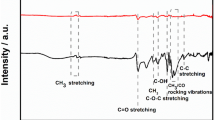
Abstract
Nanoparticle-based materials have played an important role in the development of new electrochemical sensors and received recently tremendous attention for the detection of toxic ions such as nitrate molecules (\({{\text{NO}}}_{3}^{-}\) and \({{\text{NO}}}_{2}^{-}\)). Here, we employ La1.7Sr0.3CuO4 (LSCu) and La0.6Sr0.4Co0.8Fe0.2O3 (LSCF) low-cost, highly sensitive nanoparticles modified with black carbon as sensors for the detection of nitrate ions. The modified nanooxides were synthesized by a simple citrate method then prepared with black carbon powder and nafion solution as a sensing matrix on a glassy carbon electrode for the determination of nitrates ions in water using cyclic voltammetry, differential pulse voltammetry, and electrochemical impedance spectroscopy as electrochemical techniques. X-ray diffraction (XRD) and scanning electron microscopy (SEM) were used for structural and morphological characterization. The calculated crystallite size d, using the Debye–Scherrer equation was found to be 325,193 nm for LSCu and 208,317 nm for LSCF by XRD technique. The grain sizes are, respectively, 47.80 nm and 65.05 nm which were extracted by SEM analysis. In this work, the modified sensors based on LSCu and LSCF demonstrate satisfactory response and sensitivities toward nitrate molecules compared with previous works. They characterized with very low detection limits of 0.0014 nM and 0.02 nM, high sensitivities of 58.8 and 57.3 µA.µM−1, respectively, and recorded a wide linear range from 1 M to 10–12 M for LSCF and 4 M to 10–13 M for LSCu.
Both of the modified electrodes demonstrated excellent results in real river water sample with low detection limits of 3.1 nM for LSCu and 3.5 nM for LSCF and very good recoveries of 100.6% and 101.65%, respectively.










Similar content being viewed by others
Data availability
No datasets were generated or analysed during the current study.
References
Aragay G, Pons J, Merkoçi A (2011) Recent trends in macro-, micro-, and nanomaterial-based tools and strategies for heavy-metal detection. Chem Rev 111(5):3433–3458. https://doi.org/10.1021/cr100383r
Benounis M, Jaffrezic-Renault N, Bonnamour I, Messai N (2015) Detection of sodium ions by SPR sensor using modified self-assembled calix [4] arene derivative monolayer. App Mech and Mater 799:915–918. https://doi.org/10.4028/www.scientific.net/AMM.799-800.915
Ren W, Mura S, Irudayaraj JM (2015) Modified graphene oxide sensors for ultra-sensitive detection of nitrate ions in water. Talanta 143:234–239. https://doi.org/10.1016/j.talanta.2015.05.073
Bagheri H, Hajian A, Rezaei M, Shirzadmehr A (2017) Composite of Cu metal nanoparticles-multiwall carbon nanotubes-reduced graphene oxide as a novel and high-performance platform of the electrochemical sensor for simultaneous determination of nitrite and nitrate. J Hazard mater 324:762–772. https://doi.org/10.1016/j.jhazmat.2016.11.055
Kanter DR, Zhang X, Mauzerall DL, Malyshev S, Shevliakova E (2016) The importance of climate change and nitrogen use efficiency for future nitrous oxide emissions from agriculture. Environ Res Lett 11(9):094003. https://doi.org/10.1088/1748-9326/11/9/094003
Knobeloch L, Salna B, Hogan A, Postle J, Anderson H (2000) Blue babies and nitrate-contaminated well water. Environ Health Perspect 108(7):675–678. https://doi.org/10.1289/ehp.00108675
Bosnir DBJ, Bevardi M, Boskovic AG, Lasic SMD, Krivohlavek A, Racs A, Mojosovic-Cuic A, Trstenjak NU (2017) Nitrate in leafy green vegetables and estimated intake. Afr J Tradit Complementary Altern Med 14:31–41. https://doi.org/10.21010/ajtcam.v14i3.4
Nujić M, Milinković D, Habuda-Stanić M (2017) Nitrate removal from water by ion exchange. J Food Sci Technol 9(2):182–186. https://doi.org/10.17508/CJFST.2017.9.2.15
Choi BC (1985) N-nifrroso compounds and human cancer: a molecular epidemiologic approach. Am J Epidemiol 121(5):737–743. https://doi.org/10.1093/aje/121.5.737
Liang J, Zheng Y, Liu Z (2016) Nanowire-based Cu electrode as electrochemical sensor for detection of nitrate in water. Sens Actuators B: Chem 232:336–344. https://doi.org/10.1016/j.snb.2016.03.145
Reyter D, Bélanger D, Roué L (2008) Study of the electroreduction of nitrate on copper in alkaline solution. Electrochim Acta 53(20):5977–5984. https://doi.org/10.1016/j.electacta.2008.03.048
Lee YY, Sriram B, Wang SF, Kogularasu S, Chang-Chien GP (2023) A comprehensive review on emerging role of rare earth oxides in electrochemical biosensors. Microchem J 109140. https://doi.org/10.1016/j.microc.2023.109140
Ferkhi M, Khelili S, Zerroual L, Ringuedé A, Cassir M (2009) Synthesis, structural analysis and electrochemical performance of low-copper content La2Ni1−xCuxO4+δ materials as new cathodes for solid oxide fuel cells. Electrochim Acta 54 (26):6341-6346https://doi.org/10.1016/j.electacta.2009.05.082
Ferkhi M, Ringuedé A, Khaled A, Zerroual L, Cassir M (2012) La1.98Ni04±δ, a new cathode material for solid oxide fuel cell: impedance spectroscopy study and compatibility with gadolinia-doped ceria and yttria-stabilized zirconia electrolytes. Electrochim Acta 75:80–87. https://doi.org/10.1016/j.electacta.2012.04.064
Ferkhi M, Yahia HA (2016) Electrochemical and morphological characterizations of La2xNiO4±d (x= 0.01, 0.02, 0.03 and 0.05) as new cathodes materials for IT-SOFC. Mater Res Bull 83:268–274. https://doi.org/10.1016/j.materresbull.2016.06.009
Ferkhi M, Rekaik M, Khaled A, Cassir M, Pireaux JJ (2017) Neodymium nickelate Nd2-xSrxNi1-yCoyO4±d (x and y= 0 or 0.05) as cathode materials for the oxygen reduction reaction. Electrochim Acta 229:281–290. https://doi.org/10.1016/j.electacta.2017.01.023
Amira S, Ferkhi M, Khaled A, Mauvy F, Grenier JC, Houssiau L, Pireaux JJ (2019) Carbon-based lanthanum nickelate material La2−x−yNdxPryNiO4+δ (x= 0, 0.3, and 0.5; y= 0 and 0.2) as a bifunctional electrocatalyst for oxygen reduction in alkaline media. Ionics 25:3809–3822. https://doi.org/10.1007/s11581-019-02963-0
Amira S, Ferkhi M, Belghobsi M, Khaled A, Mauvy F, Grenier JC (2019) Synthesis, characterization, and electrochemical behavior of a new Nd1.9Sr0.1Ni0.9Co0.1O4±δ material as electrocatalyst for the oxygen reduction reaction. Ionics 25:3799–3807. https://doi.org/10.1007/s11581-019-02922-9
Khaled A, Rekaik M, Ferkhi M, Cassir M (2019) New La2Ni1-xO4±δ (0.01≤ x≤ 0.1) materials as cathode for solid oxide fuel cells. Anal Bioanal Electrochem 11(11):1517–1535
Kogularasu S, Akilarasan M, Chen SM, Sheu JK (2021) Scalable and sustainable synthetic assessment between solid-state metathesis and sonochemically derived electrocatalysts (strontium molybdate) for the precise anti-androgen bicalutamide (Casodex™) detection. Microchem J 168:106465. https://doi.org/10.1016/j.microc.2021.106465
Si P, Huang Y, Wang T, Ma J (2013) Nanomaterials for electrochemical non-enzymatic glucose biosensors. RSC Adv 3(11):3487–3502. https://doi.org/10.1039/C2RA22360K
Bai W, Zheng J, Sheng Q (2013) A non-enzymatic hydrogen peroxide sensor based on Ag/MnOOH nanocomposites. Electroanalysis 25(10):2305–2311. https://doi.org/10.1002/elan.201300236
Chon K, Lee Y, Traber J, Von Gunten U (2013) Quantification and characterization of dissolved organic nitrogen in wastewater effluents by electrodialysis treatment followed by size-exclusion chromatography with nitrogen detection. Water Res 47(14):5381–5391. https://doi.org/10.1016/j.watres.2013.06.019
Moo YC, Matjafri MZ, Lim HS, Tan CH (2016) New development of optical fibre sensor for determination of nitrate and nitrite in water. Optik 127(3):1312–1319. https://doi.org/10.1016/j.ijleo.2015.09.072
Kodamatani H, Yamazaki S, Saito K, Tomiyasu T, Komatsu Y (2009) Selective determination method for measurement of nitrite and nitrate in water samples using high-performance liquid chromatography with post-column photochemical reaction and chemiluminescence detection. J Chromatogr A 1216(15):3163–3167. https://doi.org/10.1016/j.chroma.2009.01.096
Kim K, Kim KL, Shin KS (2012) Selective detection of aqueous nitrite ions by surface-enhanced Raman scattering of 4-aminobenzenethiol on Au. Analyst 137(16):3836–3840. https://doi.org/10.1039/C2AN35066A
Davis J, Moorcroft MJ, Wilkins SJ, Compton RG, Cardosi MF (2000) Electrochemical detection of nitrate and nitrite at a copper modified electrode. Analyst 125(4):737–742. https://doi.org/10.1039/A909762G
Manea F, Remes A, Radovan C, Pode R, Picken S, Schoonman J (2010) Simultaneous electrochemical determination of nitrate and nitrite in aqueous solution using Ag-doped zeolite-expanded graphite-epoxy electrode. Talanta 83(1):66–71. https://doi.org/10.1016/j.talanta.2010.08.042
Li Y, Li H, Song Y, Lu H, Tong J, Bian C, Xia S (2016) An electrochemical sensor system with renewable copper nano-clusters modified electrode for continuous nitrate determination. IEEE Sens J 16(24):8807–8814. https://doi.org/10.1109/JSEN.2016.2582038
Pan D, Lu W, Zhang H, Zhang L, Zhuang J (2013) Voltammetric determination of nitrate in water samples at copper modified bismuth bulk electrode. Intl J Environ Anal Chem 93(9):935–945. https://doi.org/10.1080/03067319.2012.690149
Da Silva IS, De Araujo WR, Paixão TR, Angnes L (2013) Direct nitrate sensing in water using an array of copper-microelectrodes from flat flexible cables. Sens Actuators B: Chem 188:94–98. https://doi.org/10.1016/j.snb.2013.06.094
Li Y, Sun JZ, Bian C, Tong JH, Dong HP, Zhang H, Xia SH (2015) Copper nano-clusters prepared by one-step electrodeposition and its application on nitrate sensing. AIP Adv 5(4). https://doi.org/10.1063/1.4905712
Stortini AM, Moretto LM, Mardegan A, Ongaro M, Ugo P (2015) Arrays of copper nanowire electrodes: preparation, characterization and application as nitrate sensor. Sens Actuators B: Chem 207:186–192. https://doi.org/10.1016/j.snb.2014.09.109
Li H, Li J, Yang Z, Xu Q, Hou C, Peng J, Hu X (2011) Simultaneous determination of ultra trace lead and cadmium by square wave stripping voltammetry with in situ depositing bismuth at Nafion-medical stone doped disposable electrode. J Hazard Mater 191(1–3):26–31. https://doi.org/10.1016/j.jhazmat.2011.04.020
Huang MR, Ding YB, Li XG (2013) Lead-ion potentiometric sensor based on electrically conducting micro particles of sulfonic phenylene diamine copolymer. Analyst 138(13):3820–3829. https://doi.org/10.1039/C3AN00346A
Huang MR, Ding YB, Li XG, Liu Y, Xi K, Gao CL, Kumar RV (2014) Synthesis of semiconducting polymer microparticles as solid ionophore with abundant complexing sites for long-life Pb(II) sensors. ACS Appl Mater Interfaces 6(24):22096–22107. https://doi.org/10.1021/am505463f
Huang MR, Ding YB, Li XG (2014) Combinatorial screening of potentiometric Pb (II) sensors from polysulfoaminoanthraquinone solid ionophore. ACS Comb Sci 16(3):128–138. https://doi.org/10.1021/co400140g
Bui MPN, Brockgreitens J, Ahmed S, Abbas A (2016) Dual detection of nitrate and mercury in water using disposable electrochemical sensors. Biosens Bioelectron 85:280–286. https://doi.org/10.1016/j.bios.2016.05.017
Zhang Y, Nie J, Wei H, Xu H, Wang Q, Cong Y, Wu X (2018) Electrochemical detection of nitrite ions using Ag/Cu/MWNT nanoclusters electrodeposited on a glassy carbon electrode. Sens Actuators B: Chem 258:1107–1116. https://doi.org/10.1016/j.snb.2017.12.001
Dima GE, De Vooys ACA, Koper MTM (2003) Electrocatalytic reduction of nitrate at low concentration on coinage and transition-metal electrodes in acid solutions. J Electroanal Chem 554:15–23. https://doi.org/10.1016/S0022-0728(02)01443-2
Bouzek K, Paidar M, Sadilkova A, Bergmann H (2001) Electrochemical reduction of nitrate in weakly alkaline solutions. J Appl Electrochem 31:1185–1193. https://doi.org/10.1023/A:1012755222981
Patil SB, Liu TR, Chou HL, Huang YB, Chang CC, Chen YC, Wang DY (2021) Electrocatalytic reduction of NO3– to ultrapure ammonia on 200 facet dominant Cu nanodendrites with high conversion faradic efficiency. J Phys Chem Lett 12(33):8121–8128. https://doi.org/10.1021/acs.jpclett.1c02236
Essousi H, Barhoumi H, Bibani M, Ktari M et al (2019) Ion-imprinted electrochemical sensor based on copper nanoparticles-polyaniline matrix for nitrate detection. J Sens 2019. https://doi.org/10.1155/2019/4257125
Hanane K, Messaoud B, Houcine B, Moncef T (2020) Highly sensitive modified glassy carbon sensor based on TDAN for nitrate detection in real water. Monatshefte für Chemie-Chem Mon 151:153–158. https://doi.org/10.1007/s00706-019-02547-8
Patella B, Russo RR, O’Riordan A, Aiello G et al (2021) Copper nanowire array as highly selective electrochemical sensor of nitrate ions in water. Talanta 221:121643. https://doi.org/10.1016/j.talanta.2020.121643
Ramakrishnappa T, Sureshkumar K, Pandurangappa M (2020) Copper oxide impregnated glassy carbon spheres based electrochemical interface for nitrite/nitrate sensing. Mater Chem Phys 245:122744. https://doi.org/10.1016/j.matchemphys.2020.122744
Wang J, Diao P (2020) Simultaneous detection of ammonia and nitrate using a modified electrode with two regions. Microchem J 154:104649. https://doi.org/10.1016/j.microc.2020.104649
Inam AS, Costa Angeli MA, Shkodra B et al (2021) Flexible screen-printed electrochemical sensors functionalized with electrodeposited copper for nitrate detection in water. ACS Omega 6(49):33523–33532. https://doi.org/10.1021/acsomega.1c04296
Akhter F, Siddiquei HR, Alahi MEE, Mukhopadhyay SC (2021) An IoT-enabled portable sensing system with MWCNTs/PDMS sensor for nitrate detection in water. Measurement 178:109424. https://doi.org/10.1016/j.measurement.2021.109424
Siddiqui H, Singh N, Chauhan V, Sathish N, Kumar S (2021) Electrochemical 3D printed copper garden for nitrate detection. Mater Lett 305:130795. https://doi.org/10.1016/j.matlet.2021.130795
Sriram B, Kogularasu S, Wang SF, Chang-Chien GP (2023) The fabrication of a La2Sn2O7/f-HNT composite for non-enzymatic electrochemical detection of 3-nitro-l-tyrosine in biological samples. Biosens 13(7):722. https://doi.org/10.3390/bios13070722
Muthumariappan A, Govindasamy M, Chen SM et al (2017) Screen-printed electrode modified with a composite prepared from graphene oxide nanosheets and Mn3O4 microcubes for ultrasensitive determination of nitrite. Microchim Acta 184:3625–3634. https://doi.org/10.1007/s00604-017-2379-9
Mekersi M, Ferkhi M, Savan EK (2023) Electrochemical biodetection of glucose using La0.6Sr0.4Co0.8Fe0.2O3 and La1,7Sr0,3CuO4 nano-particles modified with black carbon deposited on glassy carbon electrode. Microchem J 109346. https://doi.org/10.1016/j.microc.2023.109346
Amira S, Ferkhi M, Khaled A, Mauvy F, Bassat JM, Cassir M, Grenier JC (2023) Development of an innovative interfacial layer adapted to La2BO4±δ (B: Ni, Mn, Co) IT-SOC oxygen electrodes. Mater Res Bull 112400. https://doi.org/10.1016/j.materresbull.2023.112400
Amira S, Ferkhi M, Mauvy F, Fourcade S, Bassat JM, Grenier JC (2023) La1.5Nd0. 3Pr0.2NiO4. 16: a new cathode material for IT-solid oxide fuel cells. Electrocatalysis 1–15. https://doi.org/10.1007/s12678-023-00818-x
Zine A, Ferkhi M, Khaled A, Savan EK (2022) A2BO4±δ as new materials for electrocatalytic detection of paracetamol and diclofenac drugs. Electrocatalysis 13(5):524–538. https://doi.org/10.1007/s12678-022-00745-3
Amira S, Ferkhi M, Khaled A, Pireaux JJ (2022) Electrochemical properties of La2BO4+ δ/C electrocatalysts and study of the mechanism of the oxygen reduction reaction in alkaline medium. J Iran Chem Soc 1–17. https://doi.org/10.1007/s13738-021-02423-5
Rodríguez JE (2009) LSCuO ceramics as possible material for thermoelectric energy conversion. Momento (39):67–76. 10.15446/mo
Liotta LF, Puleo F, La Parola V et al (2015) La0.6Sr0.4FeO3‐δ and La0.6Sr0.4Co0.2Fe0.8O3‐δ perovskite materials for H2O2 and glucose electrochemical sensors. Electroanalysis 27(3):684–692. https://doi.org/10.1002/elan.201400589
Kumar Y, Pramanik P, Das DK (2019) Electrochemical detection of paracetamol and dopamine molecules using nano-particles of cobalt ferrite and manganese ferrite modified with graphite. Heliyon 5(7):e02031. https://doi.org/10.1016/j.heliyon.2019.e02031
Koh TM, Febriansyah B, Mathews N (2017) Ruddlesden-Popper perovskite solar cells. Chem 2(3):326–327. https://doi.org/10.1016/j.chempr.2017.02.015
Jeena SE, Gnanaprakasam P, Dakshinamurthy A, Selvaraju T (2015) Tuning the direct growth of Ag seeds into bimetallic Ag @ Cu nanorods on surface functionalized electrochemically reduced graphene oxide: enhanced nitrite detection. RSC Adv 5(60):48236–48245. https://doi.org/10.1039/C5RA05730B
Author information
Authors and Affiliations
Contributions
M. Mekersi did the experiments and wrote the text. M. FERKHI and E. KUYUMCU SAVAN corrected and interpreted the results ! All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Ethical approval
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Mekersi, M., Savan, E.K. & Ferkhi, M. Electrochemical simultaneous determination of nitrate ions in water using modified glassy carbon electrode based on La1.7Sr0.3CuO4 and La0.6Sr0.4Co0.8Fe0.2O3 nanomaterials and black carbon sensors. Ionics 30, 2357–2374 (2024). https://doi.org/10.1007/s11581-024-05404-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11581-024-05404-9