Abstract
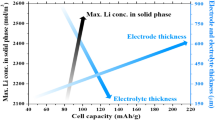
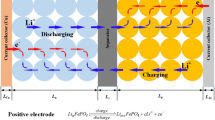
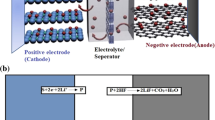
Capacity is one of the key parameters to characterize the performances of lithium-ion batteries. Heat generation analysis is essential to evaluate the safety of batteries. To figure out the effects of electrode thickness on capacity fade and thermal behaviors, a capacity fading model is proposed considering reaction kinetics and mass transfer processes on solid electrolyte interface (SEI) layers coupled with thermal evolution. Simulations are conducted on seven LiFePO4 batteries with variable electrode thicknesses. Results show that, with the increase of electrode thickness, the capacity losses of batteries deteriorate, and the total heat generation aggravates. For the battery with thick electrode, both the polarization overpotential and the gradient of lithium ion concentrations on particle surfaces of active materials increase on the edges, and then decrease perpendicularly to the cathodes. Under the adiabatic conditions, the temperature of battery (with anode 68 μm and cathode 140 μm) is increased to over 130 °C at the sixth cycle. The temperature of batteries declines when discharging in the beginning and then rises, which is noticeable for the batteries with thin electrodes. The proposed model and the simulation results would provide deep insights into both design and operation of batteries.












Similar content being viewed by others
Abbreviations
- a s :
-
Active area per unit electrode volume, m2 m−3
- c :
-
Concentration, mol m−3
- c p :
-
Specific heat capacity, J kg−1 K−1
- D :
-
Diffusion coefficient, m2 s−1
- E a :
-
Active energy, kJ mol−1
- f ± :
-
Electrolyte activity coefficient
- F :
-
Faradays constant, 98465 C mol−1
- h :
-
Convective heat transfer coefficient, W m−2 K−1
- i :
-
Reaction current, A m−2
- i app :
-
Applied current density, A m−2
- i loc :
-
Local current density, A m−2
- i Li :
-
Reaction current for intercalation reaction, A m−2
- i side :
-
Reaction current for side reaction, A m−2
- k :
-
Reaction rate constant, m s−1
- L :
-
Thickness, μm
- M :
-
Molecular weight, kg mol−1
- q rea :
-
Reaction heat generation, W m−3
- q act :
-
Irreversible polarization heat generation, W m−3
- q ohm :
-
Ohmic heat generation, W m−3
- r :
-
Radial coordinate, m
- R :
-
Universal gas constant, 8.314 J mol−1 K−1
- R s :
-
Particle radius, m
- R SEI :
-
Resistance of the SEI layer, Ω m2
- S a :
-
Active area per unit electrode volume, m2 m−3
- SOC :
-
States of charge
- t :
-
Time, s
- \( {t}_{+}^0 \) :
-
Transference number
- T :
-
Temperature, K
- u :
-
Growth rate of the SEI layer, m s−1
- U :
-
Open circuit voltage (OCV), V
- x :
-
x-coordinate, m
- β :
-
Charge transfer coefficient.
- δ :
-
Thickness of the SEI layer, nm
- ε :
-
Porosity
- η :
-
Overpotential, V
- λ :
-
Thermal conductivity, W m−1 K−1
- ρ :
-
Density, kg m−3
- σ :
-
Ionic conductivity, S m−1
- ϕ :
-
Voltage, V
- υ :
-
The product of thermodynamic factor
- eff :
-
Effective
- 1 :
-
Solid phase
- 2 :
-
Electrolyte phase
- EC :
-
Ethylene carbonate in electrolyte
- j :
-
Anode, cathode or separator
- max :
-
Maximum
- n :
-
Anode
- p :
-
Cathode
- s :
-
Separator
- side :
-
Side reaction
- SEI :
-
SEI layer
References
Purvins A, Zubaryeva A, Llorente M, Tzimas E, Mercier A (2011) Challenges and options for a large wind power uptake by the European electricity system. Appl Energy 88(5):1461–1469. https://doi.org/10.1016/j.apenergy.2010.12.017
Kil KC, Paik U (2015) Lithium salt of carboxymethyl cellulose as an aqueous binder for thick graphite electrode in lithium ion batteries. Macromol Res 23(8):719–725. https://doi.org/10.1007/s13233-015-3094-1
Franco AA (2013) Multiscale modelling and numerical simulation of rechargeable lithium ion batteries: concepts, methods and challenges. RSC Adv 3(32):13027–13058
Zheng H, Li J, Song X, Liu G, Battaglia VS (2012) A comprehensive understanding of electrode thickness effects on the electrochemical performances of li-ion battery cathodes. Electrochim Acta 71:258–265. https://doi.org/10.1016/j.electacta.2012.03.161
Hamankiewicz B, Michalska M, Krajewski M, Ziolkowska D, Lipinska L, Korona K, Kaminska M, Czerwinski A (2014) The effect of electrode thickness on electrochemical performance of LiMn2O4 cathode synthesized by modified sol–gel method. Solid State Ionics 262:9–13. https://doi.org/10.1016/j.ssi.2013.08.010
Doyle M, Fuller TF, Newman J (1993) Modeling of calvanostatic charge and discharge of the lithium polymer insertion cell. J Electrochemical Soc 140(6):1526–1533. https://doi.org/10.1149/1.2221597
Zheng Q, Li X, Cheng Y, Ning G, Xing F, Zhang H (2014) Development and perspective in vanadium flow battery modeling. Appl Energy 132:254–266. https://doi.org/10.1016/j.apenergy.2014.06.077
Jokar A, Rajabloo B, Desilets M, Lacroix M (2016) Review of simplified pseudo-two-dimensional models of lithium-ion batteries. J Power Source 327:44–55. https://doi.org/10.1016/j.jpowsour.2016.07.036
Zhao R, Liu J, Gu J (2015) The effects of electrode thickness on the electrochemical and thermal characteristics of lithium ion battery. Appl Energy 139:220–229. https://doi.org/10.1016/j.apenergy.2014.11.051
Zheng HY, Tan L, Zhang L, Qu QT, Wan ZM, Wang Y, Shen M, Zheng HH (2015) Correlation between lithium deposition on graphite electrode and the capacity loss for LiFePO4/graphite cells. Electrochim Acta 173:323–330. https://doi.org/10.1016/j.electacta.2015.05.039
Wang FM, Wang HY, Yu MH, Hsiao YJ, Tsai Y (2011) Differential pulse effects of solid electrolyte interface formation for improving performance on high-power lithium ion battery. J Power Source 196(23):10395–10400. https://doi.org/10.1016/j.jpowsour.2011.08.045
Pinson MB, Bazant MZ (2013) Theory of SEI formation in rechargeable batteries: capacity fade, accelerated aging and lifetime prediction. J Electrochem Soc 160(2):A243–A250. https://doi.org/10.1149/2.044302jes
Ramadass P, Haran B, Gomadam PM, White R, Popov BN (2004) Development of first principles capacity fade model for li-ion cells. J Electrochemical Soc 151(2):A196–A203. https://doi.org/10.1149/1.1634273
Ning G, White RE, Popov BN (2006) A generalized cycle life model of rechargeable li-ion batteries. Electrochim Acta 51(10):2012–2022. https://doi.org/10.1016/j.electacta.2005.06.033
Ploehn HJ, Ramadass P, White RE (2004) Solvent diffusion model for aging of lithium-ion battery cells. J Electrochemical Soc 151(3):A456–A462. https://doi.org/10.1149/1.1644601
Xie YY, Li JY, Yuan C (2014) Multiphysics modeling of lithium ion battery capacity fading process with solid-electrolyte interphase growth by elementary reaction kinetics. J Power Source 248:172–179. https://doi.org/10.1016/j.jpowsour.2013.09.059
Ashwin TR, Chung YM, Wang J (2016) Capacity fade modelling of lithium-ion battery under cyclic loading conditions. J Power Sources 328:586–598. https://doi.org/10.1016/j.jpowsour.2016.08.054
Colclasure AM, Smith KA, Kee RJ (2011) Modeling detailed chemistry and transport for solid-electrolyte-interface (SEI) films in li–ion batteries. Electrochim Acta 58:33–43. https://doi.org/10.1016/j.electacta.2011.08.067
Zhang Y, Song W, Lin S, Feng Z (2014) Multiparameters model of the initial SOC considering the relaxation effect. ACS Sustain Chem Eng 2(4):599–605. https://doi.org/10.1021/sc400430e
Yan J, Xia B, Su Y, Zhou X, Zhang J, Zhang X (2008) Phenomenologically modeling the formation and evolution of the solid electrolyte interface on the graphite electrode for lithium-ion batteries. Electrochim Acta 53(24):7069–7078. https://doi.org/10.1016/j.electacta.2008.05.032
Lu P, Harris SJ (2011) Lithium transport within the solid electrolyte interphase. Electrochem Commun 13(10):1035–1037. https://doi.org/10.1016/j.elecom.2011.06.026
Tang M, Lu S, Newman J (2012) Experimental and theoretical investigation of solid-electrolyte-interphase formation mechanisms on glassy carbon. J Electrochemical Soc 159(11):A1775–A1785. https://doi.org/10.1149/2.025211jes
Safari M, Morcrette M, Teyssot A, Delacourt C (2009) Multimodal physics-based aging model for life prediction of li-ion batteries. J Electrochemical Soc 156(3):A145–A153. https://doi.org/10.1149/1.3043429
Basu S, Hariharan KS, Kolake SM, Song T, Sohn DK, Yeo T (2016) Coupled electrochemical thermal modelling of a novel li-ion battery pack thermal management system. Appl Energy 181:1–13. https://doi.org/10.1016/j.apenergy.2016.08.049
Ye Y, Shi Y, Tay AAO (2012) Electro-thermal cycle life model for lithium iron phosphate battery. J Power Sources 217:509–518. https://doi.org/10.1016/j.jpowsour.2012.06.055
Wu W, Xiao XR, Huang XS (2012) The effect of battery design parameters on heat generation and utilization in a li-ion cell. Electrochim Acta 83:227–240. https://doi.org/10.1016/j.electacta.2012.07.081
Valoen LO, Reimers JN (2005) Transport properties of LiPF6-based li-ion battery electrolytes. J Electrochemical Soc 152(5):A882–A891. https://doi.org/10.1149/1.1872737
Bernardi D, Pawlikowski E, Newman J (1985) A general energy balance for battery systems. J Electrochemical Soc 132(1):5–12. https://doi.org/10.1149/1.2113792
Xu M, Zhang ZQ, Wang X, Jia L, Yang LX (2015) A pseudo three-dimensional electrochemical-thermal model of a prismatic LiFePO4 battery during discharge process. Energy 80:303–317. https://doi.org/10.1016/j.energy.2014.11.073
Safari M, Delacourt C (2011) Modeling of a commercial graphite/LiFePO4 cell. J Electrochem Soc 158(5):A562–A571. https://doi.org/10.1149/1.3567007
Srinivasan V, Wang CY (2003) Analysis of electrochemical and thermal behavior of li-ion cells. J Electrochemical Soc 150(1):A98–A106. https://doi.org/10.1149/1.1526512
Gerver RE, Meyers JP (2011) Three-dimensional modeling of electrochemical performance and heat generation of lithium-ion batteries in tabbed planar configurations. J Electrochemical Soc 158(7):A835–A843. https://doi.org/10.1149/1.3591799
Wang J, Liu P, Hicks-Garner J, Sherman E, Soukiazian S, Verbrugge M, Tataria H, Musser J, Finamore P (2011) Cycle-life model for graphite-LiFePO4 cells. J Power Sources 196(8):3942–3948. https://doi.org/10.1016/j.jpowsour.2010.11.134
Jalkanen K, Karppinen J, Skogström L, Laurila T, Nisula M, Vuorilehto K (2015) Cycle aging of commercial NMC/graphite pouch cells at different temperatures. Appl Energy 154:160–172. https://doi.org/10.1016/j.apenergy.2015.04.110
Saw LH, Ye YH, Tay AAO (2013) Electrochemical-thermal analysis of 18650 lithium iron phosphate cell. Energy Conv Manag 75:162–174. https://doi.org/10.1016/j.enconman.2013.05.040
Wang Q, Ping P, Zhao X, Chu G, Sun J, Chen C (2012) Thermal runaway caused fire and explosion of lithium ion battery. J Power Sources 208:210–224. https://doi.org/10.1016/j.jpowsour.2012.02.038
Borodin O, Smith GD, Fan P (2006) Molecular dynamics simulations of lithium alkyl carbonates. J Phys Chem B 110(45):22773–22779. https://doi.org/10.1021/jp0639142
Safari M, Delacourt C (2011) Simulation-based analysis of aging phenomena in a commercial graphite/LiFePO4 cell. J Electrochemical Soc 158(12):A1436–A1447. https://doi.org/10.1149/2.103112jes
Acknowledgements
The authors gratefully acknowledge funding by the projects (No. 21676211 and No. 21606174) sponsored by the National Natural Science Foundation of China (NSFC). The authors also gratefully acknowledge funding by the China Postdoctoral Science Foundation (Grant 2016M592793).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
ESM 1
(DOCX 413 kb)
Rights and permissions
About this article
Cite this article
Huang, X., Ke, S., Lv, H. et al. A dynamic capacity fading model with thermal evolution considering variable electrode thickness for lithium-ion batteries. Ionics 24, 3439–3450 (2018). https://doi.org/10.1007/s11581-018-2476-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11581-018-2476-8