Abstract
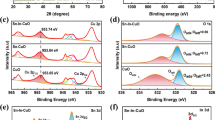
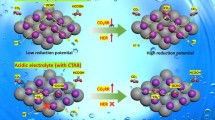
Electrode materials have an important impact on the efficiency and product selectivity of electrocatalytic CO2 reduction, and copper is favored as an electrode material capable of generating a variety of multicarbon products. However, its poor product selectivity limits its large-scale application. In order to regulate the selectivity to certain products, 1,2-cyclohexylenedinitrilotetraacetic acid (CyDTA) was employed as electrolyte additive to form Cu–N active sites by ligand chelation with copper, which improved the selectivity of CH4. Furthermore, the electrode modification was enhanced with activation using cyclic voltammetry (CV), and the results showed that the CV activation significantly strengthened the interaction between CyDTA and copper electrode, which led to the highest partial current density up to 9.3 mA cm−2 of CH4 in an H-type electrolytic cell at an electrode potential of − 1.0 V (vs. RHE). Under this condition, the Faradaic efficiency of CH4 was up to 74% and could be kept more than 50% after 8-h constant potential electrolysis. The results of this study provided a new strategy to improve the selectivity of CO2 reduction products with copper electrode, which can promote the wide application of electrocatalytic CO2 reduction process.
Graphical Abstract








Similar content being viewed by others
Data availability
Data will be made available based on request.
References
Mohsin I, Al-Attas TA, Sumon KZ et al (2020) Economic and environmental assessment of integrated carbon capture and utilization[J]. Cell Rep Phys Sci 1(7):100104
Figueres C, Le Quéré C, Mahindra A et al (2018) Emissions are still rising: ramp up the cuts[J]. Nature 564(7734):27–30
Li CW, Kanan MW (2012) CO2 reduction at low overpotential on Cu electrodes resulting from the reduction of thick Cu2O films[J]. J Am Chem Soc 134(17):7231–7234
Gao D, Arán-Ais RM, Jeon HS et al (2019) Rational catalyst and electrolyte design for CO2 electroreduction towards multicarbon products[J]. Nat Catal 2(3):198–210
Ren D, Fong J, Yeo BS (2018) The effects of currents and potentials on the selectivities of copper toward carbon dioxide electroreduction[J]. Nat Commun 9(1):925
Roberts FS, Kuhl KP, Nilsson A (2015) High selectivity for ethylene from carbon dioxide reduction over copper nanocube electrocatalysts[J]. Angew Chem 127(17):5268–5271
Li Y, Kim D, Louisia S et al (2020) Electrochemically scrambled nanocrystals are catalytically active for CO2-to-multicarbons[J]. Proc Natl Acad Sci 117(17):9194–9201
Kim D, Kley CS, Li Y et al (2017) Copper nanoparticle ensembles for selective electroreduction of CO2 to C2–C3 products[J]. Proc Natl Acad Sci 114(40):10560–10565
Wang H, Liang Z, Tang M et al (2019) Self-selective catalyst synthesis for CO2 reduction[J]. Joule 3(8):1927–1936
Zhou X, Shan J, Chen L et al (2022) Stabilizing Cu2+ ions by solid solutions to promote CO2 electroreduction to methane[J]. J Am Chem Soc 144(5):2079–2084
Kuhl KP, Hatsukade T, Cave ER et al (2014) Electrocatalytic conversion of carbon dioxide to methane and methanol on transition metal surfaces[J]. J Am Chem Soc 136(40):14107–14113
Nitopi S, Bertheussen E, Scott SB et al (2019) Progress and perspectives of electrochemical CO2 reduction on copper in aqueous electrolyte[J]. Chem Rev 119(12):7610–7672
Kim JY, Hong D, Lee JC et al (2021) Quasi-graphitic carbon shell-induced Cu confinement promotes electrocatalytic CO2 reduction toward C2+ products[J]. Nat Commun 12(1):3765
Fan Q, Zhang M, Jia M et al (2018) Electrochemical CO2 reduction to C2+ species: heterogeneous electrocatalysts, reaction pathways, and optimization strategies[J]. Mater Today Energy 10:280–301
Han Z, Han D, Chen Z et al (2022) Steering surface reconstruction of copper with electrolyte additives for CO2 electroreduction[J]. Nat Commun 13(1):3158
Jovanov ZP, Ferreira de Araujo J, Li S et al (2019) Catalyst preoxidation and EDTA electrolyte additive remedy activity and selectivity declines during electrochemical CO2 reduction[J]. J Phys Chem C 123(4):2165–2174
Fan M, Miao RK, Ou P et al (2023) Single-site decorated copper enables energy-and carbon-efficient CO2 methanation in acidic conditions[J]. Nat Commun 14(1):3314
Takashima T, Suzuki T, Irie H (2017) Acceleration of electrocatalytic CO2 reduction by adding proton-coupled electron transfer inducing compounds[J]. J Photonics Energy 7(1):012005–012005
Hsieh YC, Betancourt LE, Senanayake SD et al (2018) Modification of CO2 reduction activity of nanostructured silver electrocatalysts by surface halide anions[J]. ACS Appl Energy Mater 2(1):102–109
Simon GH, Kley CS, Roldan CB (2021) Potential-dependent morphology of copper catalysts during CO2 electroreduction revealed by in situ atomic force microscopy[J]. Angew Chem Int Ed 60(5):2561–2568
Ahmad N, Wang X, Sun P et al (2021) Electrochemical CO2 reduction to CO facilitated by MDEA-based deep eutectic solvent in aqueous solution[J]. Renew Energy 177:23–33
Ahmad N, Chen Y, Wang X et al (2022) Highly efficient electrochemical upgrade of CO2 to CO using AMP capture solution as electrolyte[J]. Renew Energy 189:444–453
Arán-Ais RM, Rizo R, Grosse P et al (2020) Imaging electrochemically synthesized Cu2O cubes and their morphological evolution under conditions relevant to CO2 electroreduction[J]. Nat Commun 11(1):3489
Singh MR, Kwon Y, Lum Y et al (2016) Hydrolysis of electrolyte cations enhances the electrochemical reduction of CO2 over Ag and Cu[J]. J Am Chem Soc 138(39):13006–13012
Christophe J, Doneux T, Buess-Herman C (2012) Electroreduction of carbon dioxide on copper-based electrodes: activity of copper single crystals and copper–gold alloys[J]. Electrocatalysis 3:139–146
Zhong D, Zhao ZJ, Zhao Q et al (2021) Coupling of Cu (100) and (110) facets promotes carbon dioxide conversion to hydrocarbons and alcohols[J]. Angew Chem Int Ed 60(9):4879–4885
Durand WJ, Peterson AA, Studt F et al (2011) Structure effects on the energetics of the electrochemical reduction of CO2 by copper surfaces[J]. Surf Sci 605(15–16):1354–1359
Shi C, Hansen HA, Lausche AC et al (2014) Trends in electrochemical CO2 reduction activity for open and close-packed metal surfaces[J]. Phys Chem Chem Phys 16(10):4720–4727
Kim YG, Javier A, Baricuatro JH et al (2016) Surface reconstruction of pure-Cu single-crystal electrodes under CO-reduction potentials in alkaline solutions: A study by seriatim ECSTM-DEMS[J]. J Electroanal Chem 780:290–295
Mistry H, Varela AS, Bonifacio CS et al (2016) Highly selective plasma-activated copper catalysts for carbon dioxide reduction to ethylene[J]. Nat Commun 7(1):12123
Peterson AA, Abild-Pedersen F, Studt F et al (2010) How copper catalyzes the electroreduction of carbon dioxide into hydrocarbon fuels[J]. Energy Environ Sci 3(9):1311–1315
Weng Z, Wu Y, Wang M et al (2018) Active sites of copper-complex catalytic materials for electrochemical carbon dioxide reduction[J]. Nat Commun 9(1):415
Zhang L, Li XX, Lang ZL et al (2021) Enhanced cuprophilic interactions in crystalline catalysts facilitate the highly selective electroreduction of CO2 to CH4[J]. J Am Chem Soc 143(10):3808–3816
Creissen CE, Fontecave M (2022) Kee** sight of copper in single-atom catalysts for electrochemical carbon dioxide reduction[J]. Nat Commun 13(1):2280
Liang X, Gao L, Yang S et al (2009) Facile synthesis and shape evolution of single crystal cuprous oxide[J]. Adv Mater 21(20):2068–2071
Yu P, Lv X, Wang Q et al (2023) Promoting Electrocatalytic CO2 Reduction to CH4 by Copper Porphyrin with Donor-Acceptor Structures[J]. Small 19(4):2205730
Wang X, Xu A, Li F et al (2020) Efficient methane electrosynthesis enabled by tuning local CO2 availability[J]. J Am Chem Soc 142(7):3525–3531
Fan Q, Zhang X, Ge X et al (2021) Manipulating Cu nanoparticle surface oxidation states tunes catalytic selectivity toward CH4 or C2+ products in CO2 electroreduction[J]. Adv Energy Mater 11(36):2101424
Xiong L, Zhang X, Chen L et al (2021) Geometric modulation of local CO Flux in Ag@ Cu2O nanoreactors for steering the CO2RR Pathway toward high-efficacy methane production [J]. Adv Mater 33(32):2101741
Xue L, Zhang C, Wu J et al (2022) Unveiling the reaction pathway on Cu/CeO2 catalyst for electrocatalytic CO2 reduction to CH4[J]. Appl Catal B 304:120951
Deng B, Huang M, Li K et al (2022) The crystal plane is not the key factor for CO2-to-methane electrosynthesis on reconstructed Cu2O microparticles[J]. Angew Chem Int Ed 61(7):e202114080
Lv J, Li W, Li J et al (2023) A triptycene-based 2D MOF with vertically extended structure for improving the electrocatalytic performance of CO2 to methane [J]. Angew Chem 135(11):e202217958
Deng H, Guo C, Shi P et al (2021) Amino assisted protonation for carbon− carbon coupling during electroreduction of carbon dioxide to ethylene on copper (I) Oxide[J]. ChemCatChem 13(20):4325–4333
Siltamaki D, Chen S, Rahmati F et al (2021) Synthesis and electrochemical study of CuAu nanodendrites for CO2 reduction[J]. J Electrochem 27(3):278
Jiao S, Fu X, Huang H (2022) Descriptors for the evaluation of electrocatalytic reactions: d-Band theory and beyond[J]. Adv Func Mater 32(4):2107651
Sun X, Sun L, Li G et al (2022) Phosphorus Tailors the d-band center of copper atomic sites for efficient CO2 photoreduction under visible-light irradiation[J]. Angew Chem Int Ed 61(38):e202207677
Yin J, Jin J, Yin Z et al (2023) The built-in electric field across FeN/Fe3N interface for efficient electrochemical reduction of CO2 to CO[J]. Nat Commun 14(1):1724
Funding
This study received support from the National Natural Science Foundation of China (No. 51208299).
Ethics declarations
Ethical approval
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yuan, Z., Gao, Y., Yang, X. et al. Facilitating electrocatalytic CO2 reduction through electrolyte additives accompanied by activation of copper electrode with cyclic voltammetry. Ionics 30, 2387–2396 (2024). https://doi.org/10.1007/s11581-024-05420-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11581-024-05420-9