Abstract
Proton diffusion in the co-PNIS85/15 membrane was investigated in the temperature range from 200 to 363 K at different water contents (4 ≤ λ ≤ 21) using 1H SFG NMR diffusometry. At high hydration values, above the threshold value λ0 = 10.5, the ln(DNMR(1/T)) dependences show two different activation modes, separated by a crossover point Tcr ≈ 250–260 K. At temperatures above Tcr, the activation energy is Ea ~ 0.20 eV, which is close to the value for bulk water (Ea ~ 0.17 eV). At temperatures below the crossover point, the ln(DNMR(1/T)) dependences for different water contents merge together into one straight line characterized by a much higher Ea = 0.46 eV. At low hydration values λ < λ0, the activation energies for the high-temperature and low-temperature modes converge, so that at λ = 4, the dependence ln(DNMR(1/T)) is described by one straight line throughout the studied temperature range with Ea = 0.38 eV. A model is proposed that phenomenologically describes the diffusion in the co-PNIS membrane at different moisture contents λ.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Numerous recent studies indicate that proton-conducting hydrocarbon membranes are a promising alternative to Nafion-type perfluorinated sulfopolymers for use in hydrogen-air proton exchange membrane fuel cells (PEMFC) [1,2,3,4,5,6,7,8,9,10]. Moreover, recent studies demonstrate the fabrications of effective PEMFC using hydrocarbon membranes [11,12,13]. Considering that the production and disposal of hydrocarbon polymers is much easier and cheaper than for perfluorinated sulfopolymers, the transition to these membranes would significantly expand the commercial use of fuel cells on their basis. Among the various classes and types of hydrocarbon polymers, the polymers based on sulfonated co-polynaphthoyleneimide (co-PNIS) are of particular interest for PEMFC application due to their good transport characteristics and particular due to oxidative, hydrolytic, and thermal stability [2, 14,15,16,17,18,19]. These polymers are formed by hydrophobic (4,4′-methylenebisanthranilic acid, MDAC) and hydrophilic (4,4′–diaminodiphenyl ether-2,2′-disulfonic acid, ODAS) blocks as presented in Fig. 1. Varying their ratio x = ODAS/MDAC changes both the mechanical properties and the transport characteristics of these membranes.
Water diffusivity is one of the most important characteristics of the proton exchange membrane for PEMFC operation, because in addition to the water produced at the cathode by the electrochemical reaction, the migrating proton also transports 3 to 5 water molecules by the electroosmotic mechanism under the influence of the electric field [20, 21]. Thus, a water concentration gradient is created in the membrane with an excess at the cathode and a deficit at the anode. This can lead both to a decrease in proton conductivity and to a slowing down of the electrochemical reaction at the cathode as a result of its flooding.
Humidity control is very important for the correct operation of the PEMFC, since with decreasing humidity, not only the transport characteristics of the membrane, but also the capacity of PEMFC based on it, decrease. Moreover, since the dependence of transport characteristics of proton exchange membranes on humidity is nonlinear, at humidity less than 50%, this effect is observed both for membranes based on sulfonated hydrocarbon polymers and on perfluorinated ones [22]. Authors in [23, 24] discuss strategies for effectively controlling and managing the amount of water during fuel cell operation.
The equalization of the H2O concentration occurs due to the reverse flux, which requires a high diffusion coefficient for optimal PEMFC performance. Thus, determining the self-diffusion coefficient of water in these polymers is an important task.
In addition to interest in applied science, the study of diffusion coefficients in hydrocarbon polymers is of undoubted interest from a fundamental point of view. Water in these membranes is in a confined state with characteristic pore sizes of ~ 2–50 nm [25,26,27,28]. The study of diffusion processes in systems with water in a confined state is important also for fundamental understanding of processes in biology, mineralogy, oil and gas industry, etc., when water is enclosed in confinements of similar size.
1H NMR diffusometry using a static or pulsed magnetic field gradient is one of the established methods of determining diffusion coefficients of protons. It allows studies in a wide temperature range and at different moisture content in the sample, which in proton exchange membranes is usually described by the number of water molecules per sulfonic group λ = NH2O/NSO3 [29,30,31,32].
Several NMR studies of Nafion proton exchange membranes show that the diffusion coefficient (DNMR) at a fixed temperature has a strongly nonlinear dependence on λ: when the membrane is saturated at 100% humidity, corresponding to values of λ between 7 and 25, depending from the wetting time, DNMR(λ) has a weak and almost linear dependence on λ. For a polymer saturated at low humidity (RH less than 80%), when the corresponding λ values vary between 4 and 6, a change in the slope of the DNMR(λ) dependence is observed. In the case of saturation at even lower humidity, when λ ≤ 3, a sharp, several orders of magnitude drop in DNMR is observed when λ changes from 3 to 1 [29,30,31,32].
This DNMR behavior correlates with the assumption that there are three types of water states in Nafion (depending on the interaction with the polymer matrix) [33,34,35]:
-
Bulk-like water in the center of the channels that is not bound to the polymer
-
Water weakly bound to the polymer
-
Water strongly bound to sulfonic groups (SO3) of polymer side chains (near wall water)
At high moisture content, the probability of finding a proton in a water molecule is 28 times higher than in any ionic states of H+ [28]. Therefore, the 1H DNMR diffusion coefficient measured at λ ≥ 6 can be associated with the self-diffusion coefficient of water molecules in the central part of the channel. At low (λ ≤ 3) and intermediate (4 ≤ λ ≤ 6) moisture content, the contribution to diffusion from intermediate near-wall regions should become more noticeable.
Computer simulations of proton dynamics in Nafion indicate that at low moisture contents (λ ≤ 3), dissociation of water molecules on sulfonic groups occurs, leading to additional proton injection into the water cluster [36]. Thus, it is expected that the contribution of H+ and/or H3O+ ions to the 1H diffusion measured by SFG NMR should increase with decreasing moisture content. This argument is partially supported by the fact the diffusion coefficient at λ ≤ 3 calculated from the proton conductivity Dσ data practically coincides with the DNMR values, i.e., DNMR/Dσ ≈1 [29, 30, 35].
At intermediate humidity (4 ≤ λ ≤ 6) in Nafion-type membranes, the influence on diffusion of weakly bound water and bulk-like water in the central part of the channel increases. In this regard, one can assume that the DNMR data correspond to the transition region (crossover) from proton H+ diffusion to bulk-like H2O self-diffusion. Indeed, such a kink in the DNMR(λ) dependences at a fixed temperature was observed in [31, 32]. The behavior of mobile water molecules and the proton diffusion coefficient in Nafion 117 as a function of temperature at different water contents were analyzed in [37]. The authors suggested that at temperatures below 0 °C, the water molecules are bound, but are unable to form an ice phase because part of their hydrogen bonds is occupied by the oxygen of the sulfonate group and the H+ cation [37].
For hydrocarbon co-PNISx/1−x membranes, it was found that the DNMR(T) dependences for the compositions 60/40, 70/30, and 85/15 at 100% humidity show qualitatively similar behavior among themselves over a wide temperature range [16, 17]. For all three compositions, two temperature intervals, separated by a crossover point around Tcr ≈ 250–260 K, have been observed with activation energy Ea ≈ 0.19 eV above and 0.46 eV below this point [16, 17]. It is important to note two important features found in co-PNIS:
-
1.
At temperatures below the crossover point, the ln(DNMR(1/T)) dependences coincide (within the experimental error) for all three compositions.
-
2.
At temperatures above the crossover point, the DNMR diffusion coefficients for co-PNIS60/40 and co-PNIS70/30 also practically coincide, whereas for the co-PNIS85/15 composition, the diffusion coefficient values are ca. 2.3 times higher than for co-PNIS60/40 and co-PNIS70/30.
The morphology of hydrocarbon membranes is similar to that of Nafion [25,26,27,28] and can also be described by a system of nanoscale channels where three main water states can be assumed at high water content: bulk-like water in the central part of the channel, weakly bound (transition layer) water, and strongly bound (near wall) water. The volume fraction of these fractions will depend on the ratio of hydrophobic and hydrophilic blocks and the fraction of near-wall water on the concentration of sulfonic SO32− groups. Measurements of the IEC value, which characterizes the concentration of sulfonic groups, show that this parameter is almost the same for all compositions studied [28] and is at a level of 2.5, so we can assume that for all compositions, the fraction of near-wall water is approximately the same. Therefore, these data allow one to assume that at low temperatures, the water in the center of the channels freezes. Below, the crossing point (which depends on the fraction of bulk-like water) proton diffusion thus only occurs in a thin non-freezable interfacial layer, where the thickness of this layer does not depend on the composition 14. If this assumption is correct, then a decrease in moisture content should also lead to an increase in the contribution of near-wall diffusion and, consequently, an increase in the activation energy to values of ~ 0.46 eV.
The goal of this work was to investigate the temperature dependences of DNMR in hydrocarbon co-PNIS membranes at different moisture contents and to clarify the micro-mechanism of the diffusion process and the water management conditions during its operation in PEMFC. The study has been done using co-PNIS85/15 membrane, which has a higher diffusion coefficient at room temperature, allowing the diffusion to be traced to lower moisture content values.
Methods
Details of the synthesis of co-PNIS85/15 membranes were presented earlier [17]. For NMR studies, 4 × 8 mm2 membrane samples were prepared, stacked 1 mm thick and tightly inserted into 5-mm NMR ampoules flattened to 1 mm from the end. The samples were dried at 150 °C in a vacuum oven at 1 mbar for 2 h. By weighing the sample on an analytical scale and knowing the weight of the NMR ampoule, the weight of the dry sample (mdry) was determined. The ampoules with the membrane samples were then sealed over cuvettes of distilled water, avoiding direct contact between the water and the membrane. Periodically, the NMR sample ampoules were weighed (mwet). Moisture saturation of the membranes was carried out until the desired values of λ were reached, using Eq. (1):
where μ = 18 g/mol is the molar mass of water, IEC = 2.66 meq/g is ion exchange capacity of the co-PNIS, and n = 0.575 relative fraction of mobile protons, excluding protons contained in the aromatic matrix of a hydrocarbon polymer. The details of determining these parameters are presented in [17], and a general characterization of membranes is presented in [14, 15].
A total of 6 samples with λ = 4.0, 6.5, 8.8, 11.6, 15.8, and 21.0 were made using this technique.
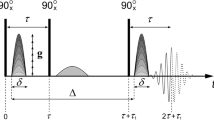
Self-diffusion coefficients were measured using 1H static field gradient (SFG) NMR diffusometry [38], which allows for higher gradient strengths and shorter T1 and T2 spin relaxation times as compared to the pulsed field gradient analog and, thus, enables measurements of samples at low temperatures with smaller diffusivities. To probe the fluctuations of the resonance frequencies arising from the diffusion process in magnetic field gradient, the three-pulse stimulated-echo (STE) experiments have been applied, which involve two evolution times tp separated by the variable mixing time tm [17]. The measurements were conducted at a resonance frequency of 173 MHz and two gradients of the magnetic field (G = 132 T/m and g = 75 T/m), using a special setup with an anti-Helmholtz arrangement of superconducting coils. The length of the 90° pulses was 0.7 μs. The temperature was set with an accuracy of ± 1 K in the range from 200 to 363 K and stabilized to ± 0.2 K using a N2 flow cryostat. To avoid effects, like polarization transfer among mobile and framework protons and spin relaxation, which, in addition to molecular diffusion, may result in a damping of the observed echo amplitude S in experimental practice, we used an approach, which takes into account exchange between fast and slow proton species. It was initially proposed in [39, 40] and proved to be well suited for SFG NMR studies of diffusion in co-PINS systems with protonated framework as we presented in the previous work [17].
In order to successfully apply this model to SFG NMR diffusometry, it is necessary to perform several STE experiments with different evolution times and gradient strengths. Exemplary, STE decays obtained for co-PNIS85/15 at T = 260 K and λ = 6.6, for evolution times tp of 50 and 100 μs and two field gradients G and g, show not only the expected dependence on the evolution time and the gradient strength but also allow to estimate additional parameters, like relaxation times and exchange rates among mobile and immobile protons (Fig. 2).
Example of STE decays for co-PNIS85/15 with λ = 6.5 at 260 K measured for two evolution times and two magnetic field gradients together with fits using model [17]
Results and discussion
The results for diffusion in the co-PNIS85/15 membrane at different moisture contents from 21 to 4 are shown in Fig. 3 as an Arrhenius plot. It can be seen that at λ values between 21 and 11.6, the ln(DNMR(1/T)) dependences have two modes with different activation energies, and a crossover point at Tcr. At temperatures above the crossover point, the temperature dependences of ln(DNMR(1/T)) are almost parallel with a slight increase in activation energy from 0.19 to 0.22 eV while decreasing λ from 21 to 11.6.
In this case, the activation energy of diffusion for the co-PNIS85/15 membrane is close to the activation energy of 0.17 eV of bulk water [41]. Consequently, in this membrane, like in Nafion, the diffusion coefficient at high λ values can be associated with self-diffusion of water molecules. In the co-PNIS85/15 membrane, this behavior is observed at λ higher than 11.6, while in Nafion, it sets in already at lower λ, starting from 6 [29, 42]. This difference in moisture content required to achieve the bulk-like behavior of DNMR probably indicates wider transport channels in co-PNIS85/15 compared to those in Nafion, since it takes more water to fill them. Figure 3 also shows that at temperatures below the crossover point and moisture contents of λ ≥ 11.6, the ln(DNMR(1/T)) dependences for the co-PNIS85/15 membrane practically coincide and are characterized by an activation energy of ~ 0.46 eV.
Similar behavior of the ln(DNMR(1/T)) dependences is observed for Nafion at T ≤ 263 K and λ in the range from 4 to 12 (6–21% by weight) [42]. The Girke cluster-channel model assumes that below the crossover point, some of the water freezes, while some remains in the liquid state [42]. From the coincidence of the dependences ln(DNMR(1/T)) below the crossover point, the assumption was made that regardless of the initial value of λ, the same amount of supercooled water remains, and the rest of the water freezes inside the hydrophilic areas of the membrane [42]. Some evidence for this assumption could be obtained by studying DNMR(T) in Nafion at low-humidity content (λ ≤ 4), but such measurements have not been carried out in [42]. We performed this study on the co-PNIS85/15 membrane to establish the proton diffusion behavior at lower hydration level.
At λ ≈ 8.8, the ln(DNMR(1/T)) dependence also shows two temperature regimes with different activation energies. However, the high-temperature regime is characterized by higher (0.25 eV) and the low-temperature regime by lower (0.40 eV) activation energies than for samples with λ ≥ 11.6 (Fig. 3). The low-temperature regime/mode no longer coincides with the regime at λ ≥ 11.6. The trend toward equalization of the activation energies for the high-temperature and low-temperature regimes continues as the moisture content decreases further, so that, at λ ≈ 4.0, the crossover point becomes indistinguishable and the ln(DNMR(1/T)) dependence is described by one line with an activation energy of 0.38 eV (Fig. 3). Similarly, it was observed for water in silica pores with diameters d > 2 nm that a water fraction in the pore center freezes and substantially affects the diffusion of the non-freezable water fraction near the pore walls, which is characterized by an activation energy of ~ 0.4 eV below the freezing point [43].
Thus, we can assume that there is some critical value of λ0 separating two different regimes of the diffusion mechanism. The value can be estimated from analysis of diffusion as a function of λ; see Fig. 4. One can see that for different temperatures above Tcr, the DNMR(λ) dependences have a kink at λ0 ≈ 10.5, which may be an indication of a change in the diffusion micromechanism. At moisture content higher than λ0, above the crossover point, the diffusion is comparable to the diffusion of bulk water. At moisture content below λ0, the DNMR is characterized by a less pronounced change in the slope at the crossover point or by the absence of this point at all. Below some threshold values of Tcr and λ0, the activation energy does not change in the entire temperature range studied, indicating that the diffusion micromechanism is identical at different temperatures in samples with low hydration.
The observed evolution of the temperature dependences ln(DNMR(1/T)) in the co-PNIS85/15 membrane for different λ can be interpreted using the model that assumes the existence of different water states in these polymers [33,34,35]: bulk water in the central part of the channels, strongly bound water on the surface and on the sulfonic groups, and weakly bound water between them. From our NMR experiment, we cannot separate the weakly bound water for the following reasons: the probably small amount of this water, the rapid exchange with other water states, and the polarization transfer among protons, which is inevitable in NMR experiment. Therefore, we will consider a simplified model with two different water states: bulk-like water in the central part of the channels and surface water.
Numerous studies show that at low temperatures in Nafion membranes, the bulk-like water is not in a crystalline, but in a glassy state [32, 44], enclosed in channels and in clusters with characteristic sizes of 1–4 nm [45, 46]. This fact is in general agreement with the observation that severely confined water undergoes a glass transition in various nanoscaled matrices with sizes below ca. 2 nm [47,48,49,50,51,52,53], while, in slightly larger pores with sizes of a few nanometers, a water fraction forms strongly disordered ice phases, e.g., stacking disordered ice comprising hexagonal and cubic forms, and coexists with a liquid water fraction [54, 55]. Assuming co-PNIS membranes, our previous study of the co-PNIS85/15 revealed no anisotropic and Q-dependent diffusion, indicating homogeneous proton transport in this membrane on a three-dimensional system of transport channels and lack of large cluster areas [17]. Therefore, based on the results obtained in this work (Figs. 3 and 4), the following generalized model of proton diffusion in co-PNIS membranes with varying temperature and/or moisture content can be assumed. The proton transport in these polymers can be considered as migration in a three-dimensional isotropic mesh formed by nanoscaled channels. Figure 5 provides a qualitative view of the cross-section of the nanoscale channel of co-PNIS membranes at different temperatures and moisture contents. In this model, it is assumed that bulk-like water is in the center (inner circle), and surface strongly bonded water is on the walls, and it is concentrated near the sulfonic groups (outer circle).
At high hydration levels (λ ≥ 10.5), water exists in both states, but the main mass of water is in the bulk-like state; see Fig. 5a. At temperatures above the crossover point (T > Tcr), protons of H2O molecules located in the central part of the pore dominate and determine the self-diffusion coefficient. The degree of filling of the central region of the channel affects the homogeneity of the hydrogen bonding network along which the proton diffusion occurs. The homogeneity of the hydrogen bonding network worsens with decreasing λ, leading to an almost parallel shift downwards of the ln(DNMR(1/T)) dependences (Fig. 3).
The bulk-like state of water in the center of the co-PNIS85/15 nanochannel is not completely identical to that of ordinary bulk water, first, because of volume limitations on the order of a few nanometers, and second, because of the large concentration of injected protons arising from the SO3H group dissociation with sufficient water content (λ ≥ 3) [35, 36, 56, 57].
Therefore, when the temperature drops below the crossover point (T < Tcr), water in the central part of the channel does not form an ordered crystal, but rather forms a substantially disordered solid state, which has a very short spin–spin relaxation time T2 so that it does not contribute to our NMR diffusion measurements. Consequently, the measured diffusion coefficients at temperatures below the crossover point characterize the transport of bound water in the near-wall region, whose movements are hindered, resulting in a higher activation energy than that of bulk water. The thickness of the non-freezable surface water layer hardly depends on the diameter of the channels. Consequently, at high moisture content, when the filled central part of the channel freezes as the temperature decreases, the thickness of the layer of mobile surface water will not change regardless of how much water is frozen in the central part. Therefore, at temperatures below the crossover point and moisture content λ ≥ 10.5, the diffusion process will be characterized by close DNMR and activation energy values, regardless of the initial membrane hydration value (Fig. 5a, T < Tcr), as observed in the experiment (Fig. 3, λ = 21, 15.8 and 11.6).
At intermediate moisture values below the boundary value λ < λ0, but still high enough to fill the entire wall area, the bulk-like water share in the channel center becomes less than the surface water share, and the bulk-like water contribution to diffusion decreases. This leads to an increase in the activation energy at temperatures above Tcr (Fig. 5b, T > Tcr). As the temperature decreases, the freezing of the water remaining in the central part of the channel will no longer have a significant effect on the measured diffusion coefficient, determined mainly by surface water and water strongly bound to sulfonic groups (Fig. 5b, T < Tcr). Therefore, the temperature dependences of ln(DNMR(1/T)) will have a milder kink, as seen in Fig. 3, for λ = 8.8 and λ = 6.5.
With further decrease in moisture content, λ ≤ 4, the thickness of the surface water layer begins to decrease and it may be interrupted. Then, over the entire temperature range, the diffusion coefficient is determined only by the mobility of surface water, which leads to the disappearance of the kink in the ln(DNMR(1/T)) dependence. The activation energy increases due to the strong interaction of water molecules with sulfonic groups; see Figs. 3 (λ = 4.0) and 5c.
An interesting point is the higher activation energy below Tcr for samples with higher moisture content (at λ = 4, Ea = 0.38 eV whereas at λ > 11.5, Ea = 0.46 eV). According to the proposed model, at low temperatures, the activation energy characterizes diffusion in a thin layer on the walls, the thickness of which does not depend significantly on the moisture content. We assume that at high λ values, water freezing in the central part of the channel increases its volume and thus creates additional pressure on the channel walls. This leads to difficulty of diffusion in the near-wall region and, accordingly, to increase of activation energy.
Conclusion
The temperature dependences of proton diffusion in the co-PNIS85/15 membrane were investigated at different moisture contents λ, ranging from 4 to 21. It was found that at λ > 10.5, there is a crossover in the ln(DNMR(1/T)) dependences at Tcr ≈ 250–260 K characterizing the change in the diffusion micromechanism.
Above the crossover point, the activation energy Ea ~ 0.2 eV is close to the activation energy of self-diffusion of H2O molecules in bulk water (~ 0.17 eV [41]). At temperatures below Tcr, the ln(DNMR(1/T)) dependences for = 11.6, 15.8, and 21 coincide in one common line with an activation energy of ~ 0.46 eV. It should be noted that similar diffusion coefficient dependencies were observed for co-PNISx/1−x membranes when varying the ratio of hydrophilic to hydrophobic blocks [17]. Such behavior of the diffusion coefficient suggests that above the crossover point, the measured diffusion coefficient corresponds to bulk-like water in the central part of the transport channel. At temperatures below the crossover point, this bulk-like water freezes and diffusion occurs only in the near-surface region of the transport channels, where water is strongly bound to sulfonic groups. In this case, the mobile near-surface water layer has almost the same thickness regardless of the moisture content, if it is above a certain value. This leads to coincidence of the dependences ln(DNMR(1/T)) at low temperatures. Consistent with these arguments, previous work on water in silica pores [51] reported that DNMR becomes independent of the pore diameter when water fractions in the pore center freeze and restrict diffusion to a narrow layer of non-freezable water at the pore wall.
Analysis of DNMR in dependence on the moisture content at fixed temperatures showed that this scenario holds up to a limit value of λ0 ≈ 10.5, which seems to correspond to the lower limit of bulk-like water presence in the sample. The existence of different types of water in proton conducting polymers has been also previously suggested [33,34,35].
At lower moisture content 4 < λ < λ0, the ln(DNMR(1/T)) dependences retain the crossover point with the two regions having different activation energies, but this difference is much smaller than at λ ≥ λ0. At the lowest studied value of λ ≈ 4, the crossover point disappears, and the diffusion coefficient is described by one activation energy over the entire temperature range. Thus, for water content λ < λ0, the measured diffusion coefficient can be related to the transport of surface protons bound to SO32− groups.
Data availability
All data sets can be accessed at the Institute of Condensed Matter Physics, Technische Universität Darmstadt, Hochschulstr. 6, 64,289, Darmstadt, Germany.
References
Kreuer KD (2001) On the development of proton conducting polymer membranes for hydrogen and methanol fuel cells. J Membr Sci 185:29–39. https://doi.org/10.1016/S0376-7388(00)00632-3
Marestin C, Gebel G, Diat O, Mercier R (2008) Sulfonated polyimides. Adv Polym Sci 216:185–258. https://doi.org/10.1007/12_2008_155
Bauer B, Jones DJ, Roziere J, Tchicaya L, Alberti G, Casciola M, Massinelli L, Peraio A, Besse S, Ramunni E (2000) Electrochemical characterisation of sulfonated polyetherketone membranes. J New Mater Electrochem Syst 3:93–98. https://doi.org/10.1515/9783110825992.93
Sakaguchi Y, Kaji A, Nagahara S, Kitamura K, Takase S, Omote K, Asako Y, Kimura K (2004) Sulfonation of fluorine-containing poly(ether ketone)s and poly(ether nitrile)s for proton-conducting membranes. Polym Prepr 45:20–21
Han J, Lee H, Kim J, Kim S, Kim H, Kim E, Sung YE, Kim K, Lee JC (2020) Sulfonated poly(arylene ether sulfone) composite membrane having sulfonated polytriazole grafted graphene oxide for high-performance proton exchange membrane fuel cells. Journal of Membrane Science 612:118428. https://doi.org/10.1016/j.memsci.2020.118428
Leea KH, Chua JY, Mohanraj V, Kim AR, Song MH, Yoo DJ (2020) Enhanced ion conductivity of sulfonated poly(arylene ether sulfone) block copolymers linked by aliphatic chains constructing wide-range ion cluster for proton conducting electrolytes. Int J Hydrogen Energy 45:29297–29307. https://doi.org/10.1016/j.ijhydene.2020.07.197
Xu S, Adamski M, Killer M, Schibli EM, Frisken BJ, Holdcroft S (2019) Sulfo-phenylated polyphenylenes containing sterically hindered pyridines. Macromolecules 52:2548–2559. https://doi.org/10.1021/acs.macromol.8b02289
Lee KH, Cho DH, Kim YM, Moon SJ, Seong JG, Shin DW, Lee YM (2017) Highly conductive and durable poly(arylene ether sulfone) anion exchange membrane with end-group cross-linking. Energy Environ Sci 10:275–285. https://doi.org/10.1039/c6ee03079c
Roy A, Hickner MA, Yu X, Li Y, Glass TE, McGrath JE (2006) Influence of chemical composition and sequence length on the transport properties of proton exchange membranes. J Polym Sci, Part B: Polym Phys 44:2226–2239. https://doi.org/10.1002/polb.20859
Simari C, Prejanò M, Lufrano E, Sicilia E, Nicotera I (2021) Exploring the structure–performance relationship of sulfonated polysulfone proton exchange membrane by a combined computational and experimental approach. Polymers 13:959–978. https://doi.org/10.3390/polym13060959
Ranganathan H, Vinothkannan M, Kim AR, Subramanian V, Oh M-S, Yoo DJ (2022) Simultaneous improvement of power density and durability of sulfonated poly(ether ether ketone) membrane by embedding CeO2 -ATiO2: a comprehensive study in low humidity proton exchange membrane fuel cells. Int J Energy Res 46(7):9041–9057. https://doi.org/10.1002/er.7781
Kim AR, Vinothkannan M, Lee KH, Chu JY, Park BH, Han MK, Yoo DJ (2022) Enhanced performance and durability of composite membranes containing anatase titanium oxide for fuel cells operating under low relative humidity. Int J Energy Res 46(4):4835–4851. https://doi.org/10.1002/er.7477
Kim AR, Poudel MB, Chu JY, Vinothkannan M, Kumar RS, Logeshwaran N, Park B-H, Han M-K, Yoo DJ (2023) Advanced performance and ultra-high, long-term durability of acid-base blended membranes over 900 hours containing sulfonated PEEK and quaternized poly(arylene ether sulfone) in H2/O2 fuel cells. Composites Part B: Engineering 254:110558. https://doi.org/10.1016/j.compositesb.2023.110558
Ponomarev II, Nikolskii OG, Volkova YuA, Zakharov AV (1994) New rigid-chain copoly(naphthoylenelmidobenzimidazoles) and their films. Polym Sci Ser A 36:1185–1192
Emets VV, Ponomarev II, Grinberg VA, Mayorova NA, Zharinova MY, Volkova YA, Nizhnikovskii EA, Skupov KM, Razorenov DY, Andreev VN (2017) Development of hydrogen–air fuel cells with membranes based on sulfonated polyheteroarylenes. Russ J Electrochem 53:86–91. https://doi.org/10.1134/S1023193517010062
Zavorotnaya UM, Ponomarev II, Volkova YA, Modestov AD, Andreev VN, Privalov AF, Vogel M, Sinitsyn VV (2020) Preparation and study of sulfonated co-polynaphthoyleneimide proton-exchange membrane for a H2/Air fuel cell. Materials (Basel) 13:5297–5308. https://doi.org/10.3390/ma13225297
Zavorotnaya UM, Privalov AF, Kresse B, Vogel M, Ponomarev II, Volkova YA, Sinitsyn VV (2022) Diffusion in sulfonated co-polynaphthoyleneimide proton exchange membranes with different ratios of hydrophylic to hydrophobic groups studied using SFG NMR. Macromolecules 55:8823–8833. https://doi.org/10.1021/acs.macromol.2c01486
Chalykh AE, Petrova TF, Ponomarev II (2022) Water sorption by polyheteroarylenes Polymers 14:2255–2272. https://doi.org/10.3390/polym14112255
Zavorotnaya UM, Ponomarev II, Volkova YA, Sinitsyn VV (2023) Development of high-performance hydrogen-air fuel cell with fluorine-free sulfonated co-polynaphthoyleneimide membrane. Membranes 13:485–498. https://doi.org/10.3390/membranes13050485
Zawodzinski TA, Derouin C, Radzinski S, Sherman RJ, Smith VT, Springer TE, Gottesfeld S (1993) Water uptake by and transport through Nafion® 117 membranes. J Electrochem Soc 140:1041–1047. https://doi.org/10.1149/1.2056194
Zhu X, Zhang H, Zhang Y, Liang Y, Wang X, Yi B (2006) An ultrathin self-humidifying membrane for PEM fuel cell application: fabrication, characterization, and experimental analysis. J Phys Chem B 110:14240–14248. https://doi.org/10.1021/jp061955s
Kim J, Kim K, Han J, Lee H, et. al. (2020) End group cross-linked membranes based on highly sulfonated poly(arylene ether sulfone) with vinyl functionalized graphene oxide as a cross-linker and a filler for proton exchange membrane fuel cell application. J Polym Sci Part A: Polym Chem.: 1–11. https://doi.org/10.1002/pol.20200665
Chen X, Xu J, Fang Y, Li W, Ding Y, Wan Z, Wang X, Tu Z (2022) Temperature and humidity management of PEM fuel cell power system using multi-input and multi-output fuzzy method Applied Thermal Engineering 203:117865. https://doi.org/10.1016/j.applthermaleng.2021.117865
Kwon JH, Eom KS (2021) Effects of oversaturated cathode humidity conditions on the performance degradation of PEMFCs and diagnostic signals of Warburg impedance under low humidity conditions. The Journal of Physical Chemistry C 125(19):10824–10834. https://doi.org/10.1021/acs.jpcc.1c02805
Weissbach T, Tsang EMW, Yang ACC, Narimani R, Frisken BJ, Holdcroft S (2012) Structural effects on the nano-scale morphology and conductivity of ionomer blends. J Mater Chem 22:24348–24355. https://doi.org/10.1039/C2JM31287E
He G, Li Z, Zhao J, Wang S, Wu H, Guiver MD, Jiang Z (2015) Nanostructured ion-exchange membranes for fuel cells: recent advances and perspectives. Adv Mater 27:5280–5295. https://doi.org/10.1002/adma.201501406
Li N, Guiver MD (2014) Ion transport by nanochannels in ion-containing aromatic copolymers. Macromolecules 47:2175–2198. https://doi.org/10.1021/ma402254h
Shin DW, Guiver MD, Lee YM (2017) Hydrocarbon-based polymer electrolyte membranes: importance of morphology on ion transport and membrane stability. Chem Rev 117:4759–4805. https://doi.org/10.1021/acs.chemrev.6b00586
Zawodzinski TA, Neeman M, Sillerud LO, Cottesfeld S (1991) Determination of water diffusion coefficients in perfluorosulfonate ionomeric membranes. J Phys Chem 95:6040–6044. https://doi.org/10.1021/J100168A060
Ochi S, Kamishima O, Mizusaki J, Kawamura J (2009) Investigation of proton diffusion in Nafion®117 membrane by electrical conductivity and NMR. Solid State Ionics 180:580–584. https://doi.org/10.1016/j.ssi.2008.12.035
Saito M, Arimura N, Hayamizu K, Okada T (2004) Mechanisms of ion and water transport in perfluorosulfonated ionomer membranes for fuel cells. J Phys Chem B 108:16064–16070. https://doi.org/10.1021/jp0482565
Saito M, Hayamizu K, Okada T (2005) Temperature dependence of ion and water transport in perfluorinated ionomer membranes for fuel cells. J Phys Chem B 109:3112–3119. https://doi.org/10.1021/jp045624w
Kim YS, Dong LM, Hickner MA, Glass TE, Webb V, McGrath JE (2003) State of water in disulfonated poly(arylene ether sulfone) copolymers and a perfluorosulfonic acid copolymer (Nafion) and its effect on physical and electrochemical properties. Macromolecules 36:6281–6285. https://doi.org/10.1021/ma0301451
Lu Z, Polizos G, Macdonald DD, Manias E (2008) State of water in perfluorosulfonic ionomer (Nafion 117) proton exchange membranes. J Electrochem Soc 155:163–171. https://doi.org/10.1149/1.2815444
Kusoglu A, Weber AZ (2017) New insights into perfluorinated sulfonic-acid ionomers. Chem Rev 117:987–1104. https://doi.org/10.1021/acs.chemrev.6b00159
Paddison SJ (2003) Proton conduction mechanisms at low degrees of hydration in sulfonic acid–based polymer electrolyte membranes. Annu Rev Mater Res 33:289–319. https://doi.org/10.1146/annurev.matsci.33.022702.155102
Chernyak AV, Vasiliev SG, Avilova IA, Volkov VI (2019) Hydration and water molecules mobility in acid form of Nafion membrane studied by 1H NMR techniques. Appl Magn Reson 50:677–693. https://doi.org/10.1007/s00723-019-1111-9
Chang FI, Fujara B, Geil G, Hinze H, Sillescu, (1994) A new perspectives of NMR in ultrahigh static magnetic field gradients. J Non-Cryst Solid 674:172–174. https://doi.org/10.1016/0022-3093(94)90563-0
Edzes HT, Samulski ET (1977) Cross relaxation and spin diffusion in the proton NMR of hydrated collagen. Nature 265:521–523. https://doi.org/10.1038/265521a0
Peschier LJC, Bouwstra JA, de Bleyser J, Junginger HE, Leyte JC (1996) Cross-relaxation effects in pulsed-field-gradient stimulated-echo measurements on water in a macromolecular matrix. J Magn Reson 110:150–157. https://doi.org/10.1006/jmrb.1996.0024
Hardy EH, Zygar A, Zeidler MD, Holz M, Sacher FD (2001) Isotope effect on the translational and rotational motion in liquid water and ammonia. J Chem Phys 114:3174–3181. https://doi.org/10.1063/1.1340584
Nicotera I, Coppola L, Rossi CO, Youssry M, Ranieri GA (2009) NMR investigation of the dynamics of confined water in Nafion-based electrolyte membranes at subfreezing temperatures. J Phys Chem B 113:13935–13941. https://doi.org/10.1021/jp904691g
Weigler M, Winter E, Kresse B, Brodrecht M, Buntkowsky G, Vogel M (2020) Static field gradient NMR studies of water diffusion in mesoporous silica. Phys Chem Chem Phys 22:13989–13998. https://doi.org/10.1039/d0cp01290d
Guillermo A, Geebel G, Mendil-Jakani H, Pinton E (2009) NMR and pulsed field gradient NMR approach of water sorption properties in Nafion at low temperature. J Phys Chem B 113:6710–6717. https://doi.org/10.1021/jp8110452
Gierke DT, Hsu WY (1982) The cluster-network model of ion clustering in perfluorosulfonated membranes. Perfluorinated Ionomer Menbranes ACS 13:283–307. https://doi.org/10.1016/S0376-7388(00)81563-X
Gebel G (2000) Structural evolution of water swollen perfluorosulfonated ionomers from dry membrane to solution. Polymer 41:5829–5838. https://doi.org/10.1016/S0032-3861(99)00770-3
Artemov VG (2017) Dynamical conductivity of confined water. Meas Sci Technol 28:014013–014018. https://doi.org/10.1088/1361-6501/28/1/014013
Chakraborty S, Kumar H, Dasgupta C, Maiti PK (2017) Confined water: structure, dynamics, and thermodynamics. Acc Chem Res 50:2139–2146. https://doi.org/10.1021/acs.accounts.6b00617
Artemov VG, Uykur E, Kapralov PO, Kiselev A et al (2020) Anomalously high proton conduction of interfacial water. J Phys Chem Lett 11:3623–3628. https://doi.org/10.1021/acs.jpclett.0c00910
Chu X-Q, Kolesnikov AI, Moravsky AP, Garcia-Sakai V, Chen S-H (2007) Observation of a dynamic crossover in water confined in double-wall carbon nanotubes. Phys Rev 76:021505–021510. https://doi.org/10.1103/PhysRevE.76.021505
Cerveny S, Mallamace F, Swenson J, Vogel M, Xu L (2016) Confined water as model of supercooled water. Chem Rev 116:7608–7625. https://doi.org/10.1021/acs.chemrev.5b00609
Weigler M, Brodrecht M, Buntkowsky G, Vogel M (2019) Reorientation of deeply cooled water in mesoporous silica: NMR studies of the pore-size dependence. J Phys Chem C 123:2123–2134. https://doi.org/10.1021/acs.jpcb.8b12204
Yao Y, Fella V, Huang W, Zhang KAI, Landfester K, Butt H-J, Vogel M, Floudas G (2019) Crystallization and dynamics of water confined in model mesoporous silica particles: two ice nuclei and two fractions of water. Langmuir 35:5890–5901. https://doi.org/10.1021/acs.langmuir.9b00496
Jazdzewska M, Domin K, Sliwinska-Bartowiak M, Beskrovnyi AI, Chudoba DM, Nagorna TV, Ludzik K, Neov DS (2019) Structural properties of ice in confinement. J Molecular Liquids 283:167–173. https://doi.org/10.1016/J.MOLLIQ.2019.03.080
Lupi L, Hudait A, Peters B, Grünwald M, Mullen RG, Nguyen AH, Molinero V (2017) Role of stacking disorder in ice nucleation. Nature 551:218–222. https://doi.org/10.1038/nature24279
Eikerling M, Kornyshev YuM (2001) Mechanisms of proton conductance in polymer electrolyte membranes. J Phys Chem B 105:3646–3662. https://doi.org/10.1021/jp003182s
Eikerling M, Kornyshev A (2001) Proton transfer in a single pore of a polymer electrolyte membrane. J Electroanal Chem 502:1–14. https://doi.org/10.1016/S0022-0728(00)00368-5
Funding
Open Access funding enabled and organized by Projekt DEAL. U. Zavorotnaya received financial support from the Interdisciplinary Science Center (DAAD, Funding Decision on Proposal M-2021b-2_d) for her research stay at the Technical University of Darmstadt. Ulyana M. Zavorotnaya and Vitaly V. Sinitsyn received financial support from NRU HSE (project No.23–00-001). Elemental analysis was supported by the RF Ministry of Science and Higher Education employing the equipment of Center for molecular composition studies of INEOS RAS.
Author information
Authors and Affiliations
Contributions
All of the authors have contributed to the development of the research concept. The synthesis of materials is done by Igor I. Ponomarev and sample preparation and measurements performed by Ulyana M. Zavorotnaya, Alexei F. Privalov, and Celine Wolter. The figures are prepared by Ulyana M. Zavorotnaya and Alexei F. Privalov. The manuscript was written by Alexei F. Privalov, Michael Vogel, and Vitaly V. Sinitsyn. All the authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethical approval
The publication has no ethnic issues applicable to both human and/ or animal studies.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zavorotnaya, U.M., Privalov, A.F., Wolter, C. et al. Humidity effect on temperature behavior of proton diffusion coefficient in sulfonated co-polynaphthoyleneimide membranes measured by 1H NMR diffusometry. Ionics 29, 3609–3617 (2023). https://doi.org/10.1007/s11581-023-05084-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11581-023-05084-x