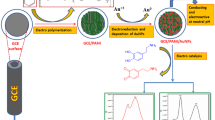
Abstract
This study presents the development of a selective sensor for the electrochemical determination of riboflavin. In the first step, electropolymerization of 3,4-ethylenedioxythiophene (EDOT) was carried out on the pencil graphite electrode to prepare the modified electrode (PEDOT/PGE). In the second step, we prepared over-oxidized poly(3,4-ethylene-dioxythiophene) pencil graphite electrode (OOPEDOT/PGE) by chronoamperometry method in pH 7.0 phosphate buffer solution. In the final step, silver nanoparticles were deposited on the OOPEDOT/PGE by chronoamperometry. The sensitivity of the developed AgNp/OOPEDOT/PGE sensor to riboflavin has been improved. Cyclic voltammetry (CV), electrochemical impedance spectroscopy (EIS), X-ray photoelectron spectroscopy (XPS), and Fourier transform infrared spectroscopy (FTIR) were used to characterize the sensor. The surface morphology of the sensor was examined using field emission scanning electron microscopy (FESEM). The limit of detection (LOD) value was determined to be 0.51 µmol L−1 and a linear range of 0.8 and 80 µmol L−1 using differential pulse voltammetry (DPV). The sensor demonstrated excellent resistance to interference from dopamine (DA), glucose (G), uric acid (UA), and lactate (LA). The study investigated the applicability of the constructed sensor to real samples and achieved good recovery values. The results indicate that the modified electrode is suitable for riboflavin determination applications.
Graphical abstract









Similar content being viewed by others
Data availability
No datasets were generated or analyzed during the current study.
References
Bandžuchová L, Šelešovská R, Navrátil T et al (2012) Voltammetric monitoring of electrochemical reduction of riboflavin using silver solid amalgam electrodes. Electrochim Acta 75:316–324. https://doi.org/10.1016/j.electacta.2012.05.009
Zarei E, Jamali MR, Bagheri J (2018) Electrochemical determination of riboflavin by an ionic liquid modified carbon paste electrode as a sensitive sensor. Anal Bioanal Electrochem 10:642–657
Derakhshan M, Shamspur T, Molaakbari E et al (2020) Fabrication of a novel electrochemical sensor for determination of riboflavin in different drink real samples. Russ J Electrochem 56:181–188. https://doi.org/10.1134/S1023193520030039
Nemomsa T, Kitte SA, Woldemariam ET, Fereja TH (2020) Indirect electrochemical determination of riboflavin (Vb2) using 1, 4-benzaquinone modified carbon paste electrode. Acta Chem Iasi 28:1–18. https://doi.org/10.2478/achi-2020-0001
Kodentsova VM, Uspenskaia ID, Vrzhesinskaia OA, Kharitonchik LA, Sokol’nikov AA, Iakushina LM, Makarova IB, Spirichev VB (1995) Features of B group vitamin metabolism and criteria for providing them to children suffering from celiac disease. Vopr Med Khimii 41(4):41–45
Brezo T, Stojanović Z, Suturović Z et al (2015) Simple, rapid and selective chronopotentiometric method for the determination of riboflavin in pharmaceutical preparations using a glassy carbon electrode. Acta Chim Slov 62:923–931. https://doi.org/10.17344/acsi.2015.1745
Marzabad MA, Jafari B, Norouzi P (2020) Determination of riboflavin by nanocomposite modified carbon paste electrode in biological fluids using fast fourier transform square wave voltammetry. Int J Eng Trans C: Asp 33:1696–1702. https://doi.org/10.5829/ije.2020.33.09c.01
Wang M, Zhao L, Liu M, Lin JM (2007) Determination of riboflavin by enhancing the chemiluminescence intensity of peroxomonosulfate-cobalt(II) system. Spectrochim Acta A Mol Biomol Spectrosc 66:1222–1227. https://doi.org/10.1016/j.saa.2006.06.010
Hu L, Yang X, Wang C et al (2007) Determination of riboflavin in urine and beverages by capillary electrophoresis with in-column optical fiber laser-induced fluorescence detection. J Chromatogr B Analyt Technol Biomed Life Sci 856:245–251. https://doi.org/10.1016/j.jchromb.2007.06.011
Kang L, Lin C, Ning F et al (2021) Rapid determination of folic acid and riboflavin in urine by polypyrrole magnetic solid-phase extractant combined ultra-performance liquid chromatography. J Chromatogr A 1648:462192. https://doi.org/10.1016/j.chroma.2021.462192
Dokur E, Gorduk O, Sahin Y (2020) Selective electrochemical sensing of riboflavin based on functionalized multi-walled carbon nanotube/gold nanoparticle/pencil graphite electrode. ECS J Solid State Sci Technol 9:121003. https://doi.org/10.1149/2162-8777/abcdff
Sá ÉS, Da Silva PS, Jost CL, Spinelli A (2015) Electrochemical sensor based on bismuth-film electrode for voltammetric studies on vitamin B2 (riboflavin). Sens Actuators B Chem 209:423–430. https://doi.org/10.1016/j.snb.2014.11.136
Blanco E, Dominguez CSH, Hernández P et al (2009) Alkanethiols modified gold electrodes for selective detection of molecules with different polarity and molecular size. Application to vitamin B2 analysis. Electroanalysis 21:495–500. https://doi.org/10.1002/elan.200804430
Yu YY, Wang JX, Si RW et al (2017) Sensitive amperometric detection of riboflavin with a whole-cell electrochemical sensor. Anal Chim Acta 985:148–154. https://doi.org/10.1016/j.aca.2017.06.053
Kotkar RM, Desai PB, Srivastava AK (2007) Behavior of riboflavin on plain carbon paste and aza macrocycles based chemically modified electrodes. Sens Actuators B Chem 124:90–98. https://doi.org/10.1016/j.snb.2006.12.004
Zhang H, Zhao J, Liu H et al (2010) Application of poly (3-methylthiophene) modified glassy carbon electrode as riboflavin sensor. Int J Electrochem Sci 5:295–301. https://doi.org/10.1016/S1452-3981(23)15285-0
Uruc S, Gorduk O, Sahin Y (2023) Electroanalytical determination of Allura Red in beverage samples using an anodically pretreated graphite electrode. Int J Environ Anal Chem 103:3544–3562. https://doi.org/10.1080/03067319.2021.1910252
Nagarajan S, Vairamuthu R (2021) Electrochemical detection of riboflavin using tin-chitosan modified pencil graphite electrode. J Electroanal Chem 891:115235. https://doi.org/10.1016/j.jelechem.2021.115235
Sedhu N, Jagadeesh Kumar J, Sivaguru P, Raj V (2023) Electrochemical detection of riboflavin in pharmaceutical and food samples using in situ electropolymerized glycine coated pencil graphite electrode. J Electroanal Chem 928:117037. https://doi.org/10.1016/j.jelechem.2022.117037
Sreelekshmi, Sajeevan B, MG G et al (2023) An electrochemical sensor based on a pencil graphite electrode modified with poly-riboflavin for 4-nitrophenol quantification. Mater Chem Phys 301:127568. https://doi.org/10.1016/j.matchemphys.2023.127568
Gorcay H, Turkoglu G, Sahin Y, Berber H (2014) Electrochemical determination of paracetamol by a novel derivative of formazan modified pencil graphite electrode. IEEE Sens J 14:2529–2536. https://doi.org/10.1109/JSEN.2014.2311296
Prinith NS, Manjunatha JG, Tigari G et al (2021) Mechanistic Insights into the voltammetric determination of riboflavin at poly (serine) modified graphite and carbon nanotube composite paste electrode. ChemistrySelect 6:10746–10757. https://doi.org/10.1002/slct.202103184
Koyun O, Sahin Y (2018) Voltammetric determination of nitrite with gold nanoparticles/poly(methylene blue)-modified pencil graphite electrode: application in food and water samples. Ionics (Kiel) 24:3187–3197. https://doi.org/10.1007/s11581-017-2429-7
Koyun O (2017) Electrochemical determination of some substances important for food safety. Dissertation, Yildiz Technical University
Tigari G, Manjunatha JG, Raril C, Hareesha N (2019) Determination of riboflavin at carbon nanotube paste electrodes modified with an anionic surfactant. ChemistrySelect 4:2168–2173. https://doi.org/10.1002/slct.201803191
Muthusankar G, Rajkumar C, Chen SM et al (2019) Sonochemical driven simple preparation of nitrogen-doped carbon quantum dots/SnO2 nanocomposite: a novel electrocatalyst for sensitive voltammetric determination of riboflavin. Sens Actuators B Chem 281:602–612. https://doi.org/10.1016/j.snb.2018.10.145
Roushani M, Abdi Z (2014) Novel electrochemical sensor based on graphene quantum dots/riboflavin nanocomposite for the detection of persulfate. Sens Actuators B Chem 201:503–510. https://doi.org/10.1016/j.snb.2014.05.054
Gorduk O (2020) Differential pulse voltammetric determination of serotonin using an acid-activated multiwalled carbon nanotube–over-oxidized poly(3,4-ethylenedioxythiophene) modified pencil graphite electrode. Anal Lett 53:1034–1052. https://doi.org/10.1080/00032719.2019.1693583
Hu X, Zheng W, Zhang R (2016) Determination of p-chloronitrobenzene by voltammetry with an electrochemically pretreated glassy carbon electrode. J Solid State Electrochem 20:3323–3330. https://doi.org/10.1007/s10008-016-3302-8
Tahtaisleyen S, Gorduk O, Sahin Y (2020) Electrochemical determination of sunset yellow using an electrochemically prepared graphene oxide modified–pencil graphite electrode (EGO-PGE). Anal Lett 0:1–23. https://doi.org/10.1080/00032719.2020.1767120
Gorduk O, Gorduk S, Sahin Y (2020) Fabrication of tetra-substituted copper(II) phthalocyanine-graphene modified pencil graphite electrode for amperometric detection of hydrogen peroxide. ECS J Solid State Sci Technol 9:061003. https://doi.org/10.1149/2162-8777/ab9c7a
Dokur E, Uruc S, Gorduk O, Sahin Y (2022) Ultrasensitive electrochemical detection of carcinoembryonic antigen with a label-free immunosensor using gold nanoparticle-decorated poly(pyrrole-co-3,4-ethylenedioxythiophene). ChemElectroChem 9:e202200121. https://doi.org/10.1002/celc.202200121
Han Y, Shen M, Wu Y et al (2013) Preparation and electrochemical performances of PEDOT/sulfonic acid-functionalized graphene composite hydrogel. Synth Met 172:21–27. https://doi.org/10.1016/j.synthmet.2013.04.001
Xie Y, Zhang SH, Jiang HY et al (2019) Properties of carbon black-PEDOT composite prepared via in-situ chemical oxidative polymerization. E-Polymers 19:61–69. https://doi.org/10.1515/epoly-2019-0008
Takcı DK, Ozdenefe MS, Genc S (2023) Green synthesis of silver nanoparticles with an antibacterial activity using Salvia officinalis aqueous extract. J Cryst Growth 614:127239. https://doi.org/10.1016/j.jcrysgro.2023.127239
Dokur E, Uruc S, Kurteli R et al (2023) Designing disposable hand-made screen-printed electrode using conductive ink for electrochemical determination of dopamine. Ionics (Kiel) 29:5465–5480. https://doi.org/10.1007/s11581-023-05239-w
Ignat EC, Lutic D, Ababei G, Carja G (2022) Novel heterostructures of noble plasmonic metals/Ga-substituted hydrotalcite for solar light driven photocatalysis toward water purification. Catalysts 12:1351. https://doi.org/10.3390/catal12111351
Qie L, Chen W, Xiong X, et al (2015) Sulfur-doped carbon with enlarged interlayer distance as a high-performance anode material for sodium-ion batteries. Adv Sci 2:1500195. https://doi.org/10.1002/advs.201500195
Tahtaisleyen S, Gorduk O, Sahin Y (2020) Electrochemical determination of tartrazine using a graphene/poly(L-phenylalanine) modified pencil graphite electrode. Anal Lett 53:1683–1703. https://doi.org/10.1080/00032719.2020.1716242
Dokur E, Uruc S, Gorduk O, Sahin Y (2023) A novel approach for ultrasensitive amperometric determination of NADH via graphene-based electrode obtained by electrochemical intercalation of tetraalkylammonium ions. Ionics (Kiel) 29:2005–2019. https://doi.org/10.1007/s11581-023-04948-6
Malinauskas A (2008) Electrochemical study of riboflavin adsorbed on a graphite electrode. Chemija 19:1–3
D’Souza ES, Manjunatha JG, Raril C et al (2020) Polymer modified carbon paste electrode as a sensitive sensor for the electrochemical determination of riboflavin and its application in pharmaceutical and biological samples. Anal bioanal chem res 7:461-472.
Sharma A, Khosla A, Arya S (2020) Synthesis of SnO2 nanowires as a reusable and flexible electrode for electrochemical detection of riboflavin. Microchem J 156:104858. https://doi.org/10.1016/j.microc.2020.104858
Nezamzadeh-Ejhieh A, Pouladsaz P (2014) Voltammetric determination of riboflavin based on electrocatalytic oxidation at zeolite-modified carbon paste electrodes. J Ind Eng Chem 20:2146–2152. https://doi.org/10.1016/j.jiec.2013.09.044
Gribat LC, Babauta JT, Beyenal H, Wall NA (2017) New rotating disk hematite film electrode for riboflavin detection. J Electroanal Chem 798:42–50. https://doi.org/10.1016/j.jelechem.2017.05.008
Huang DQ, Wu H, Song C et al (2018) The determination of riboflavin (vitamin B2) using manganese dioxide modified glassy carbon electrode by differential pulse voltammetry. Int J Electrochem Sci 13:8303–8312. https://doi.org/10.20964/2018.09.02
Acknowledgements
The authors have nothing to report.
Funding
There is no funding.
Author information
Authors and Affiliations
Contributions
Conceptualization is performed by O.G. and Y.S. All authors contributed to the study’s writing review and editing. Experiments, data collection, and analysis were performed by M.Y.A., S.U., E.D., and O.G. The first draft of the manuscript was written by M.Y.A., S.U., E.D., O.G., and Y.S. and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Acikalin, M.Y., Dokur, E., Uruc, S. et al. Silver nanoparticle–incorporated over-oxidized poly(3,4-ethylenedioxythiophene)-based electrochemical sensor for selective determination of riboflavin. Ionics (2024). https://doi.org/10.1007/s11581-024-05583-5
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11581-024-05583-5