Abstract
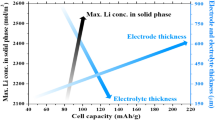
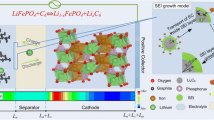
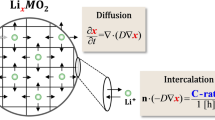
Small lithium-ion (Li-ion) batteries are often used in smart devices. Due to its low battery capacity, the lifespan of the headset needs to be improved by reducing the decay of the Li-ion battery capacity. Here, an electrochemical-thermal coupling model and a capacity fade model based on the pseudo-two-dimensional (P2D) model are established by COMSOL multi-physics field simulation software. The aging behavior of the Li-ion battery during cycling and the effects of different factors on the battery capacity are investigated; the condition of the battery is further predicted. The simulation parameters include charge–discharge rate, initial concentration of lithium ions in the liquid phase, and radius of the anode particles. The results show that the electrolyte diffusion coefficient increases with the increase of temperature in the range of 1000–1600 mol/m3 concentration. However, the initial concentration of 1600 mol/m3 leads to a higher concentration increment (421 mol/m3) during 3000 cycles; it aggravates the concentration polarization. The larger particle radius forms a thicker SEI film. When the radius rises from 2 to 12.5 μm, the thickness and resistance of the SEI film increase to 1136.51 nm and 6.685 m \(\Omega\)∙m2, respectively. It consumes more lithium ions and increases the cycle time under the same current.
Graphical Abstract














Similar content being viewed by others
Data availability
The authors confirm that the part of data supporting the findings of this study are available within the article [and/or its supplementary materials], and the remaining data that support the findings of this study are openly available in the COMSOL software database.
References
Tarascon JM, Armand M (2001) Issues and challenges facing rechargeable lithium batteries. Nature 414:359–367
Goodenough JB, Park K (2013) The Li-ion rechargeable battery: a perspective. J Am Chem Soc 135:1167–1176
Laresgoiti I, Käbitz S, Ecker M, Sauer DU (2015) Modeling mechanical degradation in lithium ion batteries during cycling: solid electrolyte interphase fracture. J Power Sources 300:112–122
Iurilli P, Brivio C, Wood V (2021) On the use of electrochemical impedance spectroscopy to characterize and model the aging phenomena of lithium-ion batteries: a critical review. J Power Sources 505:229860
Zhu J, Dewi Darma MS, Knapp M, Sørensen DR, Heere M, Fang Q, Wang X, Dai H, Mereacre L, Senyshyn A, Wei X, Ehrenberg H (2020) Investigation of lithium-ion battery degradation mechanisms by combining differential voltage analysis and alternating current impedance. J Power Sources 448:227575
Zhu J, Knapp M, Sørensen DR, Heere M, Darma MSD, Müller M, Mereacre L, Dai H, Senyshyn A, Wei X, Ehrenberg H (2021) Investigation of capacity fade for 18650-type lithium-ion batteries cycled in different state of charge (SoC) ranges. J Power Sources 489:229422
Fong R, von Sacken U, Dahn JR (1990) Studies of lithium intercalation into carbons using nonaqueous electrochemical cells. J Electrochem Soc 137:2009–13
Novak P, Joho F, Lanz M, Rykart B, Panitz J, Alliata D, Kotz R, Haas O (2001) The complex electrochemistry of graphite electrodes in lithium-ion batteries. J Power Sources 97-98:39–46
Li J, Murphy E, Winnick J, Kohl PA (2001) The effects of pulse charging on cycling characteristics of commercial lithium-ion batteries. J Power Sources 102:302–309
Liu S, Su J, Zhao J, Chen X, Zhang C, Huang T, Wu J, Yu A (2018) Unraveling the capacity fading mechanisms of LiNi0.6Co0.2Mn0.2O2 at elevated temperatures. J Power Sources 393:92–98
Zhu J, Su P, Dewi Darma MS, Hua W, Mereacre L, Liu-Théato X, Heere M, Sørensen DR, Dai H, Wei X, Knapp M, Ehrenberg H (2022) Multiscale investigation of discharge rate dependence of capacity fade for lithium-ion battery. J Power Sources 536:231516
Watanabe S, Kinoshita M, Hosokawa T, Morigaki K, Nakura K (2014) Capacity fading of LiAlyNi1−x−yCoxO2 cathode for lithium-ion batteries during accelerated calendar and cycle life tests (effect of depth of discharge in charge–discharge cycling on the suppression of the micro-crack generation of LiAlyNi1−x−yCoxO2 particle). J Power Sources 260:50–56
Amine K, Chen C, Liu J, Hammond M, Jansen A, Dees D, Bloom I, Vissers D, Henriksen G (2001) Factors responsible for impedance rise in high power lithium ion batteries. J Power Sources 97-98:684-687
Zhang D, Haran BS, Durairajan A, White RE, Podrazhansky Y, Popov BN (2000) Studies on capacity fade of lithium-ion batteries. J Power Sources 91:122–129
Shim J, Striebel KA (2003) Cycling performance of low-cost lithium ion batteries with natural graphite and LiFePO4. J Power Sources 119–121:955–958
Spotnitz R (2003) Simulation of capacity fade in lithium-ion batteries. J Power Sources 113:72–80
Sikha G, Popov BN, White RE (2004) Effect of porosity on the capacity fade of a lithium-ion battery. J Electrochem Soc 151:A1104
Ramadass P, Haran B, Gomadam PM, White R, Popov BN (2004) Development of first principles capacity fade model for Li-ion cells. J Electrochem Soc 151:A196-203
Ekstrom H, Lindbergh G (2015) A model for predicting capacity fade due to SEI formation in a commercial graphite/LiFePO4 Cell. J Electrochem Soc 162:A1003-1007
Cai L, White RE (2011) Mathematical modeling of a lithium ion battery with thermal effects in COMSOL Inc., Multiphysics (MP) software. J Power Sources 196:5985–5989
Doyle M, Fuller TF, Newman J (1993) Modeling of galvanostatic charge and discharge of the lithium/polymer/insertion cell. J Electrochem Soc 140:1526–1533
Fuller TF, Doyle M, Newman J (1994) Simulation and optimisation of the dual lithium ion insertion cell. J Electrochem Soc 141:1–10
Doyle C (1995) Design and simulation of lithium rechargeable batteries. Lawrence Berkeley National Laboratory, California
Hariharan KS, Tagade P, Ramachandran S (2018) Mathematical modeling of lithium batteries. Springer Cham, Berlin
Landegren GF (1957) Newton’s law of cooling. Am J Phys 25:648–649
Bernardi D, Pawlikowski E, Newman J (1985) A general energy balance for battery systems. J Electrochem Soc 132:5–12
Tang S, Wang Z, Guo H (2019) Systematic parameter acquisition method for electrochemical model of 4.35 V LiCoO2 batteries. Solid State Ionics 343:115083
Kumaresan K, Sikha G, White RE (2008) Thermal model for a Li-ion cell. J Electrochem Soc 155:A164
Aurbach D, Markovsky B, Shechter A, Ein-Eli Y, Cohen H (1996) A comparative study of synthetic graphite and Li electrodes in electrolyte solutions based on ethylene carbonate-dimethyl carbonate mixtures. J Electrochem Soc 143:3809–20
Wang Y, Nakamura S, Ue M, Balbuena PB (2001) Theoretical studies to understand surface chemistry on carbon anodes for lithium-ion batteries: reduction mechanisms of ethylene carbonate. J Am Chem Soc 123:11708–11718
Kim S, Duin ACTV, Shenoy VB (2011) Effect of electrolytes on the structure and evolution of the solid electrolyte interphase (SEI) in Li-ion batteries: a molecular dynamics study. J Power Sources 196:8590–8597
Tasaki K, Goldberg A, Lian J, Walker M, Timmons A, Harris SJ (2009) Solubility of lithium salts formed on the lithium-ion battery negative electrode surface in organic solvents. J Electrochem Soc 156:A1019
Ciosek Högström K, Malmgren S, Hahlin M, Rensmo H, Thébault F, Johansson P, Edström K (2013) The influence of PMS-additive on the electrode/electrolyte interfaces in LiFePO4/graphite Li-ion batteries. J Phys Chem C 117:23476–23486
Lamorgese A, Mauri R, Tellini B (2018) Electrochemical-thermal P2D aging model of a LiCoO2/graphite cell: capacity fade simulations. J Energy Storage 20:289–297
Xu K (2014) Electrolytes and interphases in Li-ion batteries and beyond. Chem Rev 114:11503–11618
Ji Y, Zhang Y, Wang C (2013) Li-ion cell operation at low temperatures. J Electrochem Soc 160:A636-649
Bai P, Li J, Brushett FR, Bazant MZ (2016) Transition of lithium growth mechanisms in liquid electrolytes. Energ Environ Sci 9:3221–3229
Zhou X, Huang J, Pan Z, Ouyang M (2019) Impedance characterization of lithium-ion batteries aging under high-temperature cycling: importance of electrolyte-phase diffusion. J Power Sources 426:216–222
Valoen LO, Reimers JN (2005) Transport properties of LiPF6-based Li-ion battery electrolytes. J Electrochem Soc 152:A882–A891
Yang X, Wang C (2018) Understanding the trilemma of fast charging, energy density and cycle life of lithium-ion batteries. J Power Sources 402:489–498
Dong T, Peng P, Jiang F (2018) Numerical modeling and analysis of the thermal behavior of NCM lithium-ion batteries subjected to very high C-rate discharge/charge operations. Int J Heat Mass Tran 117:261–272
Tran TD, Feikert JH, Pekala RW, Kinoshita K (1996) Rate effect on lithium-ion graphite electrode performance. J Appl Electrochem 26:1161–1167
Blaubaum L, Roder F, Nowak C, Chan H, Kwade A, Krewer U (2020) Impact of particle size distribution on performance of lithium-ion batteries. ChemElectroChem 7:1–23
Funding
This work was supported by the National Natural Science Foundation of China (No.. 52174338), Natural Science Foundation of Hunan Province, China (No. 2022JJ20086 & 2021JJ30796), and Central South University Innovation-Driven Research Programme (No. 2023CXQD005). This work was supported in part by the High-Performance Computing Center of Central South University, Amperex Technology Limited, and Jiangxi Provincial Key Laboratory of Flash Green Development and Recycling.
Author information
Authors and Affiliations
Contributions
Feiyang Su and Jinlin Liu wrote the main manuscript and Jin Xiao.Qifan Zhong.Liping Wang.Jindi Huang.Bo Hong.Zhenhua Zhang. reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Su, F., Xiao, J., Wang, L. et al. Simulation exploration on capacity fade and aging prediction of M1254S2 button-type lithium-ion battery. Ionics 30, 2055–2068 (2024). https://doi.org/10.1007/s11581-024-05423-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11581-024-05423-6