Abstract
The effect of doping with aluminum compounds on the crystal structure, morphology, and electrochemical properties of LiFePO4 has been investigated with aluminum stearate, alumina, aluminum sulfate, and aluminum phosphate as dopants. The contraction of unit cell observed by XRD analysis and reduced lattice spacing determined by HRTEM of the doped crystals indicate that Al3+ ions, which occupy smaller space than lithium ions, are successfully doped into the lattice of LiFePO4. Lattice doping of aluminum ions enlarges Li+ transport channels; 1%-AlP-LFP has the slowest attenuation of discharge specific capacity. After 30 cycles of charge and discharge curve test at 0.5C, the retention rate of the sample is 97.43%. Owing to the substitution of S for O sites, and SO42− has a breaking effect on the carbon layer, this accelerates the capacity decay of 1%-AlS-LFP. The discharge capacity of 1%-AlS-LFP is 132.9 mAh/g, which is lower than 139.8mAh/g of LFP. The electrochemical impedance spectroscopy (EIS) results show that the resistance of 1%-AlP-LFP is 147.1 Ω, the resistance of LFP is 138.9Ω, and the resistance of LFP is 183.9Ω. The Li+ diffusion coefficient of 1%-AlP-LFP is partially increased; the double substitution of Al3+ and S2− slows the migration rate of Li+.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
LiFePO4(LFP) with olivine structure is the first material to be studied in the orthophosphate family of cathode materials for lithium-ion batteries [1, 2]. The most important features of the material include high energy density (theoretical capacity 170 mAhg−1), as well as stable crystal structure, low production cost, and good environmental compatibility [3,4,5]. However, this material is also characterized by low intrinsic electronic conductivity and low lithium-ion diffusivity [6, 7], which constitute obstacles to optimizing electrical properties.
At present, the methods to improve the electrical properties of lithium-ion battery materials mainly focus on ion doping, particle minimization, and particle surface coating [8]. Carbon coating modification belongs to non-lattice modification, which can slow down the interface effect of lithium-ion battery reaction and improve electronic conductivity. Lattice doping uses metal cations to replace part sites of the cathode material lattice, so as to expand Li + transport channels and accelerate the diffusion rate of Li+ [9,10,11]. There are some reports on modification of lithium-ion battery cathode materials with spinel structure and olivine structure by doping with metal ions [12]. Lattice doping, doping with metal elements such as (V, Mo, Al, Mn, Ni, Mg, Nb) [13,14,15,16,17,18] is more common, while doping with anions like SO42−, Cl−, and F− has also been reported [19, 20], but relatively rare.
Ruhul found that diffusion in LFP preferentially occurs in the [010] direction, while diffusion is limited in other crystallographic directions [21]. Lattice doping causes lattice distortion, adjusts the crystal structure, and changes the particle size of material particles, thus affecting the diffusion rate of lithium ions. Andrzej found that electrical performance of LFP cathode material can be improved by partial replacement of lithium ions with aluminum ions [22]. In 2023, Qu [23] studied the effect of Al ion doping on the structure of β-Li3PS4 and the dynamics of Li+ transport. The ion transport path in this material is similar to that of LiFePO4; the introduction of Al creates vacancy, which is conducive to Li+ jump; the activation barrier in the material is significantly reduced, and the ionic conductivity of β-Li3PS4 is increased by more than three times. Hui [24] synthesized Li0.98Al0.02FePO4/C by solid-state reaction. Compared with undoped materials, doped cathode materials show improved dynamic properties in terms of capacity transport, cycle life, and rate capability. The overcharge test shows that the composite has excellent safety performance.
In this paper, the influence of Al3+ ions and aluminum compounds (Al2O3, AlPO4, Al2(SO4)3) which exist as impurities in industrial raw materials on LFP has been comprehensively studied. By adding Al(OH)(C18H35O2)2, the crystal cell, particle morphology, particle size, compaction density, and their relationship with electrical properties of LFP doped with Al ions at Li sites have been studied. The crystal structure and electrical properties of LFP whose Li and O sites are partially replaced at the same time by doping with aluminum compounds like Al2O3, Al2(SO4)3 and AlPO4 have been investigated, and the effect of PO43− and SO42− from raw materials on LFP are revealed. The optimal condition for doping of LFP by simultaneous substitution with both metal ions and anions has been determined. The doped LFP prepared under optimal conditions has a complete carbon layer, extended lithium-ion channel, more uniform particle size, and exhibits prime electrical properties.
Materials and methods
In this study, LFP was prepared by carbothermal reduction method [15], which has been applied in large-scale industrial manufacturing in China.
Synthesis of LFP
Anhydrous iron phosphate (FePO4, ≥ 99%, Guangzhou Tinci Materials Technology Co., Ltd) and lithium carbonate (Li2CO3, ≥ 99%, Tianqi Lithium Corporation) were suspended in ultrapure water with a molar ratio of Fe:Li = 1:1; anhydrous glucose (C6H12O6, ≥ 99%, Shanghai Maclin Biochemical Technology Co., Ltd.) was subsequently added in the amount of equivalent to 10 wt% (mass ratio) of theoretical LiFePO4. The suspended mixture was then milled in a planetary ball mill at 450 r/min for 3 h, and precursor powder was obtained by spray drying of milled slurry. The precursor was calcined with a heating rate of 10 °C/min to 400 °C in N2 atmosphere in a tube furnace and kept at 400 °C for 5 h, then heated to 750 °C at the same heating rate and kept at 750 °C for 5 h, and then cooled to room temperature to obtain LFP product. The prepared LFP was ground in a grinding machine for 30 min and then characterized by SEM, HRTEM while compaction density and electrical properties were also tested.
Influence of additional dosage of aluminum ions
In preparation of aluminum doped LFP, raw materials of FePO4, Li2CO3, and Al(OH)(C18H35O2)2 (≥ 99%, Shanghai Hao Hong biological medicine Technology Co., Ltd.) were suspended in ultrapure water according to molar ratio of elements of Fe: Li: Al: P = 1:(1 − 3x):x:1, where x = 0.5%, 1%, 1.5%, and 10%. The suspended mixture was then milled and spray dried to yield precursor powder for subsequent calcination to produce LFP product. The calcination conditions were the same as in the section “Synthesis of LFP.” Sample Li0.985FeAl0.005PO4/C was denoted as 0.5%-Al-LFP, Li0.97FeAl0.01PO4/C denoted as 1%-Al-LFP, Li0.955FeAl0.015PO4/C denoted as 1.5%-Al-LFP, and Li0.7FeAl0.1PO4/C denoted as 10%-Al-LFP, respectively.
Influence of types of aluminum compounds
With Al2O3 (≥ 99.99%, Shanghai Dibo Biotechnology Co., Ltd.), AlPO4 (≥ 99%, Shanghai Dibo Biotechnology Co., Ltd.), and Al2(SO4)3 (≥ 99.99%, Shanghai Maclin Biochemical Technology Co., Ltd.) as dopants to produce aluminum compound doped LFP, raw materials of FePO4, Li2CO3, and Al2O3 (AlPO4, Al2(SO4)3) were suspended in ultrapure water according to molar ratio of elements of Fe: Li: Al: P = 1:(1 − 3x):x:1, where x = 1%, 1.5%, and 10%. The preparation method of precursor powder and subsequent calcination conditions were the same as in the section “Synthesis of LFP.” LFP samples doped with Al2O3, AlPO4, and Al2(SO4)3 were denoted as AlO-LFP, AlP-LFP, and AlS-LFP, respectively, and the amount of doping is indicated before the label.
Fabrication of electrode sheet
The prepared LiFePO4 sample was weighed with acetylene black and polyvinylidene fluoride (PVDF) according to the mass ratio of 90:5:5, and an appropriate amount of N-methyl pyrrolidone (NMP) was added into the homogenizer to obtain the electrode paste. Lay the cut aluminum foil on the coater and wipe it clean with alcohol, making sure there are no bubbles between the aluminum foil and the coater. Then, scrape the electrode paste evenly on the clean flat aluminum foil with the coater. The positive electrode sheet was put into a vacuum drying oven and dried at 120 °C for 3 h to remove NMP, moisture in the electrode sheet and functional groups adsorbed on the surface. The dried positive electrode sheet was cut into a 14-mm diameter round sheet by the cutting machine, and the active substance of the electrode was weighed and transferred to a glove box under argon protection for later use.
Battery assembly
The button battery needs to be assembled in a glove box insulated from oxygen and water, and the moisture content in the glove box must be less than 0.0003%. In this experiment, CR2032 button battery was used; the negative electrode was a sheet of lithium metal, and 1 mol/L LiPF6 was dissolved in 1:1 vinyl carbonate (EC) and dimethyl carbonate (DMC) solution as the electrolyte. The assembled button battery is compacted and sealed on the packaging machine. In order to fully wet the diaphragm with the electrolyte, the battery needs to be left for 2 h before the electrochemical test.
Characterizations of LFP
The particle size distribution of LFP particles was characterized by CSD,\(CSD=\frac{{D}_{90}-{D}_{10}}{{D}_{50}}\). An icAPPRO-XP inductively coupled plasma emission spectrometer (Thermo Fisher) was used to determine the content of lithium, phosphorus, and iron in the sample, and infrared carbon and sulfur analyzer HW2000 (Wuxi Creative Analytical Instruments Co., LTD.) was used to determine the content of carbon and sulfur in the sample. The crystal structure of solid samples was characterized by Bruker D2 Phaser X-ray diffractometer (Bruker, Germany). Phenom Pharos scanning electron microscopy (Thermo Fisher) and TalosF200S high-resolution transmission electron microscopy (Thermo Fisher) were used to characterize the particle morphology and EDS date, and electronic pressure testing machine UTM7305 (Shenzhen Suns Technology Stock Co., LTD.) was used to measure the compaction density of the material. The button battery is produced by electric roller press MSK-HRP-MR100A (Hefei Kejing Material Technology Co., LTD.), casting coater MSK-AFA-1 (Shenyang Kejing Automation Equipment Co., LTD.), and button battery sealing machine MSK-110 (Shenzhen Kejing Zhida Technology Co., LTD.). Land battery test system CT-2001A (Wuhan Land Electronics Co., LTD.) was used to test the electrical performance, and electrochemical workstation ZENNIUM PRO (ZAHNER, Germany) was used to test the impedance performance.
Results and discussions
Effect of aluminum ion (aluminum stearate) doping on LFP
XRD characterization
Figure 1a is an XRD pattern of 0.5%-Al-LFP, 1-%-Al-LFP, 1.5%-Al-LFP, and 10%-Al-LFP; Fig. 1b is the magnification of 2θ in the range from 34.8 to 36.2°. The results show that the diffraction peak position of all samples corresponds to the diffraction peak position of the LFP standard card PDF#83–2092, and no aluminum peak is found, which indicates that the crystal structure of LFP is essentially unaffected within the range of aluminum doping amount set in this study. It can be seen from the local magnification diagram (Fig. 1b) that with the increase of aluminum addition, the position of the diffraction peak at about 35° is shifted. Neutron diffraction experiment proved that aluminum ions replace the Li site of LFP lattice rather than the Fe site [22]. Since the ionic radius of Al (0.51 Å) is smaller than that of Li (0.76 Å), when Al replaces the Li position [25], LFP unit cell shrinks and the crystal plane spacing d decreases. The corresponding EDS data gives the composition of the samples, and the presence of Fe, P, O, and Al is confirmed in 0.5%-Al-LFP (Fig. 1c). In case of Li0.985FeAl0.005PO4, the Fe:Al ratio is 0.994:0.006; it is fairly similar to the nominal ratio (1:0.005).
HRTEM characterization
Figure 2 is an image of 10%-Al-LFP by high-power resolution electron microscope (TEM). With the aid of Digital Micrograph software, the crystal plane spacing is determined to be d = 3.440 Å, corresponding to the (101) plane, which is smaller than the crystal plane spacing of d = 3.705 Å in pure LFP, indicating that Al element has successfully entered the LFP lattice.
Particle size distribution and compaction density
The relationship between LFP particle size and aluminum doping amount is shown in Fig. 3. With the increase of aluminum content of LFP, D10, D50, and D90, all show a decrease, and especially the decrease of D90 is more significant. Doping with aluminum significantly inhibits the growth of LFP particles. When the dosage of dopant is less than 1.5%, the CSD value decreases with the increase of aluminum ion doping content, and the particle size distribution is more uniform. With the doping amount up to 10%, D10 decreases significantly, but D50 and D90 change little, so CSD increases, and the variance of particle size distribution becomes prominent.
The data in Table 1 show that with the increase of aluminum content, the compaction density presents a trend of first decreasing and then increasing, which is consistent with the trend of particle size changing with the amount of aluminum doping. Compared with other samples, 1%-Al-LFP has the smallest compaction density and the smallest CSD value. The fewer large particles in LFP, the more uniform the particle size distribution and the smaller the compaction density are. The phenomenon can be explained as follows: with the increase of Al doped, the growth activation energy of LFP crystals is increased, slowing down the crystal growth rate, reducing the formation of large particles, and weakening the agglomeration effect between large LFP particles (D50 < size < D90).
The proper compaction density helps to increase the discharge capacity of the battery, reduce the internal resistance, reduce the polarization loss, lengthen the cycle life of the battery, and improve the utilization rate of the lithium battery. The data in Table 1 is the average of multiple parallel experiments; it can be concluded that when the compaction density is about 2.143 g/cm3, 0.1C discharge capacity/(mAh/g) is the best. When the compaction density is too large or too small, it is not conducive to the embedding of Li+.
Electrochemical characterization
Figure 4 shows the first charge and discharge curve of Li1-3xAlxFePO4/C at 25 °C and 0.1C. All samples have a long, uniform discharge voltage plateau at around 3.4 V, corresponding to the REDOX reaction of Fe2+/Fe3+.The discharge capacity of undoped LFP is 150.8 mAh/g, that of 0.5%Al-LFP sample is increased to 152.2 mAh/g, and that of 1%Al-LFP sample is increased to 155.1 mAh/g, indicating that low dose of aluminum doping significantly enhances the initial discharge capacity of LFP. Subsequently, when the amount of aluminum addition reaches 1.5%, the discharge capacity decays to 154.3 mAh/g and that of 10%Al-LFP is rapidly reduced to 150.1 mAh/g, and the stability of the charging and discharging platform is also deteriorated.
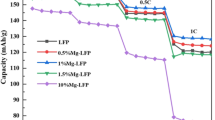
Figure 5 shows the rate performance test results of LFP samples. It can be seen from the single rate cycle performance Fig. 5a that when the doping amount of aluminum is less than 1.5%, the cycle specific capacity depletion rate of the doped sample is slower than that of the undoped LFP, indicating that aluminum doping is beneficial to maintaining the cycle performance of the battery. With the extension of the cycle time, the positive electrode sheet is gradually soaked by the electrolyte, and the battery is activated. At the same time, Al3+ with a small ionic radius enters the material lattice to replace the Li+ site, and there is a vacancy on the Li site, which increases the gap in the lattice and provides more channels for the movement of electrons and ions, so that the conductivity of the material is increased, thereby improving the specific discharge capacity. However, the charge and discharge voltage difference gradually increase in cycles, and the specific discharge capacity of the battery will start to deteriorate after many cycles.
Figure 5b shows that aluminum doping is beneficial to delay the capacity decay of LFP under high magnification conditions and improve the capacity retention rate at 1C. For the assembled button battery, 0.1C cycle is basically the battery activation stage, and the specific discharge capacity of each sample presents a slow-rising trend. The specific discharge capacity of 0.5%-Al-LFP and 1%-Al-LFP is greater than that of undoped LFP, and the discharge specific capacity of 1.5%-Al-LFP is the same as that of undoped LFP. The discharge capacity of 1%-Al-LFP is the largest, reaching about 155 mAh/g. In the 0.2C cycle, when the aluminum doping amount is less than 1.5%, the cyclic specific capacity of the doped sample is higher than that of the LFP sample. After 30 cycles, the capacity retention rates of LFP, 0.5%-Al-LFP, 1%-Al-LFP, 1.5%-Al-LFP, and 10%-Al-LFP are 95.53%, 96.57%, 96.34%, 97.36%, and 98.28%, respectively. The difference of specific discharge capacity between samples increases with the discharge rate increasing to 0.5C and 1C; the specific discharge capacity of the undoped LFP samples begins to decline at an accelerated rate. All the aluminum-doped LFP is superior to the undoped LFP samples, and 1%-Al-LFP samples maintain the best electrical properties with a final discharge specific capacity of 144 mAh/g at 0.5C. After five cycles of 1C, the difference between the doped and undoped samples increases significantly, and the specific discharge capacities of LFP, 0.5%-Al-LFP, 1%-Al-LFP, 1.5%-Al-LFP, and 10%-Al-LFP are 103.4, 124, 126.6, 123.7, and 121.4 mAh/g, respectively. The corresponding 1C cycle capacity retention rates are 94.68%, 97.79%, 97.23%, 98.33%, and 96.27%. The attenuation of LFP doped with Al is slowed down during the rate cycle, which can be explained by the presence of a large number of lithium vacancies caused by doping, which enables efficient electron transport at a large discharge rate.
Effect of aluminum compound doping on LFP
Elemental analysis of LFP doped with aluminum compounds
The uniform coating of carbon on the surface of LFP particles can improve the surface conductance between particles and reduce the electrochemical reaction impedance. The comparison of S and C contents of precursor and sample in Table 2 shows that sulfate reacts with carbon source at high temperature, and part of products escape through the gas phase. Loss of C content leads to an uneven coating of the carbon layer on the surface of LFP particles. Figure 6 shows the TEM characterization of three doped LFP samples with a magnification of 400,000 times. The light shade outer layer is the surface carbon layer, and the dark inner particle layer is LFP. It can be seen that the carbon layer of 10%-AlS-LFP is thin and unevenly distributed, indicating obvious C loss. The carbon layer of 10%-AlO-LFP is partially lost, and the thickness of the carbon layer is not uniform. The carbon layer of 10%-AlP-LFP is thick and smooth.
XRD calculations of 1%-Al-LFP and 1%-AlS-LFP show that the cell volume of 1%-Al-LFP is 290.583 Å and that of 1%-AlS-LFP is 290.616 Å, which is larger than that of the former. This is due to the fact that the diameter of S atom is larger than that of O atom, and S partially replaces the O site, resulting in an increase in crystal volume. Previous research also led to results consistent with this work [15]. Carbon in the form of carbon is coated on the surface of lithium iron phosphate particles. Carbon is coated on the surface of LFP particles in the form of elemental carbon.
XRD characterization
X-ray diffraction in Fig. 7a of LFP doped with aluminum compounds indicates that all samples are olivine structures with small fluctuations in crystallinity ranging from 88.4 to 91.5%. The local magnification (Fig. 7b) of the diffraction peak (311) shows that the diffraction peak of doped LFP shifts to a higher angle with the increase of dopants, indicating that Al3+ occupies the Li+ position and causes cell shrinkage. The ionic radius of S2− (1.84 Å) is slightly larger than that of O2− (1.40 Å); in AlS-LFP, S is doped in the O site, resulting in the increase of the cell volume.
However, in the preparation of LFP by carbothermal reduction, the reaction material is calcined at high temperature, part of S in the dopant is consumed by the C source reaction, and the actual S2− content entering the lattice will be less than the doping amount. Therefore, in this work, AlS-LFP cells exhibit contraction after Li+ and O2− are partially replaced by Al3+ and S2−, respectively. The weak impurity peak of 35.68° in Fig. 7a and b is Fe3(PO4)2, which is the reaction product of trace Fe and glucose in the precursor during the calcination process. In the case of sufficient solid reaction, this peak will not occur.
The corresponding EDS data gives the composition of the LFP samples, all of which confirm the presence of Fe, P, O, S, and Al (Fig. 7c–e). The mass ratio of elements obtained by EDS analysis is very close to the doping amount of the sample. The presence of Al is also confirmed in EDS of 10%-AlO-LFP, 10%-AlP-LFP, and 10%-AlS-LFP, respectively.
HRTEM characterization
Figure 8 shows the high-power TEM images of 10%-AlS-LFP and 10%-AlO-LFP, respectively. The crystal plane spacing d (101) of 10%-AlS-LFP is 3.5181 Å, and the crystal plane spacing d (101) of 10%-AlO-LFP is 3.5891 Å, both of which are smaller than 3.705 Å (101) of pure LFP, indicating that Al3+ and S2− enter the LFP lattice, resulting in a decrease in lattice spacing.
Particle size distribution and compaction density characterization
Figure 9 shows that the CSD value of AlO-LFP increases slowly with the doping amount, while the CSD value of AlS-LFP only increases slightly due to the antagonism of Al and S elements. The CSD value of the AlP-LFP is significantly reduced, because the substitution of Li+ by Al3+ is unhindered, and there is no lattice expansion caused by other substitutions.
Table 3 shows the data of particle size D90 and compaction density of the samples. The doped samples D90 decrease with the increase of the amount of doped aluminum, and the compaction density decreases with particle size decrease. Among them, the compaction density of AlP-LFP samples decreases most significantly, and the compaction density of 10%-AlP-LFP is 2.1381 g/cm3, which is 11.8% lower than that of the LFP sample, indicating that aluminum phosphate inhibits the formation of large particles more obviously.
Electrochemical characterization
Figure 10 shows the initial charge and discharge curve of LFP doped with aluminum compounds like Al2O3, AlPO4, and Al2(SO4)3 at 0.1C. The specific discharge capacity of undoped LFP is 148.5 mAh/g and that of 1%-AlO-LFP decreases to 145.8 mAh/g. The specific discharge capacity of 1%-AlS-LFP is 148.1 mAh/g, which is not much different from that of undoped LFP. Doping a small amount of aluminum phosphate is beneficial to increase the specific discharge capacity of LFP; when the doping content is 1.5%, it has the best effect on the electric performance gain of LFP. The doping of alumina and aluminum sulfate has a negative effect on LFP. The specific discharge capacities of 1.5% AlS-LFP and 1.5% AlO-LFP are 140 mAh/g and 100 mAh/g, respectively, and that of 10%-AlS-LFP and 10%-AlO-LFP are 74.6 mAh/g and 50.3 mAh/g, which are significantly smaller than that of LFP.
Figure 11a shows the cycle performance of each LFP sample at 0.5C. In the first 20 cycles, the discharge specific capacities of 1%-AlP-LFP and 1.5%-AlP-LFP are higher than that of LFP. Then, the discharge capacities decrease slowly with the increase of cycles. After 30 cycles, the capacity retention rates of 1%-AlP-LFP, 1.5%-AlP-LFP, and LFP are 97.43%,96.5%, and 96.8%, respectively. The results show that 1%AlPO4 doping can improve the discharge capacity retention rate. Aluminum phosphate and LFP are both orthophosphates and have similar chemical properties. As a dopant, the lattice cell of phosphate formed by doping with Al3+ is smaller in volume than the lattice cell of LFP, which is more conducive to aluminum ions entering the LFP lattice to replace Li+.
Doping with Al2(SO4)3 and Al2O3 shows a significant negative effect, and the discharge specific capacities of doped LFP decreased significantly with the increase of doping amount. In particular, the 0.5C discharge specific capacities of 1%-AlS-LFP, 1.5%-AlS-LFP, and 10%-AlS-LFP are significantly reduced to 130 mAh/g, 121 mAh/g, and 41 mAh/g, respectively.
In the case of excessive doping, some alumina and aluminum sulfate particles are distributed among the LFP particles, blocking the conduction routes of electrons and ions. And both substances destroy the carbon layer to a certain extent, further causing the deterioration of electrical properties. At the same time, it should be noted that the sample of 1%-AlS-LFP has better discharge performance at 1C rate. The substitution O site by S2− is considered to be the reason for inhibiting the Fe-Li anti-site defect. Specifically, the presence of S on the O site shared by LiO6 and FeO6 octahedrons squeezes the LiO6 octahedron. The strong affinity between highly electropositive Li+ and highly electronegative S2− may affect the occupation of iron and lithium, which is not conducive to the formation of Fe-Li anti-position defects [26].
According to Fig. 11b, when the doping content is less than 1.5%, the multiple rate discharge capacity of LFP can be significantly increased by aluminum phosphate, especially at 1C rate. Although aluminum oxide and aluminum sulfate are not conducive to the discharge of LFP, they can shorten the capacity gap between the LFP doped and LFP at 0.5C conditions, and the specific discharge capacity at 1C exceeds that of the LFP sample. When the doping content is 10%, all of the three aluminum compounds adversely affect the electrical properties of LFP.
EIS characterization
Electrochemical impedance spectroscopy (EIS) is used to evaluate the reaction and diffusion kinetics of lithium ions between the electrode and the electrolyte, and the results are shown in Fig. 12. The semi-circular part of Fig. 12a of the high frequency region represents the charge transfer impedance (Rct) of the charged electrons transferred from the electrolyte to the electrode, which can be fitted by Zview software. The results show that the resistance of 1%-AlS-LFP is 197.1 Ω, the resistance of 1%-AlO-LFP is the highest (724.4 Ω), the resistance of 1%-AlP-LFP is 147.1 Ω, and the resistance of undoped LFP is 183.9 Ω. The oblique line of Fig. 12b in the low frequency region represents the diffusion impedance, often called the Warburg impedance, which is formed by the diffusion process of Li+ between the electrode surface and the electrolyte and is calculated by the formula \(Z \, ^{\prime} = R + \sigma *\omega^{ - 0.5}\), where \(\omega = 2\pi f\), σ being the Warburg factor, which can be calculated by the slope in Fig. 12c. The σ of LFP, 1%-AlP-LFP, and 1%-AlS-LFP is 95.05, 62.60, and 98.40, respectively. Table 4 shows that aluminum doping significantly accelerates the reaction kinetics of LFP, speeds up the diffusion rate of lithium ions, and reduces Warburg impedance. Substitution of aluminum ions at the lithium site can increase the lithium vacancy and broaden the migration channel of lithium ions, thus improving the electrical properties of materials.
Conclusions
The samples of Al-LFP, AlO-LFP, AlS-LFP, and AlP-LFP doped with compounds Al(OH)(C18H35O2)2, Al2O3, Al2(SO4)3, and AlPO4 have been prepared by high temperature solid phase method, and the following conclusions are obtained:
In doping with Al(OH)(C18H35O2)2, Al3+ replaces Li+, which leads to the contraction of LFP cells and the reduction of lattice spacing. The particle size, CSD, and compaction density of 1%-Al-LFP are also decreased. The specific discharge capacity of 1%-Al-LFP reaches 155 mAh/g at 0.1C, and the capacity retention rate of 0.2C is 96.57% after 30 cycles.
The results show that when the doping amount is less than 1.5%, the charge–discharge performance, cycle performance, and rate performance of doped LFP are optimized compared with LFP. However, the charge–discharge performance, cycle performance, and rate performance of 10%-Al-LFP are decreased, which can be attributed to the excessive doping of Al.
With Al2O3 and Al2(SO4)3 acting as dopants, part of Al3+ and S2− enters the lattice, and the crystal cell shrinks. The (011) crystal face spacing of 10%-AlS-LFP is reduced to 3.5181 Å, and the (011) crystal face spacing of 10%-AlO-LFP is reduced to 3.5891 Å. The reduction of particle size leads to a decrease in CSD and compaction density. The carbon layer damage of 10%-AlO-LFP and 10%-AlS-LFP is obvious, and the combined effect of particle agglomeration and carbon layer damage results in a significant decrease in the cycling and rate properties of AlO-LFP and AlS-LFP.
After doping with AlPO4, the particle size of AlP-LFP decreases slightly. A small amount of doping (x ≤ 1.5%) is conducive to the improvement of electrical properties; the retention rate of 1.5%-AlP-LFP at 0.5C is 96.0%. Excessive doping of aluminum compounds (x = 10%) can lead to a decrease in electrical properties.
EIS detection of 1%-AlO-LFP and 1%-AlS-LFP shows that the presence of Al2O3 and Al2(SO4)3 increases Warburg impedance and reduces Li+ diffusion, possibly because alumina does not enter the lattice; Al3+ and S2− simultaneously replace Li+ and O2− sites, which may cause migration channel contraction.
For 1%-AlP-LFP, the doping of AlPO4 reduces Warburg impedance because Al3+ replaces Li+ sites, the Li+ migration channel is extended, the impedance factor of 1%-AlP-LFP is reduced to 62.60, the Li+ diffusion coefficient is enhanced, and DLi+ = \(2.8268 \times 10^{ - 16}\) cm2 s−1.
Data availability
All data generated and analyzed during this study are available from the corresponding author on reasonable request.
References
Padhi AK, Nanjundaswamy KS, Goodenough JB (1997) Phospho-olivines as positive electrode materials for rechargeable lithium batteries. J Electrochem Soc 144:1188. https://doi.org/10.1149/1.1837571
Liu Y, Liu H, An L (2019) Blended spherical lithium iron phosphate cathodes for high energy density lithium–ion batteries. Ionics 25:61–69. https://doi.org/10.1007/s11581-018-2566-7
Muruganantham R, Sivakumar M, Subadevi R (2016) Synthesis and electrochemical characterization of olivine-type lithium iron phosphate cathode materials via different techniques. Ionics 22:1557–1565. https://doi.org/10.1007/s11581-016-1676-3
Wang Y, Zhang J, Xue J (2021) LiFePO4/C composites with high compaction density as cathode materials for lithium-ion batteries with high volumetric energy density. Ionics 27:4687–4694. https://doi.org/10.1007/s11581-021-04078-x
Zhu YM, Ruan ZW, Tang SZ (2014) Research status in preparation of FePO4: a review. Ionics 20:1501–1510. https://doi.org/10.1007/s11581-014-1241-x
Ding KQ, Guo HB, Zheng CB, Lu L, Liu LK, Yan XR, Guo ZH (2014) Octagonal prism shaped lithium iron phosphate composite particles as positive electrode materials for rechargeable lithium-ion battery. Electrochim Acta 146:585–590. https://doi.org/10.1016/j.electacta.2014.08.141
Rakesh S, Amrish KP, Yogesh S (2017) Physicochemical and electrochemical performance of LiFe1-xNixPO4(0≤x≤1.0) solid solution as potential cathode material for rechargeable lithium-ion battery. Ceram Int 43:5734–5742. https://doi.org/10.1016/j.ceramint.2017.01.115
Chung SY, Bloking JT, Chiang YM (2002) Electronically conductive phosphor-olivines as lithium storage electrodes. Nat Mater 1:123–128. https://doi.org/10.1038/nmat732
Cheng YZ, Yan NZ, Xiao HY, Peng D, Jian GD, Jia ML, Jian XL, Ying JZ (2020) Controllable fabrication and li storage kinetics of 1d spinel limn2o4 positive materials for li-ion batteries: an exploration of critical diameter. Chemsuschem 13:803–810. https://doi.org/10.1002/cssc.201902846
Bao Z, Yan NZ, Bo WZ, Jian GD, Xue L, Xiao YZ, Zheng L, Rui G, Kai Z, Zheng WW, Yi FG, Peng D, Ying JZ (2022) Beneficial impact of incorporating spinel lithium manganate and samarium oxide into high performance positive materials through ultrasonic cavitation strategy. Colloids Surf, A 646:128985. https://doi.org/10.1016/j.colsurfa.2022.128985
Cheng YZ, Jian XL, Xiao HY, Ying JZ, Peng D, Xiao W, Yan NZ (2019) Boosting the stable Li storage performance in one-dimensional LiLaxMn2-xO4 nanorods at elevated temperature. Ceram Int 45:19351–19359. https://doi.org/10.1016/j.ceramint.2019.06.187
Medvedeva A, Makhonina E, Pechen L, Politov Y, Rumyantsev A, Koshtyal Y, Goloveshkin A, Maslakov K, Eremenko I (2022) Effect of Al and Fe doping on the electrochemical behavior of Li1.2Ni0.133Mn0.534Co0.133O2 Li-rich cathode material. Materials 15:8225. https://doi.org/10.3390/ma15228225
Chiang CY, Su HC, Wu PJ, Liu HJ, Hu CW, Sharma N, Peterson VK, Hsieh HW, Lin YF, Chou WC, Lee CH, Lee J, Shew BY (2012) Vanadium substitution of LiFePO4 cathode materials to enhance the capacity of LiFePO4-based lithium-ion batteries. J Phys Chem C 116:24424–24429. https://doi.org/10.1021/jp307047w
Sattar T, Lee SH, Jin BS, Kim HS (2020) Influence of Mo addition on the structural and electrochemical performance of Ni-rich cathode material for lithium-ion batteries. Sci Rep 10:8562. https://doi.org/10.1038/s41598-020-64546-8
Mao HQ, Chen K, Luo XM (2023) Effect of magnesium compounds on crystal structure, morphology, and electrochemical properties of LiFePO4/C. Ionics 29:3525–3535. https://doi.org/10.1007/s11581-023-05089-6
Sun HH, Kim UH, Park JH, Park SW, Seo DH, Heller A, Mullins CB, Yoon CS, Sun YK (2021) Transition metal-doped Ni-rich layered cathode materials for durable Li-ion batteries. Nat Commun 12:6552. https://doi.org/10.1038/s41467-021-26815-6
Zeng HG, Liu DY, Zhang YC, See KA, Jun YS, Wu G, Gerbec JA, Ji X, Stucky GD (2015) Nanostructured Mn-Doped V2O5 cathode material fabricated from layered vanadium jarosite. Chem Mater 27:7331–7336. https://doi.org/10.1021/acs.chemmater.5b02840
Zhao WG, Zou LF, Jia HP, Zheng J, Wang D, Song J, Hong C, Liu R, Xu W, Yang Y, Xiao J, Wang C, Zhang J (2020) Optimized Al doping improves both interphase stability and bulk structural integrity of Ni-Rich NMC cathode materials. Energy Mater 3:3369–3377. https://doi.org/10.1021/acsaem.9b02372
Dragana J, Miodrag M, Miloš M, Nikola C, Bojan J, Ana U, Dragan U (2017) The influence of fluorine doping on the structural and electrical properties of the LiFePO4 powder. Ceram Int 43:3224–3230. https://doi.org/10.1016/j.ceramint.2016.11.149
Seaby T, Lin TE, Hu YX, Yuan QH, Wang LZ (2022) An analysis of F-doping in Li-rich cathodes. Rare Met 41:1771–1796. https://doi.org/10.1007/s12598-021-01883-1
Ruhul A, Palani B, Joachim M (2006) Anisotropy of electronic and ionic transport in LiFePO4 single crystals. Electrochem Solid-State Lett 10:A13. https://doi.org/10.1149/1.2388240
Andrzej K, Artur B, Wen HT, Anna W, Marcin TK, Andrzej S, Dominika B, Wojciech Z, Konrad Ś, Janina M (2015) Evidence for Al doping in lithium sublattice of LiFePO4. Solid State Ionics 270:33–38. https://doi.org/10.1016/j.ssi.2014.12.004
Qu H, Wang Y, Ju J, Eck E, Cui G, Kentgens A (2023) Aluminium ion doping mechanism of lithiumthiophosphate based solid electrolytes revealed withsolid-state NMR. Phys Chem Chem Phys 25:4997–5006. https://doi.org/10.1039/D2CP04670A
Hui X, Zhen TZ (2006) Physical and electrochemical properties of mix-doped lithium iron phosphate as cathode material for lithium ion battery. Electrochim Acta 51:2063–2067. https://doi.org/10.1016/j.electacta.2005.07.014
Hui X, Zhentao Z (2006) Physical and electrochemical properties ofmix-doped lithium iron phosphate ascathode material for lithium ion battery. Electrochim Acta 51:2063–2067. https://doi.org/10.1016/j.electacta.2005.07.014
Radhamani AV, Karthik C, Ubic R, Ramachandrarao MS, Sudakar C (2013) Suppression of Fe·Li antisite defects in fluorine-doped LiFePO4. Scripta Mater 69:96–99. https://doi.org/10.1016/j.scriptamat.2013.02.015
Funding
We thank the Sichuan Provincial Science and Technology Bureau for financial support of major science and technology projects 2023YF0080.
Author information
Authors and Affiliations
Contributions
Gongsheng Zou: methodology, project administration, data curation, validation, investigation, software/writing—original draft. Kui Chen: conceptualization, funding acquisition, supervision, validation, writing—review and editing. Xianming Luo: resources, methodology. Quanjun Fu: resources, methodology. Bin Wu: validation, writing—review and editing.
Corresponding author
Ethics declarations
Ethics approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zou, G., Chen, K., Luo, X. et al. Crystal structure, morphology, and electrical properties of aluminum-doped LFP materials. Ionics 30, 2549–2563 (2024). https://doi.org/10.1007/s11581-024-05489-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11581-024-05489-2