Abstract
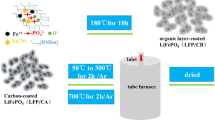
The further development of electrode materials with both high capacity and rate capability is necessary for meeting the continuing requirement for increasing high-energy density and long-cycle life of lithium-ion batteries (LIBs). Herein, a cathode material of LIBs, LiFePO4/C modified with high electrical conductivity compound tantalum carbide (TaC) is successfully synthesized by a hydrothermal method. The co-coating of nano-sized TaC and amorphous carbon layer on the surface of LiFePO4 particles can facilitate good electrons and Li ions transfer, leading to faster electrochemical kinetics. As the cathode material for LIBs, this composite presents both excellent electrochemical performances with high reversible capacity (159.0 mAh g−1, 0.1C) and improved rate capacity. The methodology developed in this paper demonstrates a new prospect for the application of transition metal carbides (TMCs) in the modification of battery electrode materials.





Similar content being viewed by others
References
Wang Y, Wang Y, Hosono E, Wang K, Zhou H (2008) The design of a LiFePO4/carbon nanocomposite with a core-shell structure and its synthesis by an in situ polymerization restriction method. Angew Chem Int Ed Engl 47(39):7461–7465. https://doi.org/10.1002/anie.200802539
Goodenough JB, Kim Y (2010) Challenges for rechargeable Li batteries. Chem Mater 22(3):587–603. https://doi.org/10.1021/cm901452z
Yang L, Tian Y, Chen J, Gao J, Long Z, Deng W, Zou G, Hou H, Ji X (2021) A high-rate capability LiFePO4/C cathode achieved by the modulation of the band structures. J Mater Chem A 9(43):24686–24694. https://doi.org/10.1039/D1TA07757K
Duan J, Tang X, Dai H, Yang Y, Wu W, Wei X, Huang Y (2019) Building safe lithium-ion batteries for electric vehicles: a review. Electrochemical Energy Reviews 3(1):1–42. https://doi.org/10.1007/s41918-019-00060-4
Qian H, Ren H, Zhang Y, He X, Li W, Wang J, Hu J, Yang H, Sari HMK, Chen Y, Li X (2022) Surface doping vs. bulk doping of cathode materials for lithium-ion batteries: a review. Electrochem Energy Rev 6(1):2. https://doi.org/10.1007/s41918-022-00155-5
Xia X, Yang J, Liu Y, Zhang J, Shang J, Liu B, Li S, Li W (2022) Material choice and structure design of flexible battery electrode, Advanced. Science:e2204875. https://doi.org/10.1002/advs.202204875
Saikia D, Deka JR, Chou C-J, Lin C-H, Yang Y-C, Kao H-M (2019) Encapsulation of LiFePO4 nanoparticles into 3D interpenetrating ordered mesoporous carbon as a high-performance cathode for lithium-ion batteries exceeding theoretical capacity. ACS Appl Energy Mater 2(2):1121–1133. https://doi.org/10.1021/acsaem.8b01682
Su D, Liu L, Liu Z, Dai J, Wen J, Yang M, Jamil S, Deng H, Cao G, Wang X (2020) Electrospun Ta-doped TiO2/C nanofibers as a high-capacity and long-cycling anode material for Li-ion and K-ion batteries. J Mater Chem A 8(39):20666–20676. https://doi.org/10.1039/d0ta06327d
Jarolimek K, Risko C (2021) Modification of the LiFePO4 (010) surface due to exposure to atmospheric gases. ACS Appl Mater Interfaces 13(24):29034–29040. https://doi.org/10.1021/acsami.1c01394
Lin Y, Zhang X, Liu Y, Wang Q, Lin C, Chen S, Zhang Y, Li U-s (2022) LiFePO4 batteries via advanced designing of localized high concentration electrolyte. J Colloid Interface Sci 628(Pt B):14–23. https://doi.org/10.1016/j.jcis.2022.08.018
Sun C, Zhang Y, Zhang X, Zhou Z (2010) Structural and electrochemical properties of Cl-doped LiFePO4/C. J Power Sources 195(11):3680–3683. https://doi.org/10.1016/j.jpowsour.2009.12.074
Liu X, Zhang Y, Meng Y, Kang T, Gao H, Huang L, Zhu F (2022) Influence mechanism of Mg2+ doping on electrochemical properties of LiFePO4 cathode materials. ACS Appl Energy Mater 5(7):8452–8459. https://doi.org/10.1021/acsaem.2c00986
Zhang B, Ma X, Hou W, Yuan W, He L, Yang O, Liu Y, Wang J, Xu Y Revealing the ultrahigh rate performance of the La and Ce co-doping LiFePO4 composite. ACS Appl Energy Mater. https://doi.org/10.1021/acsaem.2c02035
Cui Z, Guo X, Ren J, Xue H, Tang F, La P, Li H, Li J, Lu X (2021) Enhanced electrochemical performance and storage mechanism of LiFePO4 doped by Co, Mn and S elements for lithium-ion batteries. Electrochim Acta:388. https://doi.org/10.1016/j.electacta.2021.138592
Oh SW, Myung ST, Oh SM, Oh KH, Amine K, Scrosati B, Sun YK (2010) Double carbon coating of LiFePO4 as high rate electrode for rechargeable lithium batteries. Adv Mater 22(43):4842–4845. https://doi.org/10.1002/adma.200904027
Hu Y, Wang G, Liu C, Chou S-L, Zhu M, Jin H, Li W, Li Y (2016) LiFePO4/C nanocomposite synthesized by a novel carbothermal reduction method and its electrochemical performance. Ceram Int 42(9):11422–11428. https://doi.org/10.1016/j.ceramint.2016.04.075
Li X, Meng Y, Chen X, Wang Y, Xiao D (2022) Nano-LiFePO4/C derived from gaseous-oxidation engineering-synthesized amorphous mesoporous nano-FePO4 for high-rate Li-ion batteries. Ind Eng Chem Res 61(26):9311–9321. https://doi.org/10.1021/acs.iecr.2c01006
Cao J, Qu Y, Guo R (2012) La0.6Sr0.4CoO3-δ modified LiFePO4/C composite cathodes with improved electrochemical performances. Electrochim Acta 67:152–158. https://doi.org/10.1016/j.electacta.2012.02.031
Jin Y, Yang C, Rui X, Cheng T, Chen C (2011) V2O3 modified LiFePO4/C composite with improved electrochemical performance. J Power Sources 196(13):5623–5630. https://doi.org/10.1016/j.jpowsour.2011.02.059
Yin Y, Gao M, Pan H, Shen L, Ye X, Liu Y, Fedkiw PS, Zhang X (2012) High-rate capability of LiFePO4 cathode materials containing Fe2P and trace carbon. J Power Sources 199:256–262. https://doi.org/10.1016/j.jpowsour.2011.10.042
Pan F, Chen X, Li H, Xin X, Chang Q, Jiang K, Wang W (2011) Influence of carbon coating porosity on the electrochemical performance of LiFePO4 cathode. Electrochem Commun 13(7):726–729. https://doi.org/10.1016/j.elecom.2011.04.021
Lin Y, Lin Y, Zhou T, Zhao G, Huang Y, Huang Z (2013) Enhanced electrochemical performances of LiFePO4/C by surface modification with Sn nanoparticles. J Power Sources 226:20–26. https://doi.org/10.1016/j.jpowsour.2012.10.074
Zhang M, Garcia-Araez N, Hector AL, Owen JR (2017) A sol-gel route to titanium nitride conductive coatings on battery materials and performance of TiN-coated LiFePO4. J Mater Chem A 5(5):2251–2260. https://doi.org/10.1039/c6ta09572k
Ren X, Li Y, Xi X, Yang J, Wang S, Liu S, Zheng J, He Z, Yang Y, Wang T, Wu Q (2023) High performance LiFePO4 nanomaterial obtained by a tavorite-to-olivine phase transition at low-temperature. Chem Eng J:453. https://doi.org/10.1016/j.cej.2022.139611
Liu C, An J, Guo R, Li Y, Liu L (2013) Enhanced electrochemical performance of LiFePO4/C cathode material modified with highly conductive TiN. J Alloys Compd 563:33–38. https://doi.org/10.1016/j.jallcom.2013.02.080
Huang H, Feng T, Gan Y, Fang M, Xia Y, Liang C, Tao X, Zhang W (2015) TiC/NiO core/shell nanoarchitecture with battery-capacitive synchronous lithium storage for high-performance lithium-ion battery. ACS Appl Mater Interfaces 7(22):11842–11848. https://doi.org/10.1021/acsami.5b01372
Hunt ST, Nimmanwudipong T, Roman-Leshkov Y (2014) Engineering non-sintered, metal-terminated tungsten carbide nanoparticles for catalysis. Angew Chem Int Ed Engl 53(20):5131–5136. https://doi.org/10.1002/anie.201400294
Naguib M, Halim J, Lu J, Cook KM, Hultman L, Gogotsi Y, Barsoum MW (2013) New two-dimensional niobium and vanadium carbides as promising materials for Li-ion batteries. J Am Chem Soc 135(43):15966–15969. https://doi.org/10.1021/ja405735d
Liu Y, Kelly TG, Chen JG, Mustain WE (2013) Metal carbides as alternative electrocatalyst supports. ACS Catal 3(6):1184–1194. https://doi.org/10.1021/cs4001249
Vallance SR, Round DM, Ritter C, Cussen E, Kingman S, Gregory DH (2009) Ultrarapid microwave synthesis of superconducting refractory carbides. Adv Mater 21(44):4502–4504. https://doi.org/10.1002/adma.200900968
Kim B-R, Woo K-D, Doh J-M, Yoon J-K, Shon I-J (2009) Mechanical properties and rapid consolidation of binderless nanostructured tantalum carbide. Ceram Int 35(8):3395–3400. https://doi.org/10.1016/j.ceramint.2009.06.012
Tao X, Du J, Li Y, Yang Y, Fan Z, Gan Y, Huang H, Zhang W, Dong L, Li X (2011) TaC nanowire/activated carbon microfiber hybrid structures from bamboo fibers. Adv Energy Mater 1(4):534–539. https://doi.org/10.1002/aenm.201100191
Snyder MQ, Trebukhova SA, Ravdel B, Wheeler MC, DiCarlo J, Tripp CP, DeSisto WJ (2007) Synthesis and characterization of atomic layer deposited titanium nitride thin films on lithium titanate spinel powder as a lithium-ion battery anode. J Power Sources 165(1):379–385. https://doi.org/10.1016/j.jpowsour.2006.12.015
Yang LX, Liu RJ, Bu HP, Liu HJ, Zeng CL, Fu C (2022) TiC Nanomaterials with varying dimensionalities as anode materials for lithium-ion batteries. ACS Appl Nano Mater 5(8):11787–11796. https://doi.org/10.1021/acsanm.2c02781
Liu Y, Zhang M, Li Y, Hu Y, Zhu M, Jin H, Li W (2015) Nano-sized LiFePO4/C composite with core-shell structure as cathode material for lithium ion battery. Electrochim Acta 176:689–693. https://doi.org/10.1016/j.electacta.2015.07.064
Gaberscek M, Dominko R, Jamnik J (2007) Is small particle size more important than carbon coating? An example study on LiFePO4 cathodes. Electrochem Commun 9(12):2778–2783. https://doi.org/10.1016/j.elecom.2007.09.020
Liu H, Wang G, Wexler D, Wang J, Liu H-K (2008) Electrochemical performance of LiFePO4 cathode material coated with ZrO2 nanolayer. Electrochem Commun 10(1):165–169. https://doi.org/10.1016/j.elecom.2007.11.016
Song G-M, Wu Y, Xu Q, Liu G (2010) Enhanced electrochemical properties of LiFePO4 cathode for Li-ion batteries with amorphous NiP coating. J Power Sources 195(12):3913–3917. https://doi.org/10.1016/j.jpowsour.2009.12.089
Belharouak I, Johnson C, Amine K (2005) Synthesis and electrochemical analysis of vapor-deposited carbon-coated LiFePO4. Electrochem Commun 7:983–988. https://doi.org/10.1016/j.elecom.2005.06.019
Chang H-H, Chang C-C, Su C-Y, Wu H-C, Yang M-H, Wu N-L (2008) Effects of TiO2 coating on high-temperature cycle performance of LiFePO4-based lithium-ion batteries. J Power Sources 185(1):466–472. https://doi.org/10.1016/j.jpowsour.2008.07.021
Meng H, Zhou P, Zhang Z, Tao Z, Chen J (2017) Preparation and characterization of LiNi0.8Co0.15Al0.05O2 with high cycling stability by using AlO2- as Al source. Ceram Int 43(4):3885–3892. https://doi.org/10.1016/j.ceramint.2016.12.054
Zhang Y, Wang W, Li P, Fu Y, Ma X (2012) A simple solvothermal route to synthesize graphene-modified LiFePO4 cathode for high power lithium ion batteries. J Power Sources 210:47–53. https://doi.org/10.1016/j.jpowsour.2012.03.007
Jin B, Jin EM, Park K-H, Gu H-B (2008) Electrochemical properties of LiFePO4-multiwalled carbon nanotubes composite cathode materials for lithium polymer battery. Electrochem Commun 10(10):1537–1540. https://doi.org/10.1016/j.elecom.2008.08.001
Dathar GKP, Sheppard D, Stevenson KJ, Henkelman G (2011) Calculations of Li-ion diffusion in olivine phosphates. Chem Mater 23(17):4032–4037. https://doi.org/10.1021/cm201604g
Funding
The authors are grateful for the financial support from the National Natural Science Foundation of China (No. 21908142).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Liu, Y., Qi, C., Cai, D. et al. TaC-modified LiFePO4/C composite as cathode material for high-performance lithium-ion batteries. Ionics 29, 2191–2198 (2023). https://doi.org/10.1007/s11581-023-04969-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11581-023-04969-1