductivity of water-rich electrolytes might undergo appreciable loss upon heating, which may be linked to the evaporation of water and/or HTf2N and, hence, the elimination of the hydrogen-bonded networks. Concerning the temperature-induced issue, PILC electrolyte is supposed to be synthesized via ultra-violet radiation [170]. A 1 to 2 order of magnitude larger ionic conductivities could be achieved in the cross-linking polymeric Film-H1 and Film-Lα synthesized from 3-(1-vinyl-3-imidazolio) propane sulfonate and 4-dodecyl benzenesulfonic acid in comparison with the one of the Nafion 117. The enhanced ion conductive capability could be ascribed to the ion transport on a series of hopping within cylinder or layer units and diffusion by water among cylinder or layer units [171].
Furthermore, the researchers Luo et al. [172] produced a self-standing quasi-solid-state electrolyte, a polypropylene membrane soaked with the ionic liquid crystal electrolyte. A proton conductivity as high as 210 mS.cm−1 at 25 °C could be obtained, which was comparable to the one achieved with a membrane formed by alkyl sulfonic acid in which the protons conduct by hopping via hydrogen bonding and by the diffusion of the hydronium ions. This could be attributed to the freely migratable HSO− in the two-dimensional layer of water. The open-circuit voltage of a single fuel cell employing this ionic liquid crystal electrolyte loaded on a porous polypropylene membrane was high and equal to 0.84 V, indicating its ability to suppress the crossover of the hydrogen and oxygen.
Moreover, the researchers Al-Othman et al. [173] developed composite membranes composed of zirconium phosphate and imidazolium-based ionic liquids, supported on polytetrafluoroethylene and evaluated their potential use in proton exchange membrane fuel cells operating at 200 °C. The results showed that the produced membrane had a high proton conductivity of 0.07 S.cm−1, which is about 70% of the one observed for the Nafion polymer. Also, the membranes exhibited a very high proton conductivity of 0.06 S.cm−1 when processed at a temperature of 200 °C under anhydrous conditions. The scanning electron microscopy images indicated the formation of very small particles, with diameters between 100 and 300 nm, within the confined pores of the polytetrafluoroethylene. The thermogravimetric analysis revealed a maximum of 20% weight loss up to 500 °C for the synthesized membrane. The enhancement in the proton conductivity was attributed by the authors to the production of multiple protons conducting paths within the membrane matrix. The ionic component is acting as a proton bridge. In sum, the authors concluded that these membranes have enough potential to be applied in proton exchange membrane fuel cells functioning at around 200 °C.
Dye-sensitized solar cells
The ionic liquids have been incorporated as electrolytes in dye-sensitized solar cells to replace the organic solvents due to their unique properties of ionic liquids embracing negligible volatility, very high thermo- and electrochemical stability, the ability to dissolve a wide range of organic and inorganic compounds attract much interest of the researcher teams dealing with dye-sensitized solar cells. The ionic liquids in general can be denoted tunable solvents—their physicochemical properties can be adjusted by modification of the anionic and cationic components. Relatively low viscosity and hydrophobicity, desirable for solar cell applications, may be achieved using this approach. It has been shown that ionic liquids are improved solvents for liquid electrolytes in dye-sensitized solar cells and irreplaceable components of gel-like or quasi-solid-state electrolytes. The absolute efficiency values of a dye-sensitized solar cell should be taken with some reservation. By optimization of the conditions of efficiency determination, the values could be enhanced by at least 1–2%, and parameters, such as solar cell area, quality of glass and type of conducting glass, counter electrodes, light filtering, and masking are all performance influential parameters. Since it is normally not known to which degree such optimizations have been made, differences of a few percent in reported efficiencies may be insignificant in the sense that they do not communicate any information regarding the inherent properties of the components used or tested. The long-term instability of the dye-sensitized solar cells remains one of the most relevant factors limiting their industrialization. Overall, the long-term instability of the dye-sensitized solar cells may be due to the poor encapsulation of the cell conducting to the evaporation of the electrolyte, and the chemical processes taking place in the electrolyte or solid–liquid interfaces conducting to the deterioration of the dye, electrolyte components, and the counter electrode surface. The utilization of the ionic liquids can mitigate some of these limitations.
In this sense, the researchers Hilmy et al. [174] A series of eutectic ionic liquid mixtures composed of 1-ethyl-3-methylimidazolium iodide, [C2C1im]I, 1-ethylpyridinium iodide [C2py]I, 1-propylpyridinium iodide, [C3py]I and 1-butylpyridinium iodide, [C4py]I was developed. The physicochemical features of these ionic liquid mixtures including viscosity and ionic conductivity were evaluated for their potential use as electrolytes in dye-sensitized solar cells. The melting point of the neat ionic liquids and corresponding mixtures were determined using differential scanning calorimetry in the temperature range between 123.15 K and 393.15 K. It was found that the eutectic mixtures were formed at the molar ratios of {0.75[C2py]I:0.25[C2C1im], {0.25 [C2py]I:0.75[C2C1im]I}, {0.50[C2py]I:0.50[C2C1im]I}, {0.25[C2C1im]I:0.75 [C4py]I}, {0.75[C2C1im]I:0.25[C4py]I}, and {0.50[C2C1im]I:0.50[C4py]I} with the latter forming a highly stable liquid without melting maximum in the studied temperature range. To better understand the formation of the eutectic composition, a correlation between the molecular structures and the interaction energies of the cations and anions of the ionic liquids was performed using the Conductor-like Screening Model for Real Solvents COSMO-RS. The highest ionic conductivity was exhibited by the electrolyte {0.50[C2C1im]I:0.50[C4py]I:I2(50 wt%)} at 9.67 mS.cm−1 showing its potential to be used as the electrolyte in dye-sensitized solar cells.
Moreover, the authors Lin et al. [175] developed a novel polymeric ionic liquid, polyoxyethylene-imide-imidazolium selenocyanate POEI-IS, to be employed as an operating gel electrolyte for quasi-solid-state dye-sensitized solar cells. The POEI-IS was composed of polyoxyethylene-imide imidazolium polymeric cation and selenocyanate anions. The polyoxyethylene-imide imidazolium segment worked as a gelling agent and provided a retardation effect for the recombination reactions. The selenocyanate anion not only benefits the interfacial contacts between the POEI-IS electrolytes and the electrodes without the self-aggregation of the polymer but also had a reversible redox couple of SeCN−/SeCN3− with a more positive standard potential than one of iodide species. The POEI-IS showed only a 5% weight loss at 340 °C, indicating enhanced thermal stability, and thereby rendered the best POEI-IS dye-sensitized solar cell a prolonged durability, given that the cell efficiency maintains 95% of its starting value after 1000 h. Although the inclusion of the non-conductive polyoxyethylene-imide imidazolium segment in the electrolyte provoked a reduction in the ionic conductivity, it was found that the addition of 10–30 wt % POEI-IS in the electrolyte was adequate for dye-sensitized solar cells. Also, the best dye-sensitized solar cell reached an efficiency of approximately 8.2%, open-circuit voltage of 826 mV, short-circuit current of 13.9 mA.cm−2, and a fill factor of 0.7 via employing an electrolyte containing 30% wt. of the POEI-IS; as compared to the cell with an electrolyte containing 0 wt % POEI-IS (6.58%), a much higher cell efficiency was achieved.
Furthermore, the authors Lau et al. [176] prepared novel ionic liquid-sulfolane composite electrolytes based on the 1,2,3-triazolium family of ionic liquids developed for dye-sensitized solar cells. The best-performing device exhibited a short circuit current density of 13.4 mA.cm−2, an open-circuit voltage of 713 mV, and a fill factor of 0.65, corresponding to an overall power conversion efficiency of 6.3%. In addition, the devices proved to be highly stable, retaining above 95% of the initial device power conversion efficiency after 1000 h of light- and heat stress. These composite electrolytes show great promise for industrial application as they allow for a 14.5% improvement in power conversion efficiency, compared to the solvent-free eutectic ionic liquid electrolyte system, without compromising the stability of the cell.
Moreover, the researchers Sharma et al. [177] synthesized a polymer electrolyte based on polymethyl methacrylate doped with the ionic liquid 1-ethyl-3-methylimidazoliumtricyanomethanide via the solution cast technique. The authors found that the inclusion of the ionic liquid enhanced the ionic conductivity up to a peak of 8.83 × 10−5 S.cm−1 at 30% wt. of ionic liquid fraction in the polymer. The polarized optical microscopy, thermogravimetric analysis, Fourier transform infrared spectroscopy, and X-ray diffraction were employed to reveal the amorphous region by doping ionic liquid, composite nature, crystalline nature, and thermal stability of the developed ionic liquid-doped polymer electrolyte films. Also, the authors used the maximum ionic liquid doped with 30% wt. in polymer matrix electrolyte, film sandwiched in between carbon electrodes to fabricate an efficient supercapacitor, which showed the specific capacitance of 130 mF/g at 50 mVs−1 scan rate. Additionally, the research team developed a dye-sensitized solar cell employing the optimized ionic liquid doped polymer electrolyte that exhibited a short circuit current density of 7.23 mA/cm2, short-circuit current voltage of 0.61 V with a global efficiency of nearly 3.5% at 1 sun. Figure 15 schematically represents a typical dye-sensitized solar cell working with ionic liquids.
Perovskite solar cells
The ionic liquids can be utilized to ameliorate the performance of perovskite solar cells in many ways including passivating defects, optimizing crystallization, regulating energy levels, improving stability, and enhancing charge transport capabilities. The functions of the ionic liquids are closely linked to their cations and anions, and they can be used as precursor solvents, additives, or interface modification layers between perovskite and other functional layers. The cation contains specific groups like sulfur, nitrogen, and oxygen, that can interact with PbI2. Adding an additive to the precursor solution can form an intermediate adduct, which can improve the solubility of the precursor. The introduced intermediate adduct transformation process can effectively control the crystallization and crystal growth process and realize a perovskite film with few grain boundaries and large grain sizes. At the same time, the ionic liquids can interact with uncoordinated Pb2+ by special functional groups to achieve passivation effects on defects and decrease non-radiative recombination. Large cations usually cannot enter the perovskite crystal lattice and gather on the grain boundary or interface. The cations containing long hydrophobic alkyl chains or functionalized groups can effectively prevent the penetration of water and improve the resistance to moisture of the perovskite film. In the interface modification between the perovskite and the charge transport layer, the cation forms a dipole by coordinating with the anion to optimize the energy level arrangement and promote the extraction of the carrier charge. The hydrophobic character of certain ionic liquids will protect the perovskite layer, prevent the decomposition of the perovskite due to moisture, and improve the stability of the solar cell.
The ionic liquids can be used as a precursor solution alone or mixed with DMSO and DMF. The ionic liquids form a mesophase or complex with the precursor and introduce a reaction replacement process, which delays the crystallization process and prepares high-quality perovskite film with uniform large grain size and compactness. In the precursor solution, it can coordinate with PbI2 to form an intermediate phase, delay the crystallization process, and form large-sized perovskites. The halogen anions can fill the vacancies of halide and reduce perovskite defects. Sometimes the lattice strain can be reduced, the crystal quality can be improved, and the performance at the interface can be optimized. When the ionic liquids are employed as solvents, it is widely used in planar devices at present, and the performance of the perovskite solar cells can be considerably improved. In the mesoporous structure, due to the influence of the viscosity of the solvent, solvent residue, and other problems, the crystallization problem still needs to be further explored. At the same time, because of the in-depth study of the reaction mechanism, for example, the solute dissolution, the ion exchange, and the intermediate phase transition process. Combined with the traditional crystallization nucleation theory, the ionic liquid-induced perovskite crystallization and crystal growth theory were further improved from the perspective of crystallization kinetics. Whether the anion of the ionic liquid will exchange with the perovskite X-site anion to enter the lattice and change the crystal structure. The existing state of the anion also needs further discussion, whether it escapes by evaporation during the heat treatment or remains in the perovskite. Also, the roles of cations in the ionic liquids should be explored in depth to form a complete selection system. One view is that cations can enter the crystal lattice and replace the A-site cations, and the other view is that they exist at grain boundaries and interfaces, passivating defects through interactions.
The ionic liquids can be considered as additives in the perovskite layer and showed that the ionic liquids could improve the perovskite film quality through suppression of nucleation, and retarding crystal growth and recrystallization. The interaction between the ionic liquids and perovskites can be modulated by altering the backbone, length of the alkyl chain, and functional groups. It can be stated that the distribution position of the ionic liquids determines its role in passivating defects, tuning work function, and inhibiting ion migration. To solve the shortcomings of the common ionic liquids, such as easy aggregation and migration, a promising class of candidate materials, namely, polymeric ionic liquids, have been presented. It was already shown that the carboxyl ionic liquids are very suitable to perform as solvents and possess diverse functions such as regulating the crystallinity passivation of the defects. The ionic liquids can increase the carrier transport in the buried and top interfaces because of the dipolar polarization. For the buried interface, the ionic liquids can regulate the hydrophobicity of substrates, thus facilitating the formation of improved perovskite films. At the top interface, the ionic liquids can react with perovskite to form complexes, which can effectively improve the performance and long-term stability of the perovskite solar cells.
Moreover, the ionic liquids can also be explored as dopants to improve the morphology and electrical characteristics of the charge transport layer. Also, the ionic liquids can increase the size of the perovskite grain through suppressing nucleation, delaying crystal growth, and recrystallization. The ionic liquids have been shown to influence the preferred crystal orientation. A clear understanding of the role played by the ionic liquids in preferred crystal orientation will facilitate the production of high-quality perovskite films. The synthesis of the actual ionic liquids still entails limitations and challenges including the required synthesis easiness and cost reduction. Influence will be brought on the direction of ionic liquid molecules and the electrostatic interaction between ions after exposing to water A deeper understanding of the chemical interactions between the ionic liquids and the perovskite precursor solution is needed, and this should be supplemented by in situ monitoring of the evolution of the new precursor phase in the spin-coating process.
Considering the role of the ionic liquids in the nucleation and growth of perovskite crystals, and in the evolution of the ionic liquid precursor phases, it is foreseen as an effective means to control the formation energy of perovskites and stabilization of the perovskite phase. Figure 16 shows the main function of the ionic liquids in perovskite solar cells.
Moreover, the researchers Bai et al. [178] used ionic liquids containing triple-cation perovskite absorbers in positive–intrinsic–negative planar solar cells. Efficiency and stability of the solar cells were increased by them by adding the ionic liquid 1-butyl-3-methylimidazolium tetrafluor oborate BMIMBF4 in perovskite precursors. The short-circuit current, open-circuit voltage, and a high fill factor, were measured as approximately 23.8 mA.cm−2 and 1.1 and 0.8 V, respectively. For the device, which contains 0.3 mol%, the BMIMBF4 ionic liquid provided a power conversion efficiency of 19%. The cell functioning without the ionic liquid showed lower open-circuit voltage and fill factor of 1 and 0.8 V, respectively. The power conversion efficiency for the same cell was 18.5%. It was observed that the BMIMBF4-containing perovskite film has a minor change in film absorbance but a higher photoluminescence intensity and longer photoluminescence lifetime which keep uniform with fewer flaws in the film. It was interesting to know the impact of which component of the ionic liquid-BMIM+ or BF4− was significant for enhancing the efficiency of the cell and the long-term stability of the film. The cells were characterized with FABF4, finding that their efficiencies are equivalent to the control devices and found no improvement in the stability of the film. The authors enhanced the stability of the perovskite films by changing the BF4 with halide anions like iodide, bromide, and chloride, whereas maintaining the [BMIM]+ cation constant. However, the device efficiencies are significantly reduced. As a result, both [BMIM]+ and [BF4]− are very useful for enhancing the stability of the film and the efficiency of the cell.
On the other hand, the authors Akin et al. [179] mixed 1-hexyl-3-methylimidazolium iodide HMII ionic liquid with a long alkyl chain with the FAPbI3 precursor at concentrations ranging from 0.1 to 2.0 mol% to operate in a perovskite solar cell. It was observed that the addition of 0.5 mol% of the ionic liquid HMII was determined to be the best value for making an HMII-doped FAPbI3 cell. The open-circuit voltage, short-circuit current, and fill factor were found to be 1.07 V, 24.9 mA.cm−2, and 0.78 cm2, respectively. The cell based on HMII ionic liquid-modified perovskite showed a power conversion efficiency of 20.6%, while the control device achieved only 17.1% under equal conditions. The photovoltaic parameters were improved in the presence of HMII, and this improvement can be caused by the optoelectronic capabilities of the FAPbI3 material. The addition of larger concentrations of HMII drastically reduced the performance of the perovskite solar cell to below 7%. To infer the influence of the ionic liquid cation on the photovoltaic performance of the cell, it was also employed the 1-butyl-3-methylimidazolium iodide BMII ionic liquid with a shorter carbon chain length than HMII. The efficiency of the BMII working cells was only about 19.4% under similar conditions, which was lower than that of the HMII operating cells.
Also, the researchers Yang et al. [180] reported employing a solid-state ionic liquid 1-benzyl-3-methylimidazolium chloride as the ETM and low-temperature processing. The power conversion efficiency increased initially and subsequently dropped according to the SS-IL concentration, peaking at 0.2% wt. SS-IL concentration. The cells utilized the low-temperature solution-processed 1-benzyl 3-methylimidazolium chloride solid-state ionic liquids as an ETM. The efficiency of the cell has been increased to 15% when a thin layer of solid-state ionic liquids is introduced between the ITO cathode and the CH3NH3PbI3 absorber, with the associated fill factor, open-circuit voltage, and short-circuit current, rising to 0.7, 1 V, and 20.6 mA.cm−2, respectively.
The power conversion efficiency of the perovskite solar cell utilizing the (HC(NH2)2PbI3)0.85(CH3NH3PbBr3)0.15 as absorber layers is enhanced to around 16.1%. In addition, the researchers Wu et al. [181] tried to use ionic liquids as both a titanium dioxide-modifying layer and an independent electron transport layer of perovskite solar cells. Two novel ionic liquids were chosen, each having identical cation 1-ethyl-3-methylimidazolium, [EMIM]+ but distinct anions. The titanium dioxide electron transport layer modified with [EMIM]PF6-ionic liquid showed a short-circuit current of 22.9 mA.cm−2, an open-circuit voltage of 1.1 V, a fill factor of 73.7%, and a power conversion efficiency of 18.5%, whilst the open-circuit voltage of the control device based on the titanium dioxide electron transport layer was approximately 1.1 V, the short-circuit current was 20.3 mA.cm−2, the fill factor was 65.6%, and the power conversion efficiency 14.3%. Upon using the EMIM-PF6-ionic liquid as an independent ETL, the efficiency of the perovskite solar cell was around 13.3%. Other parameters observed were the short-circuit current was 22.2 mA/cm−2, open-circuit voltage of 1.04 V, and a fil factor of around 57.7%. 1-ethyl-3-methylimidazolium iodide EMIM-I, a smaller anion than EMIM-PF6, was also investigated. The power conversion efficiency of a device based on an independent [EMIM]I-ionic liquid electron transport layer was around 9.2%, which is significantly lower than that of a device based on the ionic liquid EMIM-PF6.
Moreover, the authors Gu et al. [182] produced four distinct ionic liquids of methylammonium formate, methylammonium acetate, methylammonium propionate (MAP), and methylammonium isobutyrate were prepared as the perovskite precursor solvents. The interaction between the functional groups of the solvents and Pb2+ in the precursor solution is studied, which has a direct impact on the morphology and crystallization of the perovskite film. It was found that the methylammonium propionate solvent produced a high-quality perovskite film, which leads to the best photovoltaic performance with the best power conversion efficiency of around 20.6% in comparison to the solar cells based on the other ionic liquid solvents. Moreover, the MAP cell kept 88% of its original power conversion efficiency after 1000 h of storage in a nitrogen atmosphere, indicating excellent stability of the cell. Therefore, the authors stated that methylammonium propionate ionic liquid was the best solvent for MAPbI3 films with respect to photovoltaic applications as compared to the other ionic liquids.
Thermo-electrochemical cells
The thermo-electrochemical cells represent a promising technology to convert waste heat energy into electrical energy, generating power with minimal material consumption and carbon footprint. Recently, the adoption of ionic liquids has pushed both the operational temperature range and the power output of the thermo-electrochemical cells. In its simplest form, a thermo-electrochemical cell is a thermally insulating container filled with an electrolyte with dissolved redox couple salts. Two sides of the cell are sealed hermetically with conducting electrodes, across which a temperature gradient is applied. The dominant phenomena for the generation of electric potential and current are the thermogalvanic and Soret effects.
The thermogalvanic effect describes the temperature-dependent oxidation and reduction potentials of dissolved redox species when they react with the electrodes. The thermoelectric voltage may be further enhanced by the Soret effect when the liquid mixtures contain multiple types (size and charge signs) of molecules and particles. As each particle/molecule responds differently to a temperature gradient, an additional electric potential difference can be generated. Despite the difference in the physical or chemical origins of the voltage creation between the solid thermoelectric materials and liquid thermogalvanic systems, it is usually the analysis of the Seebeck coefficient for describing the voltage-to-temperature ratio.
Furthermore, the researchers Abraham et al. [183] demonstrated that the thermoelectrochemical cells with ionic liquid electrolytes showed to be very promising to be applied at high temperatures superior to 100 °C and for thermal energy harvesting purposes. The I−/I3− dispersed in [C2mim][BF4] electrolytes attained power densities up to 29 mW/m2 in unoptimized cells working with the hot side at 130 °C. The authors achieved an adjusted thermoelectric figure of merit to infer the characteristics of the electrolytes and this comparison needed the measurement of the thermal conductivity of the electrolyte, redox couple diffusivity, and Seebeck coefficient of the electrolyte. In this regard, Fig. 15 summarizes the main possible methodologies for enhancing the Seebeck effects with ionic liquids.
The Seebeck effect occurs when two different metals are connected in a closed circuit, and the junction points are at different temperatures, generating an electric current in the circuit. This phenomenon explains, for example, the functioning of thermocouples. The use of ionic liquids as solvents and support electrolytes in thermoelectrochemical cells can be applied, for instance, in generators based on this effect, which are promising technologies for converting low-temperature heat into electricity. The use of ionic liquids as solvents in these devices is promising as it expands the temperature range and temperature differences between the electrodes where these thermos electrochemical cells can operate. In this regard, Fig. 17 summarizes the main possible methodologies for enhancing the Seebeck effects with ionic liquids.
Moreover, the authors Cheng et al. [184] regulated the ion–dipole interactions between an ionic liquid and a gel matrix and obtained a very large thermopower of 26.1 mVK−1. The thermopower can also be increased by adjusting the small molecule electrolyte interactions with their vicinities. For instance, the fixation of Na+ cations in the polyvinyl alcohol hydrogel matrix by coordinating interaction with the hydroxyl groups conducted to the migration of the anions OH− in the polyvinyl alcohol matrix, and a large thermopower value of 19.69 mV K1 was attained [185].
Also, the authors Laux et al. [186] examined the imidazolium-, ammonium- and choline-based ionic liquids in combination with different redox LiI/I2, I2, Co2+/3+ and demonstrated the possibility of obtaining enhanced negative Seebeck coefficients. Having both high positive and negative Seebeck coefficients will allow the matching of internal resistances of thermo-electrochemical generators, therefore optimizing the overall maximum power output given by serial connections in a highly integrated thermos-electrochemical generator. The authors argued that the Seebeck coefficient depends on the selection of the anion, even changing the sign of the coefficient for the same cation and redox couple ethylammonium formate, − 1451 µV/K; ethylammonium nitrate, 679 µV/K. The authors also confirmed that the choice of the redox couple had a strong influence going as far as changing the Seebeck coefficient considerably: ethylammonium nitrate + LiI/I2 0.2 M, 614 µV/K; ethylammonium nitrate + I2 0.2 M, − 55 µV/K. A special role of Co2+/3+ redox couple was verified by enhancing the Seebeck coefficients, both in the negative choline lactate + LiI/I2 0.2 M, − 1502 µV/K; choline lactate + Co2 + /3+, − 3630 µV/K and positive choline trifluoroacetate + LiI/I2 0.2 M 187 µV/K; choline trifluoroacetate + Co2+/3+, 1545 µV/K values. These facts contradicted the published findings in which adding redox couples LiI/I2 reduced the Seebeck coefficients. The authors stated that this could be explained by the attachment model, indicating that the potential between the electrodes is due to the specific temperature-dependent attachment of ions to the electrodes.
In addition, the researchers Xiao et al. [187] prepared a nanocomposite ionogel with skin-like stretchability, high ionic thermoelectric performance, thermostability, and durability through hybridizing ionic liquid and laponite nano-scaled sheets into waterborne polyurethane. This last one, with multiple hydrogen bond crosslinking, can accommodate a high ionic liquid content of around 70%, thereby improving the ionic conductivity of the ionogel. After the cation exchange between the ionic liquid and the laponite, the negatively charged laponite sheets and released sodium ion enhance the ionic Seebeck coefficient by enlarging the thermophoretic mobility difference between the cations and anions in the ionogel. The incorporation of the laponite nanosheets may suppress the thermal diffusion because of the enhanced size and interface effects and, hence, decrease the thermal conductivity. Consequently, the waterborne polyurethane-ionic liquid-laponite ionogel had a high ionic thermopower of 44.1 mV.K−1 and ionic conductivity of 14.1 mS.cm−1 and low thermal conductivity of 0.43 W.m−1.K−1. Apart from this, the laponite nanosheets were physical crosslinkers to improve the thermal stability and robustness of the ionogel.
Moreover, the ionogel demonstrates excellent durability during repeated stretching processes and, consequently, it can be employed as a power supply component for wearable and IoT devices. The gel ionic thermoelectric conversion systems have attracted more attention to harvest energy from ambient waste heat to power sensors in IoT systems, due to their high ionic thermopower, cost-effectiveness, and environmental friendliness.
In this sense, the researchers Zhu et al. [188] designed an ionic thermoelectric electrochemical cell by combining two asymmetric gels into a double sandwich structure, resulting in improved thermoelectric behavior. By adjusting the gel concentration and ratio of redox couples and additive amounts, a peak output power density Pmax/(DT)2 of 10 mW.m2.K2, a cell ionic thermopower of 5.2 mV.K−1, and an energy density of 3.4 J.m2K2 in 2 h were achieved at 313 K for the thermoelectric electrochemical cell composed of Gp-G-m/n FeCN4/3-Gp-G-x/y I/I3-z CF3SO3K-Gp with m/n = 0.175/0.025 M, x/y = 0.10/0.05 M, and z = 0.4 M. Moreover, the Pmax/(DT)2 increased to 20.5 mW.m2.K 2 for the cell employing a CEM as a built-in electrode. A gel-device assembled by nine ionic thermoelectric electrochemical cells generated a voltage of approximately 1.6 V and a high Pmax/(DT)2 of around 5 mW.m2 K2 at a temperature of 313 K and DT = 5 K.
Electrochemical low-grade heat harvesting
The thermally regenerative electrochemical cycle (TREC) constitutes a very promising technological solution for low-grade heat harvesting through exploring the thermogalvanic effect of the electrodes. Whereas the electrolytes applied in TREC systems have a negligible response to temperature variation. In the study conducted by the authors Wu et al. [189], a thermoresponsive ionic liquid was added to an electrolyte to endow it with temperature-driven phase change behavior, and the electrolyte was then utilized in a copper hexacyanoferrate-based TREC system for ultralow-grade heat harvesting. The TREC system was operated between 10 and 30 °C across the phase change critical point, so that the solvation states of the ions varied during the charging and discharging process, and a high energy density of 1.3 J.g−1 and high energy conversion efficiency of 1.32% (20% for the Carnot efficiency) were obtained. The energy efficiency was ten times higher than that obtained with a traditional system operating without a thermo-responsive ionic liquid under the same working conditions. Moreover, the phase change critical point of the thermo-responsive ionic liquid can be adjusted according to the species and concentrations of the electrolyte salt, which enhances the feasibility and resilience of the TREC system with the thermo-responsive ionic liquid. A thermo-responsive ionic liquid was applied in a CuHCFe half-cell TREC system to harvest ultralow-grade heat sources with narrow temperature differences.
The immiscibility of HbetTFSI in the NaTFSI aqueous solution electrolyte varied with temperature. The phase change of the electrolyte impacted on the solvation states of the dissolved ions. The TREC system operated at narrow temperature ranges could harvest considerable specific work when a phase change was involved during the temperature alteration. The TREC system operated between 10 °C and 30 °C and 0.5 °C in an electrolyte composed of 0.6 molar-NaTFSI-water-HbetTFSI mixture (HOMO phase) yielded a high-energy density of around 1.1 Jg−1, achieving an absolute heat to electricity conversion efficiency and relative efficiency, of nearly 1% and 15.4%, respectively. The improved performance could be attributed to the enhanced cycling Coulombic efficiency related to the behavior of the phase change and the relatively low specific heat of the electrolyte. At a lower current density of the cycles, a higher absolute heat-to-electricity conversion efficiency of around 1.3% and a relative efficiency of 20% were obtained under 0.2 C due to the smaller hysteresis and voltage drop. Under the same temperature conditions, the system exhibited much higher energy harvesting efficiency than other TREC systems with non-thermo-responsive electrolytes. Moreover, the critical point of the thermo-responsive ionic liquid can be adjusted according to the concentration and salt species, which enables the design and implementation of TREC systems for diverse temperature ranges. For example, the 0.42 MNaTFSI-water-HbetTFSI mixture system with a critical point equal to 33 °C operated at between 25 and 45 °C exhibited a high energy density of around 0.8 J.g−1, which was four times that of the HOMO phase system (Tc = 18 °C) operated at the same temperature range. The use of an adequate counter electrode to compose a full cell could further enhance the energy conversion efficiency. Furthermore, the application of other UCST- and LCST-type ionic liquids other than HbetTFSI in TREC systems should be studied in-depth.
Furthermore, the researchers Hsiao et al. [190] synthesized a hydrogel composed of polyvinyl alcohol, sodium alginate, and polyethylene glycol by blending and freeze–thaw method. The goal was to use ionic thermoelectric materials for harnessing low-grade waste heat because of their significant ionic Seebeck coefficient. By immersing the prepared hydrogel in the NaBF4 ionic liquid, the properties of the final product could be tuned. The polyvinyl alcohol-sodium alginate-polyethylene glycol-NaBF4 1.5 M hydrogel demonstrated much-improved mechanical properties presenting a tensile stress and strain reaching 69 kPa and 114%, respectively. Moreover, the developed hydrogel exhibited a high ionic conductivity value of nearly 31 mS.cm−1, a peak Seebeck coefficient of around 67 mV.K−1, and an increased power factor of around 14 mW.m−2.K−2. The authors attributed this enhanced behavior to the synergistic effect of Manning’s counterion condensation, which was promoted by the sodium alginate and crystalline polyvinyl alcohol chains. The application of the final hydrogel was demonstrated by an ionic thermoelectric supercapacitor. In the case where the external load resistance was 90 kΩ, the energy collected in one thermal cycle reached 4 mJ.
Supercapacitors
Supercapacitors possess the unique advantages of fast charging and ultra-long cycling life with simple and flexible design and minimum maintenance cost [191]. The employment of ionic liquid crystal electrolytes can reduce charge transfer resistance and raise the charge stored in the capacitor and discharge time, thereby facilitating an enhanced performance [192]. The supercapacitors can store energy through ion dynamics and physicochemical interactions at the electrolyte–electrode interface [193]. The double-layer charging within the ionic liquids is realized physically through the capacitive electrosorption between bare electrolyte ions and inert electrode surfaces, without the aid of additional solvent media for charge transfer [194]. The good chemical stability, a broad liquid temperature range, a highly ionized environment, and nonflammability of the ionic liquids are applied as safe and solvent-free electrolytes to afford the interfacial charge accumulation under large working voltages [195]. The thermal stability and electrochemical windows of the ionic liquids can also be adjusted by multiple couplings of cations and anions to control the voltage and temperature ranges of the supercapacitors [196].
The ionic liquids used in supercapacitors are usually based on the combined arrangement of imidazolium, tetraalkylammonium, pyrrolidinium, and pyridinium cations and versatile anions covering bis(trifluoromethane sulfonyl)imide TFSI−, bis(fluorosulfonyl)imide FSI−, hexafluorophosphate PF6−, tetrafluoroborate BF4−, and Cl− [197]. Also, the researchers Pan et al. [198] studied the subzero electrochemical performance of symmetric supercapacitors assembled with nitrogen-doped porous carbon electrodes and ionic liquid electrolytes. Benefiting from the broad temperature and potential adaptability, the assembled device delivered a large energy density under 3.5 V of 48.3 Wh·kg−1 at 1.75 kW·kg−1 and 18.6 Wh·kg−1 under 35 kW·kg−1 and − 40 °C.
Additionally, the researchers Molinari et al. [199] demonstrated that the charging and discharging processes at the La0.74Sr0.26MnO3-ionic liquid interface entailed electrostatic accumulation and faradaic electrochemical reactions in a hybrid supercapacitor, and the physical capacitive contribution of 10 μF.cm−2 was gradually replaced by a pseudocapacitive one up to 180 μF.cm−2 under an increasing external voltage. The cyclic voltammetry profile of the 1-cholesteryloxycarbonyldecyl-3-methylimidazolium bromide having the Sm phase 0.5% in ethanol displayed a quasi-rectangular shape, representing an excellent capacitive behavior. Consequently, a high specific capacitance of 157.5 F.g−1 was reported at 0.5 A.g−1, when coupled with the graphene oxide-manganese-dioxide-carbon fiber electrode. Moreover, the imidazolium ionic liquid crystal with the SmA phase could also perform a fast switching at the interface between the mesoporous carbon electrode and the electrolyte and fast charge transfer at the interface as indicated by the great knee frequency of 251 Hz and 13.2 s short response period. Thus, a high specific capacitance of 131.4 F.g−1, high energy density of 33.8 Wh.kg−1, and power density of 1033 W.kg−1 were obtained at 0.37 A.g−1, higher than that of other capacitors. The almost rectangular shape of the cyclic voltammetry curves without any redox peaks remained unchanged with increasing scan rate, confirming the exquisite capacitive behavior and excellent performance.
Moreover, the modification of the ionic liquid electrolytes with other ionic liquids, conductive salts, and nonaqueous solvents has lately been at the forefront of active research to ameliorate the relatively high viscosity and unsatisfactory conductivity for neat ionic liquids like, for instance, 0.014 S.cm−1 at 25 °C for EMIMBF4 [200]. A strong cation–anion attraction in neat ionic liquids confines the mobility of ions, leading to sluggish ion-transfer dynamics in viscous media [201]. For example, the researchers Wang et al. [202] conducted molecular dynamics simulations to study the correlation between anion types and electrochemical performances for ionic liquids supercapacitors. Consequently, both double layer thickness and charging time increased by the anion diameter BF4 − < PF6 − < OTf − < TFSI − , which also indicated the restrained ion migration due to the higher diameter of the anion. The ionic liquid eutectic mixtures can considerably reduce the melting temperature or even exhibit glass transition [203].
Additionally, the researchers Dou et al. [204] proposed an approach to employ the whole operational potential of carbon-based supercapacitors by adding a silica-grafted ionic liquid into the 1-butyl-3-methyl imidazolium bis-trifluoromethanesulfonyl-imide-propylene carbonate EMIMNTf2/PC electrolyte. The operational potential of the activated carbon device increased from 2.8 to 3.2 V, contributing to a nearly 39% energy capacity enhancement and remarkable cycling lifespan. Also, the redox-active mediators mixed with ionic liquid electrolytes are very interesting for applications like hybrid capacitors and pseudocapacitors [205]. The inclusion of pseudocapacitive species into the medium may offer extra capacitance and reduce the charge-transfer resistance for enhanced energy storage capability.
Recently, the authors Fleischmann et al. [206] used the 1-methyl-1-propylpyrrolidinium bis-trifluoromethylsulfonyl-imide (PMPyrrTFSI) ionic liquid electrolytes with soluble alkali ions in an asymmetric hybrid capacitor with a negative intercalation material. Through integrating the considerable improvements given by the ionic liquid electrolytes, the 4 V prototype devices achieved elevated energy densities of 100 Wh.kg−1, whilst upholding the specific power up to 2 kWh.kg−1, high voltage cycling tolerance, and a stable cycling performance at a temperature of 80 °C. Such a novel assembly concept demonstrated the great potential of alkali-ion hybrid capacitor systems, calling for the development of further ionic liquid mixtures toward superior energy storage.
Moreover, the authors Zhou et al. [207] prepared N/P co-doped porous carbon materials with developed pore structures from the phosphoric acid protic ionic liquid of arginine Arg[H2PO4]2 and NH42HPO4. The former acted as the carbon precursor, heteroatom source, and mesopore generator, whereas the latter worked as the activator which had a great impact on the pore distribution and microstructure. It exhibited high specific capacitance retention of 94% upon the completion of 10,000 cycles with stable electric double-layer capacitors. The assembled symmetrical supercapacitors exhibited a wide voltage window in alkaline electrolyte and neutral aqueous electrolyte, displaying high energy density and power density, respectively. In addition, the solid-state supercapacitors were prepared and showed good flexibility after bending the flexible supercapacitor cell at different angles. The results demonstrated the successful synthesis of N/P co-doped porous carbon materials form Arg[H2PO4]2. The N/P co-doping porous carbon materials having a hierarchical structure were produced from the Arg[H2PO4]2 proton ionic liquid. As an electrode, the Arg-2–900 had improved electric conductivity and high stability in a three-electrode system, which benefited from the fast electrolyte ions transmission in a hierarchically porous structure. The results showed that the Arg-2–900 had improved electrochemical performance in symmetric supercapacitors in alkaline and neutral electrolytes, possessing high energy density and power density. Moreover, the assembled devices showed good flexibility in all-solid-state supercapacitors, suggesting the exploration of Arg-2–900 in energy harvesting equipment. It was respected that hierarchically porous carbon materials with excellent electrochemical performance can be further applied in wearable and portable electronics.
Also, the researchers Xiong et al. [208] prepared two-hybrid ionic liquids comprising trifluoromethylsulfonyl and p-tosylsulfonyl, and two symmetric PTS2N ionic liquids were prepared for comparison purposes. The ionic liquids decomposition temperatures were superior to 570 K, indicating enhanced thermal stability. The Pyrr14PTSNTF ionic liquid showed the highest ionic conductivity of around 0.2 mS.cm−1 at 303 K, which is an adequate level for supercapacitors. The electrochemical stability window of Pyrr14PTSNTF reached 5.3 V. The specific capacitance of the Pyrr14PTSNTF supercapacitor was 28.4 F.g−1 at a current density of 0.05 A.g−1. The capacitance retention rate of the supercapacitor after 10,000 cycles at a current density of 0.1 A.g−1 reached approximately 91%, and the coulombic efficiency remained at 97% during the charge − discharge process.
Electrochemical sensing
Flexible iontronic sensors
Iontronics, a field that explores the interactions between electrons and ions, is fundamental to various physical, chemical, and life science processes. This area has the potential to convert ion gradients into electrical energy. However, traditional energy sources in iontronics typically suffer from low power output, are sensitive to humidity, and cannot operate at low temperatures [209]. In 202, the authors Wei et al. [209] introduced a groundbreaking device capable of functioning at temperatures as low as -40 °C and overcoming humidity limitations, making it ideal for powering future implantable electronics in human–machine interfaces. The researchers developed an ultrathin osmotic energy source using 2D nanofluidic graphene oxide, achieving a voltage of 1.5 V, a volumetric specific energy density of 6 mW.h.cm-3, and a power density of 28 mW.cm-3. When combined with a triboelectric nanogenerator, it forms a flexible, self-charging triboiontronic device.
Ion selective sensors
The ion-selective electrodes, also known as specific ion electrodes, are a type of sensor that converts changes in the concentration of specific ions dissolved in a solution into an electrical potential. These electrodes are used in analytical chemistry and biochemical/biophysical research where measurements of ionic concentration in aqueous solutions are required [210]. In chemistry, these sensors offer the ability to detect elements with reasonable selectivity and a low detection limit [211].
According to Sohail and de Marco [212], there are several types of ion-selective electrodes classified based on the type of membrane used: (i) homogeneous crystalline membrane electrodes, such as those using LaF3 for F−, Ag2S for Ag+ or S2−, and AgCl/Ag2S for Cl−; (ii) heterogeneous crystalline membrane electrodes, which can be made of silicone rubbers including crystalline powders like LaF3, Ag2S, and AgCl/Ag2S; (iii) rigid non-crystalline membrane electrodes that combine silicate and chalcogenide glasses; (iv) non-rigid non-crystalline electrodes that employ plasticized polymer membranes using neutral ionophores as receptor molecules; (v) sensitized electrodes, such as those for carbon dioxide or ammonia, which separate a filling solution containing a pH indicator electrode immersed in the sample solution using a gas diffusion membrane. There are also enzyme-sensitive electrodes, which incorporate a pH indicator electrode encapsulated by an enzyme gel film (e.g., glucose oxidase) for the selective detection of specific analytes, such as glucose.
According to the authors Sohail and de Marco [212], the future of ISEs in analytical chemistry is very promising, with the opportunity to use these electrochemical sensors in challenging applications, such as the detection of key chemical species in battery electrolytes or deep ocean analyses, where physically robust electrodes are required. They also highlight the detection of heparin in whole blood as an example of difficult samples that could benefit from these sensors.
Gas sensors
More stable electrochemical sensors for gas analysis are possible when using ionic liquids, as they eliminate the need to add a supporting electrolyte. Their intrinsic conductivity and low vapor pressure make them ideal for gas analyses of O2, CO2, and NH3 [213,214,215]. Other examples of precise gas detection include ammonia, based on the electro-oxidation of hydroquinone in dimethylformamide (DMF) and [EMIM][TF2N], as reported by the authors Mellah et al. [215]. In this study, the authors explain that ammonia can reversibly remove protons from hydroquinone molecules, thereby facilitating the oxidation process and producing a new wave with fewer positive potentials in the cyclic voltammogram. The detection limit for ammonia using this method is 4.2 ppm in DMF. Oxygen monitoring is crucial in various applications, ranging from packaging industries, manufacturing, steel, and mining to hospital use [216]. In the field of liquid ionic electrochemistry, gas sensors, especially for oxygen, have been widely studied due to their importance. As oxygen is a widely used gas, sensors for its electrochemical analysis are constantly evolving.
For instance, the researchers Wang et al. [217] developed a robust amperometric oxygen sensor, composed of a platinum mesh working electrode and using three distinct ionic liquids. This sensor demonstrated a steady-state response time of 2 min, with a detection limit of 0.05% by volume and a measurement range from 0 to 20% by volume. Furthermore, the device managed to maintain thermal stability above 150 °C over a period of 10 months.
Biosensors
Ionic liquids have been widely used in the design of electrochemical biosensors. These analytical devices can detect a wide variety of molecules, including biological elements such as enzymes, antibodies, nucleic acids, cellular receptors, and microorganisms, among others [216]. Furthermore, to be continued Niranjan et al. [216], biosensors based on ionic liquids are used to discover molecules such as adenine, antigen, cholesterol, choline, dopamine, sodium nitrite, pesticides, catechol, and glucose. Due to their sensitivity, low cost, operational timeliness, and selectivity, they can determine analytes with great precision [218, 219]. Generally, these devices are composed of three parts: (1) a biological component, which can be an enzyme, nucleic acid, or antibody, acting as the analyte detector; (2) a signal transducer to convert the interface signal between the target analyte and the biomaterial into an electrical signal; and (3) an electronic reader [218, 220, 221]. An example of application demonstrates the use of ionic liquid-based biosensors, developed to create a label-free electrochemical immunosensor for detecting the PthA protein. This was employed in diagnosing citrus canker, a disease caused by the bacterium Xanthomonas axonopodi, which affects citrus trees [218].
Recommendations for further research studies
The recommendations for further research studies can be summarized in the following topics:
-
Further experimental and numerical works on the usage of liquid electrolytes are welcome, given that it is still hindered by the strong scarcity of room-temperature ionic liquid electrolytes. Also, further studies are needed to achieve a better knowledge of the ionic conductivity, safety, stability, and interfacial compatibility of the ionic liquid electrolytes.
-
Further research endeavors should be made to urgently replace the first-generation ionic liquids with comparatively less costly ionic liquid electrolytes while improving their features for energy conversion and storage purposes applications such as the ability to withstand a wide temperature range, decreased viscosity, and increased voltage capability.
-
The development of ionic liquid precursors exhibiting synthesis easiness like, for instance, imidazolium bio ionic liquids and porous ionic liquids still needs further advances to obtain a sustained enhanced capacitive activity derived from the charging mechanism of the electric double layer.
-
Further research endeavors should attempt to integrate diverse ionic liquid-derived electrolyte–electrode components in a single device for the design and implementation of all-in-one ionic liquid supercapacitor systems soon. The cation–anion asymmetry and designability in the ionic liquids entail complex interactions of Van der Waals forces, electrostatic attractions, and hydrogen-bonding forces and contribute to the widespread ionic liquid applications in electrolytes and electrode synthesis for supercapacitors. By regulating the Lewis acidity and basicity, the alkyl chain substituents, and other task-specific functionalities between parent ions, the solid–liquid phase transitions (thermal stabilities) of the ionic liquids can be realized and are expected to adapt integrated component requirements in one single device.
-
It is notable that a major part of the dye-sensitized solar cells studied so far are essentially the same as the ones optimized for the utilization of a volatile organic solvent electrolyte. Only minor efforts have already been dedicated to the optimization of the electrode materials or sensitizing dyes to be used together with ionic liquids electrolytes. Hence, further studies should be conducted to analyze the interactions between the ionic liquid electrolytes and working, and counter electrodes. Such investigation works should include additives/dopants to the electrolytes, redox agents, innovative suitable dyes, and electrode materials to fully exploit the potentials of the ionic liquid electrolytes and corresponding derivatives when used on dye-sensitized solar cells.
-
Further research works are needed to better understand the functionalities and benefits associated with the use of ionic liquids in gas sensing applications. Hence, the structure of the ionic liquids at the electrodes requires in-depth analysis. The ionic liquids are tunable moieties, which is something that can be further explored for gas sensing, where the chemical species could be attached to the structure of the anion or cation to enhance the solubility of the gases in the electric double layer in reference to the bulk phase. This will lead to lower limits of detection and higher sensitivities.
-
More studies should be dedicated to the development of zinc ion batteries, which are safe and cost-effective means for enhanced energy storage. Care must be taken to select the most adequate ionic liquid electrolytes for rechargeable zinc-ion cells to tackle the problems associated with zinc passivation, dendritic growth, and electrode shape alterations that strongly limit the stability in time of the batteries.
-
There should be more research attempts to develop modified ionic liquid electrolytes to be utilized under extreme conditions like high temperatures of around 200 °C and very low temperatures of nearly − 100 °C. Considering that the pyrrolidinium and phosphonium ionic liquid electrolytes exhibit very high thermal stability, the high-temperature behavior of such electrolytes may pass by the selective modification of these two cations.
-
It is recommended that the scientific community pursuit the identification of a sustainable alternative to the cobalt redox couple in the ionic liquid thermoelectric generators. This would represent a great step forward because of the environmental toxicity and reduced availability associated with cobalt. The biggest challenges associated with the efficiency enhancement of the ionic liquid thermoelectric generators are the solubility of the redox salts and the high viscosity, together with the low rates of electrode reactions that limit the power output and efficiency of the thermo-electrochemical cells. Overall, the system must be sustainable from an environmental and economic point of view, the utilized materials should be recyclable, non-toxic, and not based on the exploration of vital raw materials.
Conclusions
The fundamental conclusions of this work of review can be summarized in the following topics:
-
The ionic liquids are very suitable fluids to be applied in electrochemical processes like the ones described in this overview, given that they all possess improved electrochemical properties like enhanced electrical conductivity and capacitance.
-
Because of the extended tunability of the ionic liquids, there should be further analyzed the correlations between structures and final properties, facilitating the design and implementation of improved energy harvesting applications under harsh conditions. Indeed, the physiochemical properties of the ionic liquid electrolytes can be designed by customized synthesis of targeted ionic liquids, tuning the concentration and type of functional components, and modulating temperature, where intermolecular interaction between the constituents plays a key role. Also, alterations in the characteristics of the anion and cation including size, charge distribution, metal complexing, hydrophilicity, among others, and the type of the substituted functional groups may conduct different improvements.
-
The pyrolysis of ionic liquids precursors has been considered a key research topic for fabricating functionalized carbon electrodes depending on negligible vapor pressure and molecular designability, wherein the ionic liquids undertake multiple roles like carbon supplies, microstructure-directing agents, and heteroatom dopants. The structure–property correlation between the ionic liquid precursors and ultimate carbon materials is vital to improving the performance of the electrode through multiple coupling of cations and anions.
-
The ionic liquids cation–anion designability entails the complex van der Walls interactions, electrostatic attractions, and hydrogen-bonding forces and generalized the exploration of the ionic liquids in electrolyte and electrode synthesis for supercapacitors. Also, by adjusting the pH and controlling the alkyl chain substituents, and other factors between the ions, the solid–liquid phase transitions of the ionic liquids can be performed.
-
In the case of pseudocapacitive electrodes, the energy storage capability can be strongly increased by protic ionic liquids electrolytes and redox-active mediators based on pseudocapacitive hybrid energy storage processes.
-
There is a growing use of ionic liquids in the design of high-energy supercapacitors because of their much appealing characteristics such as minor vapor pressure, good chemical and thermal stabilities, and molecular designability.
-
In terms of electrode synthesis, the ionic liquids featuring universal miscibility, negligible volatility, and multiple cation/anion coupling are involved as microstructure-directing agents, heteroatom dopants, and carbon precursors to give functionalized electrode materials toward superior capacitive performances.
-
For inert electrodes, the interfacial ion packing (double-layer capacitance) can be enhanced by improving the pore compatibility of electrode structures with the ionic liquid electrolyte ions or varying the blending ratios of the ionic liquid eutectic mixtures. For pseudocapacitive electrodes, the energy-storage capability can be profoundly increased through innovative protic ionic liquid electrolytes and versatile redox-active mediators based on inspiring pseudocapacitive-hybrid energy storage mechanisms. In addition, the porous ionic matrices as (quasi-) solid-state candidates emerge to relieve the intrinsic environmental risks associated with liquid leakage, component corrosion, and assembly issues for compact energy storage.
-
Although ionic liquids electrolytes own intrinsic safety, the leakage concern still needs to be considered in quasi-solid-state electrolytes. Thus, solid-state ionic liquid electrolytes are desirable. Nonetheless, the rapid ion transport can be minorly deteriorated. To this extent, the coordination sites and continuous ion conduction pathways at phase domain interfaces are critical. It is efficient to employ polymers with rich coordination sites in ionic liquid crystals.
-
To further promote ionic conductivity, traditional plasticizers such as water can be introduced to enhance the mobility of the ions. Also, exploring polymers to bridge the ion transport pathways between the adjacent ionic liquid crystal domains can also be a very suitable and effective way.
-
Loading ionic liquid crystal electrolytes at commercially available separators has been proved to be useful for manufacturing flexible ionic liquid crystal membranes. Besides, blending ionic liquid crystals with flexible polymers or in situ polymerization of the ionic liquid crystals with unsaturated bonds can also be a very useful strategy. The interface plays an important role in the charge transfer across the bulk electrolytes and electrodes, and, consequently, the direct contact between the ionic liquid crystal electrolytes and the electrodes, the suppression of interfacial side reactions, and the formation of beneficial interphase layers are vital. Hence, it is required to employ in situ polymerization methodologies to decrease the interfacial resistance, incorporate functional groups to interact with solvents to eliminate parasite reactions, and employ film-forming additives to produce highly ion-conductive interphase layers.
-
For enhancing the ionic conductivity, the production of three-dimensional ion conductive channels for ion hopping via customized design of ionic liquid crystal molecules and tuning of the temperature and concentration of the solvent can be very much advantageous.
-
The features underlying non-metallic redox couples dissolved in pure thermo-electrochemical cells are still not totally well understood, and basic research is needed to understand the changes in the sign of the Seebeck effect of the thermo-electrochemical cells containing iodide/triiodide redox couples in halides. Changes observed might be caused by the alterations in the redox reaction or ion transport mechanisms through the ionic liquid. Current density limitation can be overcome by dissolving ionic liquids, along with the redox couples, in traditional solvents, in this way enhancing the efficiency and power output of the cells. In certain cases, the ionic liquid increases the Seebeck coefficient of the redox couple in water or other solvents, being this enhancement generally attributed to the stronger interactions of the ionic liquid with the redox couple than those with solvent molecules, increasing the ΔSRC.
-
Considering the hydrophobicity of the ionic liquids, the ionic liquid electrolytes often need to operate under anhydrous conditions, given that the hydrophilic BF4 and PF6 are prone to hydrolysis at elevated potentials, which induces the harsh working environment and very high working cost. Apart from this, although the TFSI has a hydrophobic nature, the existence of water will impact the electrochemical performance. It has already been demonstrated that the superfluoro boron anions like BF3CF3 and BF3C2F6, and supernitrile anions like BF3C(CN)3 present increased hydrophobic character, low viscosity, and wide working potential window in comparison with BF4 or TFSI. Thus, improving the water-sensitive issue of the ionic liquid electrolytes should proceed from the anion design in the future.
References
Kaur G, Kumar H, Singla M (2022) Diverse applications of ionic liquids: a comprehensive review. J Mol Liq 351:118556. https://doi.org/10.1016/j.molliq.2022.118556
de Souza JP, Pivnic K, Bazant MZ, Urbakh M, Kornyshev AA (2022) Structural forces in ionic liquids: the role of ionic size asymmetry. J Phys Chem B 126:1242–1253. https://doi.org/10.1021/acs.jpcb.1c09441
Cabry CP, D’Andrea L, Shimizu K, Grillo I, Li P, Rogers S, Bruce DW, Canongia Lopes JN, Slattery JM (2018) Exploring the bulk-phase structure of ionic liquid mixtures using small-angle neutron scattering. Faraday Discuss 206:265–289. https://doi.org/10.1039/C7FD00167C
Kurnia KA, Neves CMSS, Freire MG, Santos LMNBF, Coutinho JAP (2015) Comprehensive study on the impact of the cation alkyl side chain length on the solubility of water in ionic liquids. J Mol Liq 210(Part B):264–271. https://doi.org/10.1016/j.molliq.2015.03.040
Russina O, Triolo A, Gontrani L, Caminiti R (2012) Mesoscopic structural heterogeneities in room-temperature ionic liquids. J Phys Chem Lett 3:27–33. https://doi.org/10.1021/jz201349z
Duan X, Ma J, Lian J, Zheng W (2014) The art of using ionic liquids in the synthesis of inorganic nanomaterials. CrystEngComm 16:2550–2559. https://doi.org/10.1039/C3CE41203B
Fellinger T-P, Thomas A, Yuan J, Antonietti M (2013) 25th anniversary article: “cooking carbon with salt”: carbon materials and carbonaceous frameworks from ionic liquids and poly(ionic liquid)s. Adv Mater 25:5838–5855. https://doi.org/10.1002/adma.201301975
Yuan J, Mecerreyes D, Antonietti M (2013) Poly(ionic liquid)s: an update. Prog Polym Sci 38:1009–1036. https://doi.org/10.1016/j.progpolymsci.2013.04.002
Sakaebe H, Matsumoto H, Tatsumi K (2005) Discharge–charge properties of Li/LiCoO2 cell using room temperature ionic liquids (RTILs) based on quaternary ammonium cation – effect of the structure. J Power Sources 146:693–697. https://doi.org/10.1016/j.jpowsour.2005.03.071
Nakamoto H, Watanabe M (2007) Brønsted Acid–base ionic liquids for fuel cell electrolytes. Chem Commun 24:2539–2541. https://doi.org/10.1039/B618953A
Kawano R, Matsui H, Matsuyama C, Sato A, Susan MABH, Tanabe N, Watanabe M (2004) High performance dye-sensitized solar cells using ionic liquids as their electrolytes. J Photochem Photobiol A Chem 164:87–92. https://doi.org/10.1016/j.jphotochem.2003.12.019
Galal A, Atta NF (2022) Chapter twelve - use of ionic liquids in electrochemical sensors. In: Carda-Broch S, Ruiz-Angel M, BT-IL in AC (eds) Ionic Liquidsin Analytical Chemistry - New Insights and Recent Developments. Elsevier, pp 343–368. https://doi.org/10.1016/B978-0-12-823334-4.00013-8
Ueno K (2018) Soft materials based on colloidal self-assembly in ionic liquids. Polym J 50:951–958. https://doi.org/10.1038/s41428-018-0083-1
Fonseca GS, Machado G, Teixeira SR, Fecher GH, Morais J, Alves MCM, Dupont J (2006) Synthesis and characterization of catalytic iridium nanoparticles in imidazolium ionic liquids. J Colloid Interface Sci 301:193–204. https://doi.org/10.1016/j.jcis.2006.04.073
Zhou Y, Antonietti M (2003) Synthesis of very small TiO2 nanocrystals in a room-temperature ionic liquid and their self-assembly toward mesoporous spherical aggregates. J Am Chem Soc 125:14960–14961. https://doi.org/10.1021/ja0380998
Ravula S, Baker SN, Kamath G, Baker GA (2015) Ionic liquid-assisted exfoliation and dispersion: stripping graphene and its two-dimensional layered inorganic counterparts of their inhibitions. Nanoscale 7:4338–4353. https://doi.org/10.1039/C4NR01524J
Shim Y, Kim HJ (2009) Solvation of carbon nanotubes in a room-temperature ionic liquid. ACS Nano 3:1693–1702. https://doi.org/10.1021/nn900195b
Badgujar KC, Bhanage BM (2015) Factors governing dissolution process of lignocellulosic biomass in ionic liquid: current status, overview and challenges. Bioresour Technol 178:2–18. https://doi.org/10.1016/j.biortech.2014.09.138
Li J, Wei X, Wang Q, Chen J, Chang G, Kong L, Su J, Liu Y (2012) Homogeneous isolation of nanocellulose from sugarcane bagasse by high pressure homogenization. Carbohydr Polym 90:1609–1613. https://doi.org/10.1016/j.carbpol.2012.07.038
Zhang H, Cui H (2009) Synthesis and characterization of functionalized ionic liquid-stabilized metal (gold and platinum) nanoparticles and metal nanoparticle/carbon nanotube hybrids. Langmuir 25:2604–2612. https://doi.org/10.1021/la803347h
de Caro D, Jacob K, Hahioui H, Faulmann C, Valade L, Kadoya T, Mori T, Fraxedas J, Viau L (2011) Nanoparticles of organic conductors: synthesis and application as electrode material in organic field effect transistors. New J Chem 35:1315–1319. https://doi.org/10.1039/C0NJ00858C
Neouze M-A, Kronstein M, Tielens F (2014) Ionic nanoparticle networks: development and perspectives in the landscape of ionic liquid based materials. Chem Commun 50:10929–10936. https://doi.org/10.1039/C4CC02419B
Xin B, Hao J (2014) Imidazolium-based ionic liquids grafted on solid surfaces. Chem Soc Rev 43(20):7171–7187. https://doi.org/10.1039/C4CS00172A
Chen F-L, Sun I-W, Paul Wang H, Huang C-H (2009) Nanosize copper dispersed ionic liquids as an electrolyte of new dye-sensitized solar cells. J Nanomater 2009:472950. https://doi.org/10.1155/2009/472950
Missan HPS, Lalia BS, Karan K, Maxwell A (2010) Polymer–ionic liquid nano-composites electrolytes: electrical, thermal and morphological properties. Mater Sci Eng B 175:143–149. https://doi.org/10.1016/j.mseb.2010.07.017
Ishikawa M, Sugimoto T, Kikuta M, Ishiko E, Kono M (2006) Pure ionic liquid electrolytes compatible with a graphitizcarbon negative electrode in rechargeable lithium-ion batteries. J Power Sources 162:658–662. https://doi.org/10.1016/j.jpowsour.2006.02.077
Kerr R, Mazouzi D, Eftekharnia M, Lestriez B, Dupré N, Forsyth M, Guyomard D, Howlett PC (2017) High-capacity retention of si anodes using a mixed lithium/phosphonium bis(fluorosulfonyl)imide ionic liquid electrolyte. ACS Energy Lett 2:1804–1809. https://doi.org/10.1021/acsenergylett.7b00403
Kerner M, Plylahan N, Scheers J, Johansson P (2015) Ionic liquid based lithium battery electrolytes: fundamental benefits of utilising both TFSI and FSI anions? Phys Chem Chem Phys 17:19569–19581. https://doi.org/10.1039/C5CP01891A
Han H-B, Liu K, Feng S-W, Zhou S-S, Feng W-F, Nie J, Li H, Huang X-J, Matsumoto H, Armand M et al (2010) Ionic liquid electrolytes based on multi-methoxyethyl substituted ammoniums and perfluorinated sulfonimines: preparation, characterization, and properties. Electrochim Acta 55:7134–7144. https://doi.org/10.1016/j.electacta.2010.06.063
Pandian S, Raju SG, Hariharan KS, Kolake SM, Park D-H, Lee M-J (2015) Functionalized ionic liquids as electrolytes for lithium-ion batteries. J Power Sources 286:204–209. https://doi.org/10.1016/j.jpowsour.2015.03.130
Ohno H (2017) Physical properties of ionic liquids for electrochemical applications. In: Endres F, Abbott A, MacFarlane D (eds) Electrodeposition from ionic liquids. pp 55–94. https://doi.org/10.1002/9783527682706.ch3
Navarra MA, Fujimura K, Sgambetterra M, Tsurumaki A, Panero S, Nakamura N, Ohno H, Scrosati B (2017) New ether-functionalized morpholinium- and piperidinium-based ionic liquids as electrolyte components in lithium and lithium–ion batteries. Chemsuschem 10:2496–2504. https://doi.org/10.1002/cssc.201700346
Xu K (2014) Electrolytes and interphases in Li-ion batteries and beyond. Chem Rev 114(23):11503–11618. https://doi.org/10.1021/cr500003w
Borodin O (2019) Challenges with prediction of battery electrolyte electrochemical stability window and guiding the electrode – electrolyte stabilization. Curr Opin Electrochem 13:86–93. https://doi.org/10.1016/j.coelec.2018.10.015
Hallinan DT, Balsara NP (2013) Polymer electrolytes. Annu Rev Mater Res 43:503–525. https://doi.org/10.1146/annurev-matsci-071312-121705
Galiński M, Lewandowski A, Stępniak I (2006) Ionic liquids as electrolytes. Electrochim Acta 51:5567–5580. https://doi.org/10.1016/j.electacta.2006.03.016
Cheng FY, Liang J, Tao ZL, Chen J (2011) Functional materials for rechargeable batteries. Adv Mater 23(15):1695–1715. https://doi.org/10.1002/adma.201003587
Hamm SC, Basuray S, Mukherjee S, Sengupta S, Mathai JC, Baker GA, Gangopadhyay S (2014) Ionic conductivity enhancement of sputtered gold nanoparticle-in-ionic liquid electrolytes. J Mater Chem A 2(3):792–803. https://doi.org/10.1039/C3TA13431H
Chen F-L, Sun I-W, Wang HP, Huang C-H (2009) Nanosize copper dispersed ionic liquids as an electrolyte of new dye-sensitized solar cells. J Nanomater 2009:472950. https://doi.org/10.1155/2009/472950
Neo C, Ouyang J (2012) Functionalized carbon nanotube-induced viscosity reduction of an ionic liquid and performance improvement of dye-sensitized solar cells. Electrochim Acta 85:1–8. https://doi.org/10.1016/j.electacta.2012.08.041
Adhyaksa GWP, Baek S-W, Lee GI, Lee DK, Lee J-Y, Kang JK (2014) Coupled near- and far-field scattering in silver nanoparticles for high-efficiency, stable, and thin plasmonic dye-sensitized solar cells. Chem Sus Chem 7:2461–2468. https://doi.org/10.1002/cssc.20140214
Lu YY, Das SK, Moganty SS, Archer LA (2012) Ionic liquid-nanoparticle hybrid electrolytes and their application in secondary lithium-metal batteries. Adv Mater 24(32):4430–4435. https://doi.org/10.1002/adma.201201953
Fernandes NJ, Wallin TJ, Vaia RA, Koerner H, Giannelis EP (2014) Nanoscale ionic materials. Chem Mater 26(1):84–96. https://doi.org/10.1021/cm402372q
Moganty SS, Jayaprakash N, Nugent JL, Shen J, Archer LA (2010) Ionic-liquid-tethered nanoparticles: hybrid electrolytes. Angew Chem Int Ed 49(48):9158–9161. https://doi.org/10.1002/anie.201004551
Oster K, Hardacre C, Jacquemin J, Ribeiro APC, Elsinawi A (2019) Ionic liquid-based nanofluids (Ionanofluids) for thermal applications: an experimental thermophysical characterization. Pure and Applied Chemistry 91(B):1309–1340. https://doi.org/10.1515/pac-2018-1114
Brennecke JF, Maginn EJ (2001) Ionic liquids: innovative fluids for chemical processing. AIChE J 47:2384–2389. https://doi.org/10.1002/aic.690471102
Asleshirin S, Mazaheri H, Omidkhah MR, Joshaghani AH (2022) Investigation of thermophysical properties of io nanofluids containing multi-walled carbon nanotubes and graphene. Iran J Chem Chem Eng 41:380–391. https://doi.org/10.30492/ijcce.2020.121326.4273
Ribeiro APC, Vieira SIC, França JM, Queirós CS, Langa E, Lourenço MJV, Murshed SMS, de Castro CAN (2011) Thermal properties of ionic liquids and ionanofluids. In: Kokorin A (ed) Ionic Liquids: Theory, Properties, New Approaches. IntechOpen, Rijeka 2. https://doi.org/10.5772/13920
Chen K, Xu B, Shen L, Shen D, Li M, Guo L-H (2022) Functions and performance of ionic liquids in enhancing electrocatalytic hydrogen evolution reactions: a comprehensive review. RSC Adv 12:19452–19469. https://doi.org/10.1039/D2RA02547G
Riedl JC, Kazemi MAA, Cousin F, Dubois E, Fantini S, Loïs S, Perzynski R, Peyre V (2020) Colloidal dispersions of oxide nanoparticles in ionic liquids: elucidating the key parameters. Nanoscale Adv 2:1560–1572. https://doi.org/10.1039/C9NA00564A
Astruc D (2020) Introduction: nanoparticles in catalysis. Chem Rev 120(2):461–463. https://doi.org/10.1021/acs.chemrev.8b00696
Seitkalieva MM, Samoylenko DE, Lotsman KA, Rodygin KS, Ananikov VP (2021) Metal nanoparticles in ionic liquids: synthesis and catalytic applications. Coord Chem Rev 445:213982. https://doi.org/10.1016/j.ccr.2021.213982
Zheng Y, Wang D, Kaushik S, Zhang S, Wada T, Hwang J, Matsumoto K, Hagiwara R (2022) Ionic liquid electrolytes for next-generation electrochemical energy devices. Energy Chem 4(3):00075. https://doi.org/10.1016/j.enchem.2022.100075
Blensdorf T, Joenathan A, Hunt M, Werner-Zwanziger U, Stein BD, Mahmoud WE, Al-Ghamdi AA, Carini J, Bronstein LM (2017) Hybrid composite polymer electrolytes: ionic liquids as a magic bullet for the poly(ethylene glycol)–silica network. J Mater Chem A 5:3493–3502. https://doi.org/10.1039/C6TA09986F
Niu H, Wang L, Guan P, Zhang N, Yan C, Ding M, Guo X, Huang T, Hu X (2021) Recent advances in application of ionic liquids in electrolyte of lithium ion batteries. Journal of Energy Storage 40:102659. https://doi.org/10.1016/j.est.2021.102659
Marquina LM, Riveros LLT, Ccana VJ, Muedas-Taipe G, Isaacs M, Rosa-Toro AL (2023) Recent studies on polymer electrolytes containing ionic liquids and their applications in lithium-ion batteries. J Electroanal Chem 948:117819. https://doi.org/10.1016/j.jelechem.2023.117819
Zhu C, Yang G, Li H, Du D, Lin Y (2015) Electrochemical sensors and biosensors based on nanomaterials and nanostructures. Anal Chem 87(1):230–249. https://doi.org/10.1021/ac5039863
Chen Y, Han X, Liu Z, Li Y, Sun H, Wang H, Wang J (2022) Thermal decomposition and volatility of ionic liquids: Factors, evaluation and strategies. J Mol Liq 366:120336. https://doi.org/10.1016/j.molliq.2022.120336
Gupta P, Rani S, Sah D, Surabhi D, Shabir J, Singh B, Pani B, Mozumdar S (2023) Basic ionic liquid grafted on magnetic nanoparticles: an efficient and highly active recyclable catalyst for the synthesis of β-nitroalcohols and 4H-benzo[b]pyrans. J Mol Struct 1274(2):134351. https://doi.org/10.1016/j.molstruc.2022.134351
Mohammed KJ, Hadrawi SK, Kianfar E (2023) Synthesis and modification of nanoparticles with ionic liquids: a review. Bio Nano Sci 13:760–783. https://doi.org/10.1007/s12668-023-01075-4
Bernardo SC, Carapito R, Neves MC, Freire MG, Sousa F (2022) Supported ionic liquids used as chromatographic matrices in bioseparation—an overview. Molecules 27:1618. https://doi.org/10.3390/molecules27051618
Avirdi E, Hooshmand SE, Sepahvand H, Vishwanathan V, Bahadur I, Katata-Seru LM, Varma RS (2022) Ionic liquids-assisted greener preparation of silver nanoparticles. Curr Opin Green Sustain Chem 33:100581. https://doi.org/10.1016/j.cogsc.2021.100581
Yang L, Thérien-Aubin H (2022) Behavior of colloidal gels made of thermoresponsive anisotropic nanoparticles. Sci Rep 12:12157. https://doi.org/10.1038/s41598-022-16414-w
Opallo M, Lesniewski A (2011) A review on electrodes modified with ionic liquids. J Electroanal Chem 656(1–2):2–16. https://doi.org/10.1016/j.jelechem.2011.01.008
Saputra HA (2023) Electrochemical sensors: basic principles, engineering, and state of the art. Monatsh Chem 154:1083–1100. https://doi.org/10.1007/s00706-023-03113-z
Luczak J, Paszkiewicz M, Krukowska A, Malankowska A, Zaleska-Medynska A (2016) Ionic liquids for nano- and microstructures preparation. Part 2: application in synthesis. Adv Colloid Interface Sci 227:1–52. https://doi.org/10.1016/j.cis.2015.08.010
Sakai K, Okada K, Uka A, Misono T, Endo T, Sasaki S et al (2015) Effects of water on solvation layers of imidazolium-type room temperature ionic liquids on silica and mica. Langmuir 31(22):6085–6091. https://doi.org/10.1021/acs.langmuir.5b01184
Khatri OP, Adachi K, Murase K, Okazaki K-i, Torimoto T, Tanaka N et al (2008) Self-assembly of ionic liquid (BMI-PF6)-stabilized gold nanoparticles on a silicon surface: chemical and structural aspects. Langmuir 24(15):7785–7792. https://doi.org/10.1021/la800678m
Ninham BW (1999) On progress in forces since the DLVO theory. Adv Colloid Interface Sci 83(1–3):1–17. https://doi.org/10.1016/S0001-8686(99)00008-1
Obliosca JM, Arellano IHJ, Huang MH, Arco SD (2010) Double layer micellar stabilization of gold nanocrystals by greener ionic liquid 1-butyl-3-methylimidazolium lauryl sulfate. Mater Lett 64(9):1109–1111. https://doi.org/10.1016/j.matlet.2010.02.029
He Z, Alexandridis P (2015) Nanoparticles in ionic liquids: interactions and organization. Phys Chem Chem Phys 17(28):18238–18261. https://doi.org/10.1039/c5cp01620g
Lin YN, Smith TW, Alexandridis P (2002) Adsorption of a polymeric siloxane surfactant on carbon black particles dispersed in mixtures of water with polar organic solvents. J Colloid Interface Sci 255(1):1–9. https://doi.org/10.1006/jcis.2002.8638
Zhao Y, Hu Z (2013) Graphene in ionic liquids: collective Van der Waals interaction and hindrance of self-assembly pathway. J Phys Chem B 117(36):10540–10547. https://doi.org/10.1021/jp405660d
Sarkar B, Venugopal V, Bodratti AM, Tsianou M, Alexandridis P (2013) Nanoparticle surface modification by amphiphilic polymers in aqueous media: role of polar organic solvents. J Colloid Interface Sci 397:1–8. https://doi.org/10.1016/j.jcis.2013.01.034
Liang Y, Hilal N, Langston P, Starov V (2007) Interaction forces between colloidal particles in liquid: theory and experiment. Adv Colloid Interface Sci 134–135:151–166. https://doi.org/10.1016/j.cis.2007.04.003
Rodriguez-Arco L, Lopez-Lopez MT, Duran JDG, Zubarev A, Chirikov D (2011) Stability and magnetorheological behavior of magnetic fluids based on ionic liquids. J Phys Condens Matter 23:455101. https://doi.org/10.1088/0953-8984/23/45/455101
Gracia R, Vijayakrishna K, Mecerreyes D (2014) Poly(ionic liquid)s with redox active counter-anions: all-in-one reactants and stabilizers for the synthesis of functional colloids. React Funct Polym 79:54–58. https://doi.org/10.1016/j.reactfunctpolym.2014.03.005
Mallakpour S, Sirous F (2015) Surface coating of α-Al2O3 nanoparticles with poly(vinyl alcohol) as biocompatible coupling agent for improving properties of bio-active poly(amide-imide) based nanocomposites having L-phenylalanine linkages. Prog Org Coat 85:138–145. https://doi.org/10.1016/j.porgcoat.2015.03.021
Texter J, Ager D, Vasantha VA, Crombez R, England D, Ma X et al (2012) Advanced nanocarbon materials facilitated by novel stimuli-responsive stabilizers. Chem Lett 41(10):1377–1379. https://doi.org/10.1246/cl.2012.1377
Prabhu Charan KT, Pothanagandhi N, Vijayakrishna K, Sivaramakrishna A, Mecerreyes D, Sreedhar B (2014) Poly(ionic liquids) as “smart” stabilizers for metal nanoparticles. Eur Polym J 60:114–122. https://doi.org/10.1016/j.eurpolymj.2014.09.004
Selvam T, Machoke A, Schwieger W (2012) Supported ionic liquids on non-porous and porous inorganic materials A topical review. Appl Catal A 445–446:101. https://doi.org/10.1016/j.apcata.2012.08.007
Kim E, Han J, Ryu S, Choi Y, Yoo J (2021) Ionic liquid electrolytes for electrochemical energy storage devices. Materials (Basel) 14:4000
Morozova SM, Gevorkian A, Kumacheva E (2023) Design, characterization and applications of nanocolloidal hydrogels. Chem Soc Rev 52(15):5317–5339. https://doi.org/10.1039/d3cs00387f
Ueno K, Hata K, Katakabe T, Kondoh M, Watanabe M (2008) Nanocomposite ion gels based on silica nanoparticles and an ionic liquid: ionic transport, viscoelastic properties, and microstructure. J Phys Chem B 112(30):9013–9019. https://doi.org/10.1021/jp8029117
Hore MJA, Korley LTJ, Kumar SK (2020) Polymer-grafted nanoparticles. J Appl Phys 128:030401. https://doi.org/10.1063/5.0019326
Weeks ER (2017) Introduction to the colloidal glass transition. ACS Macro Lett 6:27–34. https://doi.org/10.1021/acsmacrolett.6b00826
Ueno K, Watanabe M (2011) From colloidal stability in ionic liquids to advanced soft materials using unique media. Langmuir 27:9105–9115. https://doi.org/10.1021/la103942f
Le Bideau J, Viau L, Vioux A (2011) Ionogels, ionic liquid based hybrid materials. Chem Soc Rev 40(2):907–925. https://doi.org/10.1039/c0cs00059k
Wang S, Hsia B, Carraro C, Maboudian R (2014) High-performance all solid-state micro-supercapacitor based on patterned photoresist-derived porous carbon electrodes and an ionogel electrolyte. J Mater Chem A 2(21):7997–8002. https://doi.org/10.1039/C4TA00570H
Yuan Z, Ding J, Zhang Y, Huang B, Song Z, Meng X, Ma X, Gong X, Huang Z, Ma S, Xiang S, Xu W (2022) Components, mechanisms and applications of stimuli-responsive polymer gels. Eur Polymer J 177:111473. https://doi.org/10.1016/j.eurpolymj.2022.111473
Fukushima T, Aida T (2007) Ionic liquids for soft functional materials with carbon nanotubes. Chem Eur J 13(18):5048–5058. https://doi.org/10.1002/chem.200700554
Ding Y, Sun X, Zhang L, Mao S, Xie Z, Liu Z-W et al (2015) Entrapping an ionic liquid with nanocarbon: the formation of a tailorable and functional surface. Angew Chem Int Ed Engl 54:231–235. https://doi.org/10.1002/anie.201408201
Liu Y, Wu G, Fu H, Jiang Z, Chen S, Sha M (2010) Immobilization and melting point depression of imidazolium ionic liquids on the surface of nano-SiOx particles. Dalton Trans 39(13):3190–3194. https://doi.org/10.1039/B924042J
Rodriguez R, Herrera R, Bourlinos AB, Li RP, Amassian A, Archer LA et al (2010) The synthesis and properties of nanoscale ionic materials. Appl Organomet Chem 24(8):581–589. https://doi.org/10.1002/aoc.1625
Perriman AW, Colfen H, Hughes RW, Barrie CL, Mann S (2009) Solvent-free protein liquids and liquid crystals. Angew Chem Int Ed 48(34):6242–6246. https://doi.org/10.1002/anie.200903100
Warren SC, Banholzer MJ, Slaughter LS, Giannelis EP, DiSalvo FJ, Wiesner UB (2006) Generalized route to metal nanoparticles with liquid behavior. J Am Chem Soc 128(37):12074–12075. https://doi.org/10.1021/ja064469r
Li D, Wu J, Xu X, Wang X, Yang S, Tang Z, Shen H, Liu X, Zhao N, Xu J (2018) Solvent free nanoscale ionic materials based on Fe3O4 nanoparticles modified with mussel inspired ligands. J Colloid Interface Sci 531:404–409. https://doi.org/10.1016/j.jcis.2018.07.042
Ivanova R, Lindman B, Alexandridis P (2001) Modification of the lyotropic liquid crystalline microstructure of amphiphilic block copolymers in the presence of cosolvents. Adv Colloid Interface Sci 89:351–382. https://doi.org/10.1016/S0001-8686(00)00049-X
Jespersen ML, Mirau PA, von Meerwall E, Vaia RA, Rodriguez R, Giannelis EP (2010) Canopy dynamics in nanoscale ionic materials. ACS Nano 4(7):3735–3742. https://doi.org/10.1021/nn100112h
Li Q, Dong L, Fang J, Xiong C (2010) Property–structure relationship of nanoscale ionic materials based on multiwalled carbon nanotubes. ACS Nano 4(10):5797–5806. https://doi.org/10.1021/nn101542v
Bhattacharjee RR, Li RP, Estevez L, Smilgies DM, Amassian A, Giannelis EP (2009) A plasmonic fluid with dynamically tunable optical properties. J Mater Chem 19(46):8728–8731. https://doi.org/10.1039/B919006F
Luo H, He X, Liu G et al (2023) Dynamics of poly(methyl methacrylate) in ionic liquids with different concentration and cationic structures. Chin J Polym Sci 41:315–324. https://doi.org/10.1007/s10118-022-2840-z
Li R, Han Y, Akcora P (2022) Ion channels in sulfonated copolymer-grafted nanoparticles in ionic liquids. Soft Matter 18:5402–5409. https://doi.org/10.1039/D2SM00725H
Fujie K, Otsubo K, Ikeda R, Yamada T, Kitagawa H (2015) Low temperature ionic conductor: ionic liquid incorporated within a metal–organic framework††Electronic supplementary information (ESI) available: experimental details, structures of ZIF-8 and EMI-TFSA, TEM image of ZIF-8 nanocrystals, results of structural characterizations Nyquist plots, IR spectra and results of elemental analysis. Chem Sci 6(7):306–4310. https://doi.org/10.1039/c5sc01398d
Chen N, Li Y, Dai Y, Qu W, Xing Y, Ye Y, Wen Z, Guo C, Wu F, Chen R (2019) A Li+ conductive metal organic framework electrolyte boosts the high-temperature performance of dendrite-free lithium batteries. J Mater Chem A 7:9530–9536. https://doi.org/10.1039/C8TA12539B
Bose P, Deb D, Bhattacharya S (2019) Lithium-polymer battery with ionic liquid tethered nanoparticles incorporated P(VDF-HFP) nanocomposite gel polymer electrolyte. Electrochim Acta 319:753–765. https://doi.org/10.1016/j.electacta.2019.07.013
Yu Z, Yang F, Dai S et al (2018) Structure and dynamics of polymeric canopies in nanoscale ionic materials: an electrical double layer perspective. Sci Rep 8:519. https://doi.org/10.1038/s41598-018-23493-1
Sarkar B, Lakshmichand J, Alexandridis P (2012) Self-assembly of amphiphilic block copolymers in ternary solvent mixtures: lyotropic liquid crystalline phase behavior and structure. Macromol Chem Phys 213(23):2514–2528. https://doi.org/10.1002/macp.201200438
Goodchild I, Collier L, Millar SL, Prokes I, Lord JCD, Butts CP et al (2007) Structural studies of the phase, aggregation and surface behavior of 1-alkyl-3-methylimidazolium halide plus water mixtures. J Colloid Interface Sci 307(2):455–468. https://doi.org/10.1016/j.jcis.2006.11.034
Greaves T, Drummond C (2013) Solvent nanostructure, the solvophobic effect and amphiphile self-assembly in ionic liquids. Chem Soc Rev 42(3):1096. https://doi.org/10.1039/c2cs35339c
Sarkar B, Alexandridis P (2015) Block copolymer-nanoparticle composites: structure, functional properties, and processing. Prog Polym Sci 40:33–62. https://doi.org/10.1016/j.progpolymsci.2014.10.009
Qiu ZM, Texter J (2008) Ionic liquids in microemulsions. Curr Opin Colloid Interface Sci 13(4):252–262. https://doi.org/10.1016/j.cocis.2007.10.005
Frost DS, Nofen EM, Dai LL (2014) Particle self-assembly at ionic liquid-based interfaces. Adv Colloid Interface Sci 206:92–105. https://doi.org/10.1016/j.cis.2013.09.004
Binks BP, Dyab AKF, Fletcher PDI (2003) Novel emulsions of ionic liquids stabilized solely by silica nanoparticles. Chem Commun 20:2540–2541. https://doi.org/10.1039/b308998c
Wang A, Chen L, Xu F, Yan Z (2014) In situ synthesis of copper nanoparticles within ionic liquid-in-vegetable oil microemulsions and their direct use as high efficient nanolubricants. RSC Adv 4:45251–45257. https://doi.org/10.1039/c4ra06785a
Gelesky MA, Scheeren CW, Foppa L, Pavan FA, Dias SLP, Dupont J (2009) Metal nanoparticle/ionic liquid/cellulose: new catalytically active membrane materials for hydrogenation reactions. Biomacromol 10:1888–1893. https://doi.org/10.1021/bm9003089
Pedro AQ, Coutinho JAP, Freire MG (2019) Immobilization of ionic liquids, types of materials, and applications. In: Zhang S (ed) Encyclopedia of ionic liquids. Springer, Singapore. https://doi.org/10.1007/978-981-10-6739-6_86-1
Zhao M, Gao Y, Zheng L (2010) Lyotropic liquid crystalline phases formed in binary mixture of 1-tetradecyl-3-methylimidazolium chloride/ethylammonium nitrate and its application in the dispersion of multi-walled carbon nanotubes. Colloids Surf, A 369(1–3):95–100. https://doi.org/10.1016/j.colsurfa.2010.08.015
Hejazifar M, Lanaridi O, Bica-Schröder K (2020) Ionic liquid based microemulsions: a review. J Mol Liq 303:112264. https://doi.org/10.1016/j.molliq.2019.112264
Kataria J, Devi P, Rani P (2021) Importance of structures and interactions in ionic liquid-nanomaterial composite systems as a novel approach for their utilization in safe lithium metal batteries: a review. J Mol Liq 2021(339):116736. https://doi.org/10.1016/j.molliq.2021.116736
Pei Y, Ru J, Yao K, Hao L, Li Z, Wang H, Zhuc X, Wang J (2018) Nanoreactors stable up to 200 °C: a class of high temperature microemulsions composed solely of ionic liquids. Chem Commun 54:6260–6263. https://doi.org/10.1039/C8CC02901F
Sun X, Qiang Q, Yin Z, Wang Z, Ma Y, Zhao C (2019) Monodispersed silver-palladium nanoparticles for ethanol oxidation reaction achieved by controllable electrochemical synthesis from ionic liquid microemulsions. J Colloid Interface Sci 557:450–457. https://doi.org/10.1016/j.jcis.2019.09.043
Dubal DP, Chodankar NR, Kim D-H, Gomez-Romero P (2018) Towards flexible solid-state supercapacitors for smart and wearable electronics. Chem Soc Rev 47(6):2065–2129. https://doi.org/10.1039/C7CS00505A
Qian W, Texter J, Yan F (2017) Frontiers in poly(ionic liquid)s: syntheses and applications. Chem Soc Rev 46(4):1124–1159. https://doi.org/10.1039/C6CS00620E
Pan Z, Yang J, Zhang Q, Liu M, Hu Y, Kou Z, Liu N, Yang X, Ding X, Chen H, Li J, Zhang K, Qiu Y, Li Q, Wang J, Zhang Y (2019) All-solid-state fiber supercapacitors with ultrahigh volumetric energy density and outstanding flexibility. Adv Energy Mater 9(9):1802753. https://doi.org/10.1002/aenm.201802753
Rana HH, Park JH, Gund GS, Park HS (2020) Highly conducting, extremely durable, phosphorylated cellulose-based ionogels for renewable flexible supercapacitors. Energy Storage Mater 25:70–75. https://doi.org/10.1016/j.ensm.2019.10.030
Qin H, Panzer MJ (2020) Zwitterionic copolymer-supported ionogel electrolytes featuring a sodium salt/ionic liquid solution. Chem Mater 32(18):7951–7957. https://doi.org/10.1021/acs.chemmater.0c02820
Li H, Feng Z, Zhao K, Wang Z, Liu J, Liu J, Song H (2019) Chemically crosslinked liquid crystalline poly(ionic liquid)s/halloysite nanotubes nanocomposite ionogels with superior ionic conductivity, high anisotropic conductivity and a high modulus. Nanoscale 11(8):3689–3700. https://doi.org/10.1039/C8NR09030K
Li L, Yang X, Li J et al (2018) A novel and shortcut method to prepare ionic liquid gel polymer electrolyte membranes for lithium-ion battery. Ionics 24:735–741. https://doi.org/10.1007/s11581-017-2254-z
Zhou D, Liu R, Zhang J, Qi X, He Y-B, Li B, Yang Q-H, Hu Y-S, Kang F (2017) In situ synthesis of hierarchical poly(ionic liquid)-based solid electrolytes for high-safety lithium-ion and sodium-ion batteries. Nano Energy 33:45–54. https://doi.org/10.1016/j.nanoen.2017.01.027
Singh VK, Shalu Y, Balo L et al (2017) Solid polymer electrolytes based on Li+/ionic liquid for lithium secondary batteries. J Solid State Electrochem 21:1713–1723. https://doi.org/10.1007/s10008-017-3529-z
Chen T, Kong W, Zhang Z, Wang L, Hu Y, Zhu G, Chen R, Ma L, Yan W, Wang Y, Liu J, Zhong J (2018) Ionic liquid-immobilized polymer gel electrolyte with self-healing capability, high ionic conductivity and heat resistance for dendrite-free lithium metal batteries. Nano Energy 54:17–25. https://doi.org/10.1016/j.nanoen.2018.09.059
Tian X, Yang P, Yi Y, Liu P, Wang T, Shu C, Qu L, Tang W, Zhang Y, Li M, Yang B (2020) Self-healing and high stretchable polymer electrolytes based on ionic bonds with high conductivity for lithium batteries. J Power Sources 450:227629. https://doi.org/10.1016/j.jpowsour.2019.227629
Hagiwara R, Matsumoto K, Hwang J, Nohira T (2019) Sodium ion batteries using ionic liquids as electrolytes. Chem Rec 19:758–770. https://doi.org/10.1002/tcr.201800119
Matsumoto K, Nishiwaki E, Hosokawa T, Tawa S, Nohira T, Hagiwara R (2017) Thermal, physical, and electrochemical properties of Li[N(SO2F)2]-[1-ethyl-3-methylimidazolium][N(SO2F)2] ionic liquid electrolytes for li secondary batteries operated at room and intermediate temperatures. J Phys Chem C 121:9209–9219. https://doi.org/10.1021/acs.jpcc.7b02296
Liu Z, Hu Y-Y, Dunstan MT, Huo H, Hao X, Zou H, Zhong G, Yang Y, Grey CP (2014) Local structure and dynamics in the na ion battery positive electrode material Na3V2(PO4)2F3. Chem Mater 26:2513–2521. https://doi.org/10.1021/cm403728w
Wohde F, Balabajew M, Roling B (2016) Li+ transference numbers in liquid electrolytes obtained by very-low-frequency impedance spectroscopy at variable electrode distances. J Electrochem Soc 163:A714–A721. https://doi.org/10.1149/2.0811605jes
Rodríguez-Palmeiro I, Rodríguez-Cabo B, Rodil E, Arce A, Saiz-Jabardo JM, Soto A (1881) Synthesis and characterization of highly concentrated AgI–[P6,6,6,14]Cl ionanofluids. J Nanoparticle Res 2013:15. https://doi.org/10.1007/s11051-013-1881-1
Chereches E, Minea AA (2020) Experimental evaluation of electrical conductivity of ionanofluids based on water–[C2mim][CH3SO3] ionic liquids mixtures and alumina nanoparticles. J Therm Anal Calorim. https://doi.org/10.1007/s10973-020-09925-z
Deb D, Dutta B, Bhattacharya S (2019) Viscosity decoupled charge transport in surface functionalized ZnS nanoparticle dispersed imidazolium ionanofluids. Mater Res Bull 116:22–31. https://doi.org/10.1016/j.materresbull.2019.03.028
Peljo P, Girault HH (2018) Electrochemical potential window of battery electrolytes: the HOMO–LUMO misconception. Energy Environ Sci 11:2306–2309. https://doi.org/10.1039/C8EE01286E
Matsumoto K, Hwang J, Kaushik S, Chen C-Y, Hagiwara R (2019) Advances in sodium secondary batteries utilizing ionic liquid electrolytes. Energy Environ Sci 12:3247–3287. https://doi.org/10.1039/C9EE02041A
Hwang J, Matsumoto K, Hagiwara R (2020) Electrolytes toward high-voltage Na3V2(PO4)2F3 positive electrode durable against temperature variation. Adv Energy Mater 10:2001880. https://doi.org/10.1002/aenm.202001880
Zhou Z-B, Matsumoto H, Tatsumi K (2006) Cyclic quaternary ammonium ionic liquids with perfluoroalkyltrifluoroborates: synthesis, characterization, and properties. Chem - Eur J 12:2196–2212. https://doi.org/10.1002/chem.200500930
Mousavi MPS, Kashefolgheta S, Stein A, Bühlmann P (2015) Electrochemical stability of quaternary ammonium cations: an experimental and computational study. J Electrochem Soc 163:H74–H80. https://doi.org/10.1149/2.0671602jes
Sakaebe H, Matsumoto H (2003) N-methyl-N-propylpiperidinium bis (trifluoromethanesulfonyl)imide (PP13–TFSI) – novel electrolyte base for Li battery. Electrochem Commun 5:594–598. https://doi.org/10.1016/S1388-2481(03)00137-1
Chen Z, Chao Y, Li W, Wallace GG, Bussell T, Ding J, Wang C (2021) Abuse-tolerant electrolytes for lithium-ion batteries. Adv Sci 8:2003694. https://doi.org/10.1002/advs.202003694
Rana S, Thakur RC, Dosanjh HS (2023) Ionic liquids as battery electrolytes for lithium-ion batteries: Recent advances and future prospects. Solid State Ionics 400:116340. https://doi.org/10.1016/j.ssi.2023.116340
Ortiz MG, Čech O, Kazda T, Čudek P, Sedlaříková M (2021) Synthesis and characterization of blended cathode materials as improvement on the electrochemical performance in Li-ion battery. J Energy Storage 42:103059. https://doi.org/10.1016/j.est.2021.103059
Tsurumaki A, Ohno H, Panero S, Navarra MA (2019) Novel bis(fluorosulfonyl)imide-based and ether-functionalized ionic liquids for lithium batteries with improved cycling properties. Electrochim Acta 293:160–165. https://doi.org/10.1016/j.electacta.2018.09.205
Nirmale TC, Khupse ND, Kalubarme RS, Kulkarni MV, Varma AJ, Kale BB (2022) Imidazolium-based dicationic ionic liquid electrolyte: strategy toward safer lithium-ion batteries. ACS Sustain Chem Eng 10(26):8297–8304. https://doi.org/10.1021/acssuschemeng.2c00767
Lei Z, Chen B, Koo Y-M, MacFarlane DR (2017) Introduction: ionic liquids. Chem Rev 117(10):6633–6635. https://doi.org/10.1021/acs.chemrev.7b00246
Kim O, Kim K, Choi UH et al (2018) Tuning anhydrous proton conduction in single-ion polymers by crystalline ion channels. Nat Commun 9:5029. https://doi.org/10.1038/s41467-018-07503-4
Mei X, Yue Z, Ma Q, Dunya H, Mandal BK (2018) Synthesis and electrochemical properties of new dicationic ionic liquids. J Mol Liq 272:1001–1018. https://doi.org/10.1016/j.molliq.2018.10.085
Dalir N, Javadian SSM, Javad Ghavam SMJ, Gharibi H (2021) The critical role of ionic liquid crystal on Mg2+ ion transport properties in magnesium ion batteries; performance and mechanism approach. J Electrochem Soc 168(7):070519. https://doi.org/10.1149/1945-7111/ac0f87
Liu X, Mariani A, Zarrabeitia M, Di Pietro ME, Dong X, Elia GA, Mele A, Passerini S (2022) Effect of organic cations in locally concentrated ionic liquid electrolytes on the electrochemical performance of lithium metal batteries. Energy Storage Mater 44:370–378. https://doi.org/10.1016/j.ensm.2021.10.034
Swiderska-Mocek A (2014) Electrolyte based on 1-ethyl-3-vinylimidazolium bis(trifluoromethanesulphonyl)imide for Li-ion batteries. Electrochim Acta 132:504–511. https://doi.org/10.1016/j.electacta.2014.03.185
Chagas LC, Jeong S, Ivana Hasa I, Passerini S (2019) Ionic Liquid-based electrolytes for sodium-ion batteries: tuning properties to enhance the electrochemical performance of manganese-based layered oxide cathode. ACS Appl Mater Interfaces 11(25):22278–22289. https://doi.org/10.1021/acsami.9b03813
Sun H, Zhu G, Xu X et al (2019) A safe and non-flammable sodium metal battery based on an ionic liquid electrolyte. Nat Commun 10:3302. https://doi.org/10.1038/s41467-019-11102-2
Duncan DT, Piper SL, Forsyth M, MacFarlane DR, Kar M (2023) Fluoroborate ionic liquids as sodium battery electrolytes. Phys Chem Chem Phys 25:27718–27730. https://doi.org/10.1039/D3CP03694D
Bi Y, He S, Fan C, Luo J, Yuan B, Liu TL (2020) A robust ionic liquid magnesium electrolyte enabling Mg/S batteries. J Mater Chem A 8:12301–12305. https://doi.org/10.1039/D0TA02041A
Zhang D, Duan S, Liu X, Yang Y, Zhang Y, Ren W, Zhang S, Cheng M, Yang W, Wang J, NuLi Y (2023) Deeping insight of Mg(CF3SO3)2 and comprehensive modified electrolyte with ionic liquid enabling high-performance magnesium batteries. Nano Energy 109:108257. https://doi.org/10.1016/j.nanoen.2023.108257
Yamamoto T, Matsubara R, Nohira T (2021) Highly conductive ionic liquid electrolytes for potassium-ion batteries. J Chem Eng Data 66(2):1081–1088. https://doi.org/10.1021/acs.jced.0c00879
Fiore M, Wheeler S, Hurlbutt K, Capone I, Fawdon J, Ruffo R, Pasta M (2020) Paving the way toward highly efficient, high-energy potassium-ion batteries with ionic liquid electrolytes. Chem Mater 32(18):7653–7661. https://doi.org/10.1021/acs.chemmater.0c01347
Zhang C, Chen Z, Zhang H, Liu Y, Wei W, Zhou Y, Xu M (2023) Uniformly dispersed Sb-nanodot constructed by in situ confined polymerization of ionic liquids for high-performance potassium-ion batteries. Molecules 28:5212. https://doi.org/10.3390/molecules28135212
Jeon J, Kang S, Koo SB, Kim H, Hong S-T, Lee H (2024) Long-life potassium metal batteries enabled by anion-derived solid electrolyte interphase using concentrated ionic liquid electrolytes. J Colloid Interface Sci 670:617–625. https://doi.org/10.1016/j.jcis.2024.05.135
Yu L, Huang J, Wang S, Qi L, Wang S, Chen C (2023) Ionic liquid “water pocket” for stable and environment-adaptable aqueous zinc metal batteries. Adv Mater 35:2210789. https://doi.org/10.1002/adma.202210789
De Anastro AF, Casado N, Wang X, Rehmen J, Evans D, Mecerreyes D, Forsyth M, Pozo-Gonzalo C (2018) Poly(ionic liquid) iongels for all-solid rechargeable zinc/PEDOT batteries. Electrochim Acta 278:271–278. https://doi.org/10.1016/j.electacta.2018.05.044
Sorenson GP, Coppage KL, Mahanthappa MK (2011) Unusually stable aqueous lyotropic gyroid phases from Gemini dicarboxylate surfactants. J Am Chem Soc 133:14928–14931. https://doi.org/10.1021/ja2063555
Ichikawa T, Kato T, Ohno H (2012) 3D continuous water nanosheet as a gyroid minimal surface formed by bicontinuous cubic liquid-crystalline Zwitterions. J Am Chem Soc 134:11354–11357. https://doi.org/10.1021/ja304124w
Lu F, Gao X, Dong B, Sun P, Sun N, Xie S, Zheng L (2014) Nanostructured proton conductors formed via in situ polymerization of ionic liquid crystals. ACS Appl Mater Interfaces 6:21970–21977. https://doi.org/10.1021/am504504m
Luo J, You J, Tan S, Wang C, Wu Y (2020) Lamellar lyotropic liquid crystal superior to micellar solution for proton conduction in an aqueous solution of 1-tetradecyl3-methylimidazolium hydrogen sulfate. ACS Appl Mater Interfaces 12:45611–45617. https://doi.org/10.1021/acsami.0c13349
Al-Othman A, Nancarrow P, Tawalbeh M, Ka’ki A, El-Ahwal K, El Taher B, Alkasrawi M (2021) Novel composite membrane based on zirconium phosphate-ionic liquids for high temperature PEM fuel cells. Int J Hydrogen Energy 46(8):6100–6109. https://doi.org/10.1016/j.ijhydene.2020.02.112
Hilmy NIMF, Yahya WZN, Kurnia KA (2020) Eutectic ionic liquids as potential electrolytes in dye-sensitized solar cells: physicochemical and conductivity studies. J Mol Liq 320(Part A):114381. https://doi.org/10.1016/j.molliq.2020.114381
Lin Y-F, Li C-T, Lee C-P, Leu Y-A, Ezhumalai Y, Vittal R, Chen M-C, Lin J-J, Ho K-C (2016) Multifunctional iodide-free polymeric ionic liquid for quasi-solid-state dye-sensitized solar cells with a high open-circuit voltage. ACS Appl Mater Interfaces 8(24):15267–15278. https://doi.org/10.1021/acsami.6b02767
Lau GP, Décoppet JD, Moehl T, Zakeeruddin SM, Grätzel M, Dyson PJ (2015) Robust high-performance dye-sensitized solar cells based on ionic liquid-sulfolane composite electrolytes. Sci Rep 16(5):18158. https://doi.org/10.1038/srep18158
Sharma T, Gultekin B, Dhapola PS, Sahoo NG, Kumar S, Agarwal D, Jun HK, Singh D, Nath G, Singh PK, Singh A (2022) Ionic liquid doped poly (methyl methacrylate) for energy applications. J Mol Liq 352:118494. https://doi.org/10.1016/j.molliq.2022.118494
Bai S, Da P, Li C et al (2019) Planar perovskite solar cells with long-term stability using ionic liquid additives. Nature 571:245–250. https://doi.org/10.1038/s41586-019-1357-2
Akin S, Akman E, Sonmezoglu S (2020) FAPbI3-based Perovskite solar cells employing hexyl-based ionic liquid with an efficiency over 20% and excellent long-term stability. Adv Func Mater 30(28):2002964. https://doi.org/10.1002/adfm.202002964
Yang D, Yang R, Ren X, Zhu X, Yang Z, Li C, Liu SF (2016) Hysteresis-suppressed high-efficiency flexible perovskite solar cells using solid-state ionic-liquids for effective electron transport. Adv Mater 28:5206–5213
Wu Q, Zhou W, Liu Q, Zhou P, Chen T, Lu Y, Qiao Q, Yang S (2016) Solution-processable ionic liquid as an independent or modifying electron transport layer for high-efficiency perovskite solar cells. ACS Appl Mater Interfaces 8(50):34464–34473. https://doi.org/10.1021/acsami.6b12683
Gu L, Ran C, Chao L, Bao Y, Hui W, Wang Y, Chen Y, Gao X, Song L (2022) Designing ionic liquids as the solvent for efficient and stable Perovskite solar cells. ACS Appl Mater Interfaces 14(20):22870–22878. https://doi.org/10.1021/acsami.1c21035
Abraham TJ, MacFarlane DR, Baughman RH, Jin L, Li N, Pringle JM (2013) Towards ionic liquid-based thermoelectrochemical cells for the harvesting of thermal energy. Electrochimica Acta 113:87–93. https://doi.org/10.1016/j.electacta.2013.08.087
Cheng H, He X, Fan Z, Ouyang J (2019) Flexible quasi-solid state ionogels with remarkable seebeck coefficient and high thermoelectric properties. Adv Energy Mater 9:1901085. https://doi.org/10.1002/aenm.201901085
Chen B, Chen Q, Xiao S, Feng J, Zhang X, Wang T (2023) Giant negative thermopower of ionic hydrogel by synergistic coordination and hydration interactions. Sci Adv 7:eabi7233. https://doi.org/10.1126/sciadv.abi7233
Laux E, Jeandupeux L, Uhl S, Keppner H, López PP, Sanglard P, Vanoli E, Marti R (2019) Novel ionic liquids for thermoelectric generator devices. Mater Today: Proceedings 8(part 2):672–679. https://doi.org/10.1016/j.matpr.2019.02.067
Xiao M, Yao Y, Liu W (2023) Durable and stretchable nanocomposite ionogels with high thermoelectric property for low-grade heat harvesting. Sci China Technol Sci 66:267–280. https://doi.org/10.1007/s11431-022-2222-5
Zhu Y, Han C-G, Chen J, Yang L, Ma Y, Guan H, Han D, Niu L (2024) Ultra-high performance of ionic thermoelectric-electrochemical gel cells for harvesting low grade heat. Energy Environ Sci. https://doi.org/10.1039/D4EE01150C
Wu A, Li X, Lee D, Li J, Yun J, Jiang C, Li Z, Lee SW (2023) Thermoresponsive ionic liquid for electrochemical low-grade heat harvesting. Nano Energy 105:108022. https://doi.org/10.1016/j.nanoen.2022.108022
Hsiao Y-C, Lee L-C, Lin Y-T, Hong S-H, Wang K-C, Tung S-H, Liu C-L (2023) Stretchable polyvinyl alcohol and sodium alginate double network ionic hydrogels for low-grade heat harvesting with ultrahigh thermopower. Materials today energy 37:101383. https://doi.org/10.1016/j.mtener.2023.101383
Yao M, Liu A, Xing C, Li B, Pan S, Zhang J, Su P, Zhang H (2020) Asymmetric supercapacitor comprising a core-shell TiNb2O7@MoS2/C anode and a high voltage ionogel electrolyte. Chem Eng J 394:124883. https://doi.org/10.1016/j.cej.2020.124883
Goossens JK, Lava K, Nockemann P, Van Hecke K, Van Meervelt L, Pattison P, Binnemans K, Cardinaels T (2009) Pyrrolidinium ionic liquid crystals with pendant mesogenic groups. Langmuir 25:5881–5897. https://doi.org/10.1021/la900048h
Wang Y, Song Y, Xia Y (2016) Electrochemical capacitors: mechanism, materials, systems, characterization, and applications. Chem Soc Rev 45(21):5925–5950. https://doi.org/10.1039/C5CS00580A
Lai F, Feng J, Yan R, Wang GC, Antonietti M, Oschatz M (2018) Breaking the limits of ionic liquid-based supercapacitors: mesoporous carbon electrodes functionalized with manganese oxide nanosplotches for dense, stable, and wide-temperature energy storage. Adv Funct Mater 28(36):1801298. https://doi.org/10.1002/adfm.201801298
Mo T, Bi S, Zhang Y, Presser V, Wang X, Gogotsi Y, Feng G (2020) Ion structure transition enhances charging dynamics in subnanometer pores. ACS Nano 14(2):2395–2403. https://doi.org/10.1021/acsnano.9b09648
Eftekhari A (2017) Supercapacitors utilizing ionic liquids. Energy storage materials 9:47–69. https://doi.org/10.1016/j.ensm.2017.06.009
Nguyen QD, Patra J, Hsieh C, Li J, Dong Q, Chang JK (2019) Supercapacitive properties of micropore- and mesopore-rich activated carbon in ionic-liquid electrolytes with various constituent ions. Chem Sus Chem 12(2):449–456. https://doi.org/10.1002/cssc.201802489
Pan H, Zhang Y, Pan Y, Lin W, Tu W, Zhang H (2020) Nitrogen-doped porous carbon with interconnected tubular structure for supercapacitors operating at sub-ambient temperatures. Chem Eng J 401:126083. https://doi.org/10.1016/j.cej.2020.126083
Molinari A, Leufke P, Reitz C, Dasgupta S, Witte R, Kruk R, Hahn H (2017) Hybrid supercapacitors for reversible control of magnetism. Nat Commun 8:15339. https://doi.org/10.1038/ncomms15339
Varela CJ, Sankar K, Hino A, Lin X, Chang W-S, Coker D, Grinstaff M (2018) Piperidinium ionic liquids as electrolyte solvents for sustained high temperature supercapacitor operation. Chem Commun 54(44):5590–5593. https://doi.org/10.1039/C8CC01093E
Yan R, Antonietti M, Oschatz M (2018) Toward the experimental understanding of the energy storage mechanism and ion dynamics in ionic liquid-based supercapacitors. Adv Energy Mater 8(18):1800026. https://doi.org/10.1002/aenm.201800026
Wang Y, Qian C, Huo F, Qin J, He H (2020) Molecular mechanism of anion size regulating the nanostructure and charging process at ionic liquid-electrode interfaces. J Mater Chem A 8(38):19908–19916. https://doi.org/10.1039/D0TA06643E
Płotka-Wasylka M, de la Guardia J, Andruch V, Vilková M (2020) Deep eutectic solvents vs ionic liquids: similarities and differences. Microchem J 159:105539. https://doi.org/10.1016/j.microc.2020.105539
Dou Q, Lian C, Lei S, Chen J, Liu H, Yan X (2019) Silica-grafted ionic liquid for maximizing the operational voltage of electrical double-layer capacitors. Energy storage mater 18:253–259. https://doi.org/10.1016/j.ensm.2018.09.004
Punnakkal N, Babu TGS, Nair BG, Suneesh PV (2022) Redox Active electrolytes in supercapacitors. In: Thomas S, Gueye AB, Gupta RK (eds) Nanostructured materials for supercapacitors. Advances in material research and technology. Springer, Cham. https://doi.org/10.1007/978-3-030-99302-3_23
Fleischmann S, Widmaier M, Schreiber A, Shim H, Stiemke FM, Schubert TJS, Presser V (2019) High voltage asymmetric hybrid supercapacitors using lithium- and sodium-containing ionic liquids. Energy Storage Mater 16:391–399. https://doi.org/10.1016/j.ensm.2018.06.011
Zhou H, Wu S, Wang H, Li Y, Liu X, Yanmei Zhou Y (2021) The preparation of porous carbon materials derived from bio-protic ionic liquid with application in flexible solid-state supercapacitors. J Hazard Mater 402(2021):124023. https://doi.org/10.1016/j.jhazmat.2020.124023
Xiong W, Yin Z, Zhang X, Tu Z, Hu X, Youting Y (2022) Ionic liquids endowed with novel hybrid anions for supercapacitors. ACS Omega 7(30):26368–26374. https://doi.org/10.1021/acsomega.2c02211
Wei D, Yang F, Jiang Z, Wang Z (2022) Flexible iontronics based on 2D nanofluidic material. Nat Commun 13:4965. https://doi.org/10.1038/s41467-022-32699-x
Bard AJ, Faulkner L (2000) Electrochemical methods: fundamentals and applications. Wiley, New York
Wei D, Ivaska A (2008) Applications of ionic liquids in electrochemical sensors. Anal Chim Acta 607:126–135. https://doi.org/10.1016/j.aca.2007.12.011
Sohail M, de Marco R (2013) Electrodes/ion-selective electrodes. In: Reference Module in Chemistry, Molecular Sciences and Chemical Engineering. Elsevier
Wang R, Okajima T, Kitamura F, Ohsaka T (2004) A Novel Amperometric O2 gas sensor based on supported room-temperature ionic liquid porous polyethylene membrane-coated electrodes. Electroanalysis 16:66–72. https://doi.org/10.1002/elan.200302919
Buzzeo MC, Hardacre C, Compton RG (2004) Use of room temperature ionic liquids in gas sensor design. Anal Chem 76(15):4583–4588. https://doi.org/10.1021/ac040042w
Mellah M, Zeitouny J, Gmouh S, Vaultier M, Jouikov V (2005) Oxidative self-coupling of aromatic compounds in ionic liquids. Electrochem commun 7:869–874. https://doi.org/10.1016/j.elecom.2005.06.002
Niranjan T, Chokkareddy R, Redhi GG, Naidu NV (2020) Chapter 11 - Ionic liquids as gas sensors and biosensors. Green Sustainable Process for Chemical and Environmental Engineering and Science - Ionic Liquids as Green Solvers. pp 319–342. https://doi.org/10.1016/B978-0-12-817386-2.00011-1
Wang Z, Lin P, Baker GA, Stetter J, Zeng X (2011) Ionic liquids as electrolytes for the development of a robust amperometric oxygen sensor. Anal Chem 83:7066–7073. https://doi.org/10.1021/ac201235w
Khorsandi D, Zarepour A, Rezazadeh I, Ghomi M, Ghanbari R, Zarrabi A, Esfahani FT, Mojahed N, Baghayeri M, Zare EN et al (2022) Ionic liquid-based materials for electrochemical biosensing. Clin Transl Discov 2:e127. https://doi.org/10.1002/ctd2.127
Santiago-Malagón S, Río-Colín D, Azizkhani H, Aller-Pellitero M, Guirado G, del Campo FJ (2021) A self-powered skin-patch electrochromic biosensor. Biosens Bioelectron 175:112879. https://doi.org/10.1016/j.bios.2020.112879
Asal M, Özen Ö, Şahinler M, Baysal HT, Polatoğlu İ (2019) An overview of biomolecules, immobilization methods and support materials of biosensors. Sens Rev 39(3):377–386. https://doi.org/10.1108/SR-04-2018-0084
Nguyen HH, Lee SH, Lee UJ, Fermin CD, Kim M (2019) Immobilized enzymes in biosensor applications. Materials 12(1):121. https://doi.org/10.3390/ma12010121
Funding
Open access funding provided by FCT|FCCN (b-on). The authors are grateful to the Fundação para a Ciência e a Tecnologia (FCT), Avenida D. Carlos I, 126, 1249–074 Lisboa, Portugal, for partially financing the Project “Estratégias interfaciais de arrefecimento para tecnologias de conversão com elevadas potências de dissipação”, Ref. PTDC/EMETED/7801/2020, António Luís Nobre Moreira, Associação do Instituto Superior Técnico para a Investigação e o Desenvolvimento (IST-ID). José Pereira also acknowledges the FCT for his PhD Fellowship (Ref. 2021. 05830.BD). The authors are also grateful for the FCT funding through 2022.03151.PTD and LA/P/0083/2020 IN + -IST-ID. A.S. Moita acknowledges the Fundação para a Ciência e Tecnologia from the support https://doi.org/10.54499/CEECINST/00043/2021/CP2797/CT0005.
Author information
Authors and Affiliations
Contributions
Conceptualization, J.P. and R.S.; methodology, J.P. and A.M. (Ana Moita); software, A.M. (Ana Moita); validation, A.M. (Ana Moita) and A.M. (António Moreira); formal analysis, A.M. (Ana Moita); investigation, J.P. and R.S.; resources, A.M. (António Moreira); data curation, J.P. and R.S.; writing—original draft preparation, J.P. and R.S.; writing—review and editing, J.P. and R.S.; supervision, A.M. (Ana Moita) and A.M. (António Moreira); project administration, A.M. (António Moreira); funding acquisition, A.M. (António Moreira). All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Pereira, J., Souza, R., Moreira, A. et al. An overview of the ionic liquids and their hybrids operating in electrochemical cells and capacitors. Ionics (2024). https://doi.org/10.1007/s11581-024-05626-x
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11581-024-05626-x