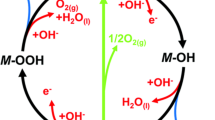
Abstract
Hydrogen is considered a key renewable energy source to meet future energy needs, and the accelerated development of hydrogen energy is largely dependent on the cost-effective and efficient production of hydrogen. Nowadays, the production of hydrogen by electrolyzing water is a generation of clean energy with great potential for development, but its industrialization requires the search for cheap and efficient catalysts. Platinum (Pt) is currently regarded as the most effective catalyst for the production of hydrogen from electrolytic water. However, its high price and scarcity limit its scale up, so the search for cheap and efficient catalysts has become a hot research topic. Nowadays, various noble/non-precious and metal-free catalysts have been developed for hydrogen evolution processes, among which ruthenium (Ru) catalysts are recognized as a promising material for hydrogen production from electrolytic water because they make a better balance between performance and price. In this review, we summarized the reported ruthenium-based catalysts with excellent hydrogen evolution reaction (HER) activity, specifically, Ru with carbon supports, Ru phosphide–based catalysts, and Ru catalysts on other metals. Finally, we discussed challenges and prospects for the development of Ru-based catalysts in the field of HER research.





Similar content being viewed by others
Data availability
No new data were created.
References
Gong L, Yang H, Wang H et al (2021) Corrosion formation and phase transformation of nickel-iron hydroxide nanosheets array for efficient water oxidation. Nano Res 14(12):4528–4533. https://doi.org/10.1007/s12274-021-3366-3
Jing H, Zhu P, Zheng X et al (2022) Theory-oriented screening and discovery of advanced energy transformation materials in electrocatalysis. Adv Powder Mater 1(1):100013. https://doi.org/10.1016/j.apmate.2021.10.004
Wei YW, Yang G, Xu XX et al (2023) Ultrafine Ru nanoparticles anchored on core–shell structured zeolite-carbon for efficient catalysis of hydrogen generation. Rare Met 2023:1–11. https://doi.org/10.1007/s12598-022-02246-0
Seh ZW, Kibsgaard J, Dickens CF et al (2017) Combining theory and experiment in electrocatalysis: insights into materials design. Science 355(6321):eaad4998. https://doi.org/10.1126/science.aad4998
Chen X, Wan J, Zheng M et al (2023) Engineering single atomic ruthenium on defective nickel vanadium layered double hydroxide for highly efficient hydrogen evolution. Nano Res 16(4):4612–4619. https://doi.org/10.1007/s12274-022-5075-y
Cui C, Hu X, Wen L (2020) Recent progress on nanostructured bimetallic electrocatalysts for water splitting and electroreduction of carbon dioxide. J Semicond 41(9):091705. https://doi.org/10.1088/1674-4926/41/9/091705
Ye S, Luo F, Zhang Q et al (2019) Highly stable single Pt atomic sites anchored on aniline-stacked graphene for hydrogen evolution reaction. Energy Environ Sci 12(3):1000–1007. https://doi.org/10.1039/c8ee02888e
Zhang X, Lai Z, Ma Q et al (2018) Novel structured transition metal dichalcogenide nanosheets. Chem Soc Rev 47(9):3301–3338. https://doi.org/10.1039/c8cs00094h
Yang H, Ma D, Li Y et al (2023) Mo doped Ru-based cluster to promote alkaline hydrogen evolution with ultra-low Ru loading. Chin J Struct Chem 2023:100031. https://doi.org/10.1016/j.cjsc.2023.100031
Zhu F, Xue J, Zeng L et al (2022) One-pot pyrolysis synthesis of highly active Ru/RuOX nanoclusters for water splitting. Nano Res 15(2):1020-1026. https://doi.org/10.1007/s12274-021-3590-x
Wang J, Yue X, Yang Y et al (2020) Earth-abundant transition-metal-based bifunctional catalysts for overall electrochemical water splitting: a review. J Alloy Compd 819:153346. https://doi.org/10.1016/j.jallcom.2019.153346
Luo W, Wang Y, Cheng C (2020) Ru-based electrocatalysts for hydrogen evolution reaction: recent research advances and perspectives. Mater Today Phys 15:100274. https://doi.org/10.1016/j.mtphys.2020.100274
Durst J, Siebel A, Simon C et al (2014) New insights into the electrochemical hydrogen oxidation and evolution reaction mechanism. Energy Environ Sci 7(7):2255–2260. https://doi.org/10.1039/c4ee00440j
Sultan S, Tiwari JN, Singh AN et al (2019) Single atoms and clusters-based nanomaterials for hydrogen evolution, oxygen evolution reactions, and full water splitting. Adv Energy Mater 9(22):1900624. https://doi.org/10.1002/aenm.201900624
Zhang W, Lai W, Cao R et al (2017) Energy-related small molecule activation reactions: oxygen reduction and hydrogen and oxygen evolution reactions catalyzed by porphyrin and corrole-based systems. Chem Rev 117:3717–3797. https://doi.org/10.1021/acs.chemrev.6b00299
Wang W, Xu X, Zhou W et al (2017) Recent progress in metal-organic frameworks for applications in electrocatalytic and photocatalytic water splitting. Adv Sci 4:1600371. https://doi.org/10.1002/advs.201600371
Zeng M, Li Y (2015) Recent advances in heterogeneous electrocatalysts for the hydrogen evolution reaction. J Mater Chem A 3(29):14942–14962. https://doi.org/10.1039/c5ta02974k
Morales-Guio CG, Stern LA, Hu X (2014) Nanostructured hydrotreating catalysts for electrochemical hydrogen evolution. Chem Soc Rev 43(18):6555–6569. https://doi.org/10.1039/c3cs60468c
Zhang G, Wang G, Liu Y et al (2016) Highly active and stable catalysts of phytic acid-derivative transition metal phosphides for full water splitting. J Am Chem Soc 138(44):14686–14693. https://doi.org/10.1021/jacs.6b08491
Burmistrz P, Chmielniak T, Czepirski L et al (2016) Carbon footprint of the hydrogen production process utilizing subbituminous coal and lignite gasification. J Clean Prod 139:858–865. https://doi.org/10.1016/j.jclepro.2016.08.112
Seyitoglu SS, Dincer I, Kilicarslan A (2017) Energy and exergy analyses of hydrogen production by coal gasification. Int J Hydrogen Energy 42(4):2592–2600. https://doi.org/10.1016/j.ijhydene.2016.08.228
Huang X, Lu R, Cen Y et al (2023) Micropore-confined Ru nanoclusters catalyst for efficient pH-universal hydrogen evolution reaction. Nano Res 2023:1–8. https://doi.org/10.1007/s12274-023-5711-1
Xu S, Niu M, Zhao G et al (2023) Size control and electronic manipulation of Ru catalyst over B, N co-doped carbon network for high-performance hydrogen evolution reaction. Nano Res 16:6212–6219. https://doi.org/10.1007/s12274-022-5250-1
Ma R, Wang X, Yang X et al (2023) Identification of active sites and synergistic effect in multicomponent carbon-based Ru catalysts during electrocatalytic hydrogen evolution. Nano Res 16:166–173. https://doi.org/10.1007/s12274-022-4643-5
Cao K, Biskupek J, Stoppiello CT et al (2020) Atomic mechanism of metal crystal nucleus formation in a single-walled carbon nanotube. Nat Chem 12(10):921–928. https://doi.org/10.1038/s41557-020-0538-9
Yi C, Yang Y, Nie Z (2019) Alternating copolymerization of inorganic nanoparticles. J Am Chem Soc 141(19):7917–7925. https://doi.org/10.1021/jacs.9b02316
Ding Y, Cao K, He J et al (2022) Nitrogen-doped graphene aerogel-supported ruthenium nanocrystals for pH-universal hydrogen evolution reaction. Chin J Catal 43(6):1535–1543. https://doi.org/10.1016/s1872-2067(21)63977-3
Ye S, Luo F, Xu T et al (2020) Boosting the alkaline hydrogen evolution of Ru nanoclusters anchored on B/N-doped graphene by accelerating water dissociation. Nano Energy 68:104301. https://doi.org/10.1016/j.nanoen.2019.104301
Wang T, Yuan H, Zhou J et al (2020) Graphite carbon nitride)assisted ruthenium/reduced graphene oxide as higheefficiency electrocatalyst for hydrogen evolution reaction under alkaline conditions. Chem Electro Chem 7(15):3269–3273. https://doi.org/10.1002/celc.202000681
Li F, Han GF, Noh HJ et al (2018) Mechanochemically assisted synthesis of a Ru catalyst for hydrogen evolution with performance superior to Pt in both acidic and alkaline media. Adv Mater 30(44):1803676. https://doi.org/10.1002/adma.201803676
Fan L, Liu PF, Yan X et al (2016) Atomically isolated nickel species anchored on graphitized carbon for efficient hydrogen evolution electrocatalysis. Nat Commun 7(1):10667. https://doi.org/10.1038/ncomms10667
Zhu QL, Xia W, Akita T et al (2016) Metal-organic framework-derived honeycomb-like open porous nanostructures as precious-metal-free catalysts for highly efficient oxygen electroreduction. Adv Mater 28(30):6391–6398. https://doi.org/10.1002/adma.201600979
Micheroni D, Lan G, Lin W (2018) Efficient electrocatalytic proton reduction with carbon nanotube-supported metal-organic frameworks. J Am Chem Soc 140(46):15591–15595. https://doi.org/10.1021/jacs.8b09521
Yan B, Liu D, Feng X et al (2020) Ru species supported on MOF-derived N-doped TiO2/C hybrids as efficient electrocatalytic/photocatalytic hydrogen evolution reaction catalysts. Adv Func Mater 30(31):2003007. https://doi.org/10.1002/adfm.202003007
Sun Y, Xue Z, Liu Q et al (2021) Modulating electronic structure of metal-organic frameworks by introducing atomically dispersed Ru for efficient hydrogen evolution. Nat Commun 12(1):1369. https://doi.org/10.1038/s41467-021-21595-5
Liu J, Li R, Yang B (2020) Carbon dots: a new type of carbon-based nanomaterial with wide applications. ACS Cent Sci 6(12):2179–2195. https://doi.org/10.1021/acscentsci.0c01306
Hu C, Li M, Qiu J et al (2019) Design and fabrication of carbon dots for energy conversion and storage. Chem Soc Rev 48(8):2315–2337. https://doi.org/10.1039/c8cs00750k
Liu ML, Chen BB, Li CM et al (2019) Carbon dots: synthesis, formation mechanism, fluorescence origin and sensing applications. Green Chem 21(3):449–471. https://doi.org/10.1039/c8gc02736f
Jiang K, Wang Y, Gao X et al (2018) Facile, quick, and gram-scale synthesis of ultralong-lifetime room-temperature-phosphorescent carbon dots by microwave irradiation. Angew Chem Int Ed 57(21):6216–6220. https://doi.org/10.1002/anie.201802441
Liu Y, Yang Y, Peng Z et al (2019) Self-crosslinking carbon dots loaded ruthenium dots as an efficient and super-stable hydrogen production electrocatalyst at all pH values. Nano Energy 65:104023. https://doi.org/10.1016/j.nanoen.2019.104023
Li W, Wei Z, Wang B et al (2020) Carbon quantum dots enhanced the activity for the hydrogen evolution reaction in ruthenium-based electrocatalysts. Mater Chem Front 4(1):277–284. https://doi.org/10.1039/c9qm00618d
Song H, Cheng Y, Li B et al (2020) Carbon dots and RuP2 nanohybrid as an efficient bifunctional catalyst for electrochemical hydrogen evolution reaction and hydrolysis of ammonia borane. ACS Sustain Chem Eng 8(9):3995–4002. https://doi.org/10.1021/acssuschemeng.0c00745
Yuan L, Wei D, Li H et al (2021) Carbon dots enhance ruthenium nanoparticles for efficient hydrogen production in alkaline. Acta Phys Chim Sin 37(7):2009082. https://doi.org/10.3866/pku.whxb.202009082
Zhou F, Zhou Y, Wang J et al (2019) Enlightening from γ, γ′ and β phase transformations in Al-Co-Ni alloy system: a review. Curr Opin Solid State Mater Sci 23(6):100784. https://doi.org/10.1016/j.cossms.2019.100784
Wu Q, Luo M, Han J et al (2019) Identifying electrocatalytic sites of the nanoporous copper-ruthenium alloy for hydrogen evolution reaction in alkaline electrolyte. ACS Energy Lett 5(1):192–199. https://doi.org/10.1021/acsenergylett.9b02374
Li W, Zhao Y, Liu Y et al (2021) Exploiting Ru-induced lattice strain in CoRu nanoalloys for robust bifunctional hydrogen production. Angew Chem 133(6):3327–3335. https://doi.org/10.1002/anie.202013985
Song H, Wu M, Tang Z et al (2021) Single atom ruthenium-doped CoP/CDs nanosheets via splicing of carbon-dots for robust hydrogen production. Angew Chem Int Ed 60(13):7234–7244. https://doi.org/10.1002/anie.202017102
Yuan W, Zhang Y, Cheng L et al (2016) The applications of carbon nanotubes and graphene in advanced rechargeable lithium batteries. J Mater Chem A 4(23):8932–8951. https://doi.org/10.1039/c6ta01546h
Gupta BD, Pathak A, Semwal V (2019) Carbon-based nanomaterials for plasmonic sensors: a review. Sensors 19(16):3536. https://doi.org/10.3390/s19163536
Ignat SR, Lazăr AD, Şelaru A et al (2019) Versatile biomaterial platform enriched with graphene oxide and carbon nanotubes for multiple tissue engineering applications. Int J Mol Sci 20(16):3868. https://doi.org/10.3390/ijms20163868
Cao Y, Lu Y, Ang EH et al (2019) MOF-derived uniform Ni nanoparticles encapsulated in carbon nanotubes grafted on rGO nanosheets as bifunctional materials for lithium-ion batteries and hydrogen evolution reaction. Nanoscale 11(32):15112–15119. https://doi.org/10.1039/c9nr05504e
Cui H, Yan X, Monasterio M et al (2017) Effects of various surfactants on the dispersion of MWCNTs–OH in aqueous solution. Nanomaterials 7(9):262. https://doi.org/10.3390/nano7090262
Arumugam S, Ramamoorthy P, Chakkarapani LD (2020) Synthesis and characterizations of biocompatible polymers and carbon nanotubes-based hybrids for biomedical applications. Int J Polym Mater Polym Biomater 69(12):786–797. https://doi.org/10.1080/00914037.2019.1616200
Wu X, Wang Z, Zhang D et al (2021) Solvent-free microwave synthesis of ultra-small Ru-Mo2C@CNT with strong metal-support interaction for industrial hydrogen evolution. Nat Commun 12(1):4018. https://doi.org/10.1038/s41467-021-24322-2
Xu W, Huang H, Wu X et al (2022) Mn-doped Ru/RuO2@CNT with strong metal-support interaction for efficient water splitting in acidic media. Compos B Eng 242:110013. https://doi.org/10.1016/j.compositesb.2022.110013
Zhou F, Sa R, Zhang X et al (2020) Robust ruthenium diphosphide nanoparticles for pH-universal hydrogen evolution reaction with platinum-like activity. Appl Catal B 274:119092. https://doi.org/10.1016/j.apcatb.2020.119092
Zhang D, Miao H, Wu X et al (2022) Scalable synthesis of ultra-small Ru2P@Ru/CNT for efficient seawater splitting. Chin J Catal 43(4):1148–1155. https://doi.org/10.1016/s1872-2067(21)64012-3
Huang Z, Chen Z, Chen Z et al (2014) Ni12P5 nanoparticles as an efficient catalyst for hydrogen generation via electrolysis and photoelectrolysis. ACS Nano 8(8):8121–8129. https://doi.org/10.1021/nn5022204
Popczun EJ, Read CG, Roske CW et al (2014) Highly active electrocatalysis of the hydrogen evolution reaction by cobalt phosphide nanoparticles. Angew Chem Int Ed 53(21):5427–5430. https://doi.org/10.1002/ange.201402646
Xiao P, Sk MA, Thia L et al (2014) Molybdenum phosphide as an efficient electrocatalyst for the hydrogen evolution reaction. Energy Environ Sci 7(8):2624–2629. https://doi.org/10.1039/c4ee00957f
Son CY, Kwak IH, Lim YR et al (2016) FeP and FeP2 nanowires for efficient electrocatalytic hydrogen evolution reaction. Chem Commun 52(13):2819–2822. https://doi.org/10.1039/c5cc09832g
Pi C, Huang C, Yang Y et al (2020) In situ formation of N-doped carbon-coated porous MoP nanowires: a highly efficient electrocatalyst for hydrogen evolution reaction in a wide pH range. Appl Catal B 263:118358. https://doi.org/10.1016/j.apcatb.2019.118358
Chang Q, Ma J, Zhu Y et al (2018) Controllable synthesis of ruthenium phosphides (RuP and RuP2) for pH-universal hydrogen evolution reaction. ACS Sustain Chem Eng 6(5):6388–6394. https://doi.org/10.1021/acssuschemeng.8b00187
Liu T, Wang J, Zhong C et al (2019) Benchmarking three ruthenium phosphide phases for electrocatalysis of the hydrogen evolution reaction: experimental and theoretical insights. Chem A Europ J 25(33):7826–7830. https://doi.org/10.1002/chem.201901215
Jiang X, Jang H, Liu S et al (2021) The heterostructure of Ru2P/WO3/NPC synergistically promotes H2O dissociation for improved hydrogen evolution. Angew Chem 133(8):4156–4162. https://doi.org/10.1002/anie.202014411
Du H, Du Z, Wang T et al (2022) Unlocking interfacial electron transfer of ruthenium phosphides by homologous core–shell design toward efficient hydrogen evolution and oxidation. Adv Mater 34(37):2204624. https://doi.org/10.1002/adma.202204624
Jiang B, Wang Z, Cheng L et al (2023) Regulating charge distribution of Ru atoms in ruthenium phosphide/carbon nitride/carbon for promoting hydrogen evolution reaction. J Alloy Compd 939:168717. https://doi.org/10.1016/j.jallcom.2023.168717
Wang YH, Li RQ, Li HB et al (2021) Controlled synthesis of ultrasmall RuP2 particles on N, P-codoped carbon as superior pH-wide electrocatalyst for hydrogen evolution. Rare Met 40:1040–1047. https://doi.org/10.1007/s12598-020-01665-1
Zhu J, Li S, Xiao M et al (2020) Tensile-strained ruthenium phosphide by anion substitution for highly active and durable hydrogen evolution. Nano Energy 77:105212. https://doi.org/10.1016/j.nanoen.2020.105212
Liu X, Liu F, Yu J et al Charge redistribution caused by S, P synergistically active Ru endows an ultrahigh hydrogen evolution activity of S-doped RuP embedded in N, P, S-doped carbon. Adv Sci 7(17):2001526. https://doi.org/10.1002/advs.202001526
Zhai Z, Wang Y, Si C et al (2023) Self-templating synthesis and structural regulation of nanoporous rhodium-nickel alloy nanowires efficiently catalyzing hydrogen evolution reaction in both acidic and alkaline electrolytes. Nano Res 16:2026–2034. https://doi.org/10.1007/s12274-022-4861-x
Mahmood J, Li F, Jung SM et al (2017) An efficient and pH-universal ruthenium-based catalyst for the hydrogen evolution reaction. Nat Nanotechnol 12(5):441–446. https://doi.org/10.1038/nnano.2016.304
Liu S, Mu X, Li W et al (2019) Cation vacancy-modulated PtPdRuTe five-fold twinned nanomaterial for catalyzing hydrogen evolution reaction. Nano Energy 61:346–351. https://doi.org/10.1016/j.nanoen.2019.04.086
Pei A, Li G, Zhu L et al (2022) Nickel hydroxide-supported Ru single atoms and Pd nanoclusters for enhanced electrocatalytic hydrogen evolution and ethanol oxidation. Adv Funct Mater 32(51):2208587. https://doi.org/10.1002/adfm.202208587
Zhang L, Si R, Liu H et al (2019) Atomic layer deposited Pt-Ru dual-metal dimers and identifying their active sites for hydrogen evolution reaction. Nat Commun 10(1):4936. https://doi.org/10.1038/s41467-019-12887-y
Wang JH, Yang SW, Ma FB et al (2023) RuCo alloy nanoparticles embedded within N-doped porous two-dimensional carbon nanosheets: a high-performance hydrogen evolution reaction catalyst. Tungsten 2023:1–10. https://doi.org/10.1007/s42864-023-00223-3
Cai C, Liu K, Zhu Y et al (2022) Optimizing hydrogen binding on Ru sites with RuCo alloy nanosheets for efficient alkaline hydrogen evolution. Angew Chem Int Ed 61(4):e202113664. https://doi.org/10.1002/anie.202113664
Yuan Y, Han W, Zhang C et al (2023) An insight into the enhanced mechanism of Ru-MoO2 interfacial chemical bonding for hydrogen evolution reaction in alkaline media. Nano Res 16(2):2230–2235. https://doi.org/10.1007/s12274-022-5013-z
Li H J W, Liu K, Fu J et al (2021) Paired Ru-O-Mo ensemble for efficient and stable alkaline hydrogen evolution reaction. Nano Energy 82:105767. https://doi.org/10.1016/j.nanoen.2021.105767
Liu Q, Zhang C, Wang P et al (2022) Nearly hollow Ru-Cu-MoO2 octahedrons consisting of clusters and nanocrystals for high efficiency hydrogen evolution reaction. J Mater Chem A 10(23):12341–12349. https://doi.org/10.1039/d2ta01699k
Wu K, Sun K, Liu S et al (2020) Atomically dispersed Ni–Ru–P interface sites for high-efficiency pH-universal electrocatalysis of hydrogen evolution. Nano Energy 80:105467. https://doi.org/10.1016/j.nanoen.2020.105467
Ren J, Liu J, Du Y et al (2023) Trace ruthenium promoted dual-reconstruction of CoFeP@C/NF for activating overall water splitting performance beyond precious-metals. Nano Res 16:10810–10821. https://doi.org/10.1007/s12274-023-5825-5
Liu Y, Liu X, Jadhav AR et al (2021) Unraveling the function of metal-amorphous support interactions in single-atom electrocatalytic hydrogen evolution. Angew Chem Int Ed 61(9):e202114160. https://doi.org/10.1002/anie.202114160
Funding
This research was supported by the Gansu key research and development program (industry field) under Grant No. 23YFGA0056, National Natural Science Foundation of China under Grant No. 22262018, and Hongliu outstanding youth talents support project.
Author information
Authors and Affiliations
Contributions
CL Li, LY Chen, D Dou and HB Wang conceived the central ideas. CL Li and LY Chen wrote the original article. QP Zhao and YY Cong supervised and revised the paper. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval
Not applicable.
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Li, C., Chen, L., Dou, D. et al. Recent research progress on ruthenium-based catalysts at full pH conditions for the hydrogen evolution reaction. Ionics 29, 4987–5001 (2023). https://doi.org/10.1007/s11581-023-05248-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11581-023-05248-9