Abstract
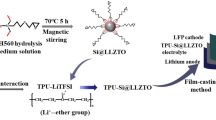
Solid-state electrolyte is a crucial component of all-solid-state batteries. Composite solid electrolytes are gaining attention for their ability to combine the high ionic conductivity and mechanical strength of inorganic solid-state electrolytes with the flexibility and interfacial compatibility of polymer solid electrolytes. However, agglomeration in composite solid electrolytes caused by inorganic fillers with high surface energy seriously affects the performance of composite solid electrolytes. To address this issue, nonionic fluorocarbon surfactant FC-4430 was added to the Li6.4La3Zr1.4Ta0.6O12 (LLZTO)/polyethylene oxide (PEO) composite solid electrolyte to enhance the wettability between LLZTO and the PEO matrix, resulting in the uniform dispersion of higher LLZTO content in PEO and improved ionic conductivity of the composite solid electrolyte. In addition, a smoother and flatter surface of the composite solid electrolyte is obtained, which improves the electrolyte/electrode interfacial contact. With the addition of 0.3 wt% FC-4430, the highest ionic conductivity of the composite solid electrolyte at 60 °C reaches 6.46 × 10−4 S/cm. The lithium-ion mobility of the composite solid-state electrolyte at room temperature is 0.69, and a voltage stability window is up to 5.0 V. The assembled LFP all-solid-state batteries show the initial discharge specific capacities of 158.4 mAh/g and 139.5 mAh/g at rate of 0.5 C and 1 C, respectively, as well as capacity retention of 96.4% and 90.9% after 300 cycles at 60 °C. Composite solid electrolytes also show improved mechanical properties and thermal stability.













Similar content being viewed by others
Data availability
No data was used for the research described in the article.
References
Tarascon JM, Armand M (2001) Issues and challenges facing rechargeable lithium batteries. Nature 414:359–367. https://doi.org/10.1038/35104644
Goodenough JB (2012) Rechargeable batteries: challenges old and new. J Solid State Electrochem 16:2019–2029. https://doi.org/10.1007/s10008-012-1751-2
Zhang XH, Li Z, Luo LA, Fan YL, Du ZY (2022) A review on thermal management of lithium-ion batteries for electric vehicles. Energy 238:12. https://doi.org/10.1016/j.energy.2021.121652
Nitta N, Wu FX, Lee JT, Yushin G (2015) Li-ion battery materials: present and future. Mater Today 18:252–264. https://doi.org/10.1016/j.mattod.2014.10.040
Fang RP, Chen K, Yin LC, Sun ZH, Li F, Cheng HM (2019) The regulating role of carbon nanotubes and graphene in lithium-ion and lithium-sulfur batteries. Adv Mater 31:22. https://doi.org/10.1002/adma.201800863
Zhang XQ, Zhao CZ, Huang JQ, Zhang Q (2018) Recent advances in energy chemical engineering of next-generation lithium batteries. Engineering 4:831–847. https://doi.org/10.1016/j.eng.2018.10.008
Gao ZH, Sun HB, Fu L, Ye FL, Zhang Y, Luo W, Huang YH (2018) Promises, challenges, and recent progress of inorganic solid-state electrolytes for all-solid-state lithium batteries. Adv Mater 30:27. https://doi.org/10.1002/adma.201705702
Zhang R, Li NW, Cheng XB, Yin YX, Zhang Q, Guo YG (2017) Advanced micro/nanostructures for lithium metal anodes. Adv Sci 4:13. https://doi.org/10.1002/advs.201600445
Li Z, Huang J, Liaw BY, Metzler V, Zhang JB (2014) A review of lithium deposition in lithium-ion and lithium metal secondary batteries. J Power Sources 254:168–182. https://doi.org/10.1016/j.jpowsour.2013.12.099
Francis CFJ, Kyratzis IL, Best AS (2020) Lithium-ion battery separators for ionic-liquid electrolytes: a review. Adv Mater 32:22. https://doi.org/10.1002/adma.201904205
Zhang HL, Zhao HB, Khan MA, Zou WW, Xu JQ, Zhang L, Zhang JJ (2018) Recent progress in advanced electrode materials, separators and electrolytes for lithium batteries. J Mater Chem A 6:20564–20620. https://doi.org/10.1039/c8ta05336g
Wan J, Xie J, Mackanic DG, Burke W, Bao Z, Cui Y (2018) Status, promises, and challenges of nanocomposite solid-state electrolytes for safe and high performance lithium batteries. Materials Today Nano 4:1–16. https://doi.org/10.1016/j.mtnano.2018.12.003
Li C, Wang ZY, He ZJ, Li YJ, Mao J, Dai KH, Yan C, Zheng JC (2021) An advance review of solid-state battery: challenges, progress and prospects. Sustain Mater Technol 29:14. https://doi.org/10.1016/j.susmat.2021.e00297
Xiao YH, Wang Y, Bo SH, Kim JC, Miara LJ, Ceder G (2020) Understanding interface stability in solid-state batteries. Nat Rev Mater 5:105–126. https://doi.org/10.1038/s41578-019-0157-5
Yu XW, Manthiram A (2021) A review of composite polymer-ceramic electrolytes for lithium batteries. Energy Storage Mater 34:282–300. https://doi.org/10.1016/j.ensm.2020.10.006
Wang CW, Fu K, Kammampata SP, McOwen DW, Samson AJ, Zhang L, Hitz GT, Nolan AM, Wachsman ED, Mo YF, Thangadurai V, Hu LB (2020) Garnet-type solid-state electrolytes: materials, interfaces, and batteries. Chem Rev 120:4257–4300. https://doi.org/10.1021/acs.chemrev.9b00427
Han G, Kinzer B, Garcia-Mendez R, Choe H, Wolfenstine J, Sakamoto J (2020) Correlating the effect of dopant type (Al, Ga, Ta) on the mechanical and electrical properties of hot-pressed Li-garnet electrolyte. J Eur Ceram Soc 40:1999–2006. https://doi.org/10.1016/j.jeurceramsoc.2019.12.054
Xu LQ, Li JY, Shuai HL, Luo Z, Wang BW, Fang SS, Zou GQ, Hou HS, Peng HJ, Ji XB (2022) Recent advances of composite electrolytes for solid-state Li batteries. J Energy Chem 67:524–548. https://doi.org/10.1016/j.jechem.2021.10.0382095-4956
Xue ZG, He D, Xie XL (2015) Poly (ethylene oxide)-based electrolytes for lithium-ion batteries. J Mater Chem A 3:19218–19253. https://doi.org/10.1039/c5ta03471j
Zhou Q, Ma J, Dong SM, Li XF, Cui GL (2019) Intermolecular chemistry in solid polymer electrolytes for high-energy-density lithium batteries. Adv Mater 31:21. https://doi.org/10.1002/adma.201902029
Dirican M, Yan CY, Zhu P, Zhang XW (2019) Composite solid electrolytes for all-solid-state lithium batteries. Mater Sci Eng R-Rep 136:27–46. https://doi.org/10.1016/j.mser.2018.10.004
Wang S, Sun Q, Peng W, Ma Y, Zhou Y, Song D, Zhang H, Shi X, Li C, Zhang L (2021) Ameliorating the interfacial issues of all-solid-state lithium metal batteries by constructing polymer/inorganic composite electrolyte. J Energy Chem 58:85–93. https://doi.org/10.1016/j.jechem.2020.09.033
Ma X, Liu M, Wu QP, Guan X, Wang F, Liu HM, Xu J Composite electrolytes prepared by improving the interfacial compatibility of organic-inorganic electrolytes for dendrite-free, long-life all-solid lithium metal batteries. ACS Appl Mater Interfaces 14:53828–53839. https://doi.org/10.1021/acsami.2c16174
Zegeye TA, Su WN, Fenta FW, Zeleke TS, Jiang SK, Hwang BJ (2020) Ultrathin Li6.75La3Zr1.75Ta0.25O12-based composite solid electrolytes laminated on anode and cathode surfaces for anode-free lithium metal batteries. ACS Appl Energ Mater 3:11713–11723. https://doi.org/10.1021/acsaem.0c01714
Tong RA, Chen LH, Shao G, Wang HL, Wang CA (2021) An integrated solvent-free modification and composite process of Li6.4La3Zr1.4Ta0.6O12/Poly(ethylene oxide) solid electrolytes: Enhanced compatibility and cycle performance. J Power Sources 492:9. https://doi.org/10.1016/j.jpowsour.2021.229672
Zuo XX, Wu JH, Ma XD, Deng X, Cai JX, Chen QY, Liu JS, Nan JM (2018) A poly(vinylidene fluoride)/ethyl cellulose and amino-functionalized nano-SiO2 composite coated separator for 5 V high-voltage lithium-ion batteries with enhanced performance. J Power Sources 407:44–52. https://doi.org/10.1016/j.jpowsour.2018.10.056
Duan H, Yin YX, Zeng XX, Li JY, Shi JL, Shi Y, Wen R, Guo YG, Wan LJ (2018) In-situ plasticized polymer electrolyte with double-network for flexible solid-state lithium-metal batteries. Energy Storage Mater 10:85–91. https://doi.org/10.1016/j.ensm.2017.06.017
Chen L, Li YT, Li SP, Fan LZ, Nan CW, Goodenough JB (2018) PEO/garnet composite electrolytes for solid-state lithium batteries: from "ceramic-in-polymer" to "polymer-in-ceramic.". Nano Energy 46:176–184. https://doi.org/10.1016/j.nanoen.2017.12.037
Kaplan WD, Chatain D, Wynblatt P, Carter WC (2013) A review of wetting versus adsorption, complexions, and related phenomena: the rosetta stone of wetting. J Mater Sci 48:5681–5717. https://doi.org/10.1007/s10853-013-7462-y
Cao L, Wu H, Yang PF, He XY, Li JZ, Li Y, Xu MZ, Qiu M, Jiang ZY (2018) Graphene oxide-based solid electrolytes with 3D prepercolating pathways for efficient proton transport. Adv Funct Mater 28:10. https://doi.org/10.1002/adfm.201804944
Bae J, Li YT, Zhang J, Zhou XY, Zhao F, Shi Y, Goodenough JB, Yu GH (2018) A 3D nanostructured hydrogel-framework-derived high-performance composite polymer lithium-ion electrolyte. Angew Chem-Int Edit 57:2096–2100. https://doi.org/10.1002/anie.201710841
Bae J, Li YT, Zhao F, Zhou XY, Ding Y, Yu GH (2018) Designing 3D nanostructured garnet frameworks for enhancing ionic conductivity and flexibility in composite polymer electrolytes for lithium batteries. Energy Storage Mater 15:46–52. https://doi.org/10.1016/j.ensm.2018.03.016
Huang Z, Pang W, Liang P, Jin Z, Grundish N, Li Y, Wang C-A (2019) A dopamine modified Li6.4La3Zr1.4Ta0.6O12/PEO solid-state electrolyte: enhanced thermal and electrochemical properties. J Mater Chem A 7:16425–16436. https://doi.org/10.1039/c9ta03395e
Zhang Z, Zhang S, Geng S, Zhou S, Hu Z, Luo J (2022) Agglomeration-free composite solid electrolyte and enhanced cathode-electrolyte interphase kinetics for all-solid-state lithium metal batteries. Energy Storage Mater 51:19–28. https://doi.org/10.1016/j.ensm.2022.06.025
Quinete N, Orata F, Maes A, Gehron M, Bauer KH, Moreira I, Wilken RD (2010) Degradation studies of new substitutes for perfluorinated surfactants. Arch Environ Contam Toxicol 59:20–30. https://doi.org/10.1007/s00244-009-9451-3
Yang Q, Hu J, Meng J, Li C (2021) C–F-rich oil drop as a non-expendable fluid interface modifier with low surface energy to stabilize a Li metal anode. Energ Environ Sci 14:3621–3631. https://doi.org/10.1039/d0ee03952g
Tonanon N, Tanthapanichakoon W, Yamamoto T, Nishihara H, Mukai SR, Tamon H (2003) Influence of surfactants on porous properties of carbon cryogels prepared by sol–gel polycondensation of resorcinol and formaldehyde. Carbon 41:2981–2990. https://doi.org/10.1016/s0008-6223(03)00422-6
Kango S, Kalia S, Celli A, Njuguna J, Habibi Y, Kumar R (2013) Surface modification of inorganic nanoparticles for development of organic–inorganic nanocomposites—a review. Prog Polym Sci 38:1232–1261. https://doi.org/10.1016/j.progpolymsci.2013.02.003
Li RG, Guo ST, Yu L, Wang LB, Wu DB, Li YQ, Hu XL (2019) Morphosynthesis of 3D macroporous garnet frameworks and perfusion of polymer-stabilized lithium salts for flexible solid-state hybrid electrolytes. Adv Mater Interfaces 6:8. https://doi.org/10.1002/admi.201900200
Lu C, Chiang SW, Du H, Li J, Gan L, Zhang X, Chu X, Yao Y, Li B, Kang F (2017) Thermal conductivity of electrospinning chain-aligned polyethylene oxide (PEO). Polymer 115:52–59. https://doi.org/10.1016/j.polymer.2017.02.024
Choi J-H, Lee C-H, Yu J-H, Doh C-H, Lee S-M (2015) Enhancement of ionic conductivity of composite membranes for all-solid-state lithium rechargeable batteries incorporating tetragonal Li7La3Zr2O12 into a polyethylene oxide matrix. J Power Sources 274:458–463. https://doi.org/10.1016/j.jpowsour.2014.10.078
Zha WP, Chen F, Yang DJ, Shen Q, Zhang LM (2018) High-performance Li6.4La3Zr1.4Ta0.6O12/poly(ethylene oxide)/succinonitrile composite electrolyte for solid-state lithium batteries. J Power Sources 397:87–94. https://doi.org/10.1016/j.jpowsour.2018.07.005
Khan K, Fu B, Xin H, Beshiwork BA, Hanif MB, Wu J, Fang Z, Yang J, Li T, Chen C, Motola M, Xu Z, Wu M (2023) Composite polymer electrolyte incorporating WO3 nanofillers with enhanced performance for dendrite-free solid-state lithium battery. Ceram Int 49:4473–4481. https://doi.org/10.1016/j.ceramint.2022.09.333
Yi SH, Xu TH, Li L, Gao MM, Du K, Zhao HL, Bai Y (2020) Fast ion conductor modified double-polymer (PVDF and PEO) matrix electrolyte for solid lithium-ion batteries. Solid State Ion 355:10. https://doi.org/10.1016/j.ssi.2020.115419
Funding
This work was supported by the National Natural Science Foundation of China (Grant Nos. 51874358 and 51772333).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wang, X., Hu, G., Peng, Z. et al. An agglomeration-free and high ion conductive ceramic-in-polymer composite solid electrolyte modified by fluorocarbon surfactant for enhancing performance of all-solid-state lithium batteries. Ionics 29, 3129–3142 (2023). https://doi.org/10.1007/s11581-023-05105-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11581-023-05105-9