Abstract
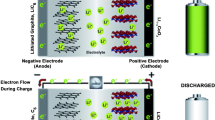
Li-ion diffusion in electrodes and separator plays a key role in determining the charge/discharge characteristics of a Li-ion cell. Past papers have analyzed solution-phase limitation diffusion in a Li-ion cell for constant current conditions. In the present work, an analytical model is presented for concentration diffusion under solution-phase limitation in a three-layer structure under arbitrary, time-dependent current conditions that may be encountered in practical scenarios. The eigenvalue-based solution is shown to agree well with numerical simulations and may be suitable for implementation in practical battery management systems since only a few eigenvalues are shown to offer excellent accuracy. Computed concentration distributions are analyzed for sinusoidal and step function current profiles. The impact of electrode porosities on concentration distribution is also investigated. This work improves the theoretical understanding of diffusion in Li-ion cells and offers practical tools for modeling and optimization of electrochemical energy conversion and storage devices.










Similar content being viewed by others
Data availability
Data and material are available upon request from the corresponding author.
Code availability
Code is available upon request from the corresponding author.
Abbreviations
- Brugg :
-
Bruggeman coefficient
- c i :
-
Concentration in region i (mol m−3)
- c i,in :
-
Initial concentration (mol m−3)
- C i :
-
Dimensionless concentration in region i
- C i,in :
-
Dimensionless initial concentration in region i
- c 0 :
-
Reference concentration (mol m-3)
- D :
-
Diffusion coefficient of lithium ions in the electrolyte (m2 s−1)
- D eff,i :
-
Effective diffusion coefficient of lithium ions in each layer (m2 s−1)
- F :
-
Faraday’s constant (C mol−1)
- i app :
-
Applied current density (A m−2)
- J i :
-
Dimensionless flux density in region i
- K :
-
Ratio of electrode thicknesses
- L :
-
Total width of cell (m)
- t :
-
Time (s)
- t + :
-
Transference number
- x :
-
Position (m)
- ε i :
-
Porosity in region i
- γ i :
-
Dimensionless position at the interface i
- λ n :
-
Eigenvalue
- τ :
-
Dimensionless time
- ξ :
-
Dimensionless position
References
Doyle M, Newman J (1997) Analysis of capacity–rate data for lithium batteries using simplified models of the discharge process. J Appl Electrochem 27:846–856. https://doi.org/10.1023/A:1018481030499
Jokar A, Rajabloo B, Désilets M, Lacroix M (2016) Review of simplified pseudo-two-dimensional models of lithium-ion batteries. J Power Sources 327:44–55. https://doi.org/10.1016/j.jpowsour.2016.07.036
Zhang J, Lee J (2011) A review on prognostics and health monitoring of Li-ion battery. J Power Sources 196:6007–6014. https://doi.org/10.1016/j.jpowsour.2011.03.101
Ramadesigan V, Northrop PWC, De S, Santhanagopalan S, Braatz RD, Subramanian VR (2012) Modeling and simulation of lithium-ion batteries from a systems engineering perspective. J Electrochem Soc 159. https://doi.org/10.1149/2.018203jes
Seaman A, Dao TS, McPhee J (2014) A survey of mathematics-based equivalent-circuit and electrochemical battery models for hybrid and electric vehicle simulation. J Power Sources 256:410–423. https://doi.org/10.1016/j.jpowsour.2014.01.057
Rahman MA, Anwar S, Izadian SA (2016) Electrochemical model parameter identification of a lithium-ion battery using particle swarm optimization method. J Power Sources 307:86–97. https://doi.org/10.1016/j.jpowsour.2015.12.083
Botte GG, Subramanian VR, White RE (2000) Mathematical modeling of secondary lithium batteries. Electrochim Acta 45:2595–2609. https://doi.org/10.1016/s0013-4686(00)00340-6
Doyle M, Fuller TF, Newman J (1993) Modeling of galvanostatic charge and discharge of the lithium/polymer/insertion cell. J Electrochem Soc 140:1526–1533. https://doi.org/10.1149/1.2221597
Fuller TF, Doyle M, Newman J (1994) Simulation and optimization of the dual lithium ion insertion cell. J Electrochem Soc 141:1–10. https://doi.org/10.1149/1.2054684
Doyle M, Fuller TF, Newman J (1994) The importance of the lithium ion transference number in lithium/polymer cells. Electrochim Acta 39:2073–2081. https://doi.org/10.1016/0013-4686(94)85091-7
Doyle M, Newman J (1995) The use of mathematical modeling in the design of lithium/ polymer battery systems. Electrochim Acta 40:2191–2196. https://doi.org/10.1016/0013-4686(95)00162-8
Newman J, Thomas-Alyea KE (2004) Electrochemical systems, 3rd edn. John Wiley & Sons
Fuller, TF, Newman, JA (1989) Concentrated solution theory model of transport in solid–polymer–electrolyte fuel cells. In: R. E. White and A. J (eds.), Proceedings of The Symposium on Fuel Cells. Appleby, Proc. Vol. 89-14. Electrochem Soc Proc
Lamorgese A, Mauri R, Tellini B (2018) Electrochemical-thermal P2D aging model of a LiCoO2/graphite cell: Capacity fade simulations. Journal of Energy Storage 20:289–297. https://doi.org/10.1016/j.est.2018.08.011
Murbach MD, Schwartz DT (2017) Extending Newman’s pseudo-two-dimensional lithium-ion battery impedance simulation approach to include the nonlinear harmonic response. J Electrochem Soc 164:E3311. https://doi.org/10.1149/2.0301711jes
Subramanian VR, Boovaragavan V, Ramadesigan V, Arabandi M (2009) Mathematical model reformulation for lithium-ion battery simulations: galvanostatic boundary conditions. J Electrochem Soc 156:A260. https://doi.org/10.1149/1.3065083
Ramadesigan V, Boovaragavan V, Subramanian VR (2009) Efficient reformulation of solid-phase diffusion in physics-based lithium-ion battery models, ECS Transactions. https://doi.org/10.1149/1.3115314
Rahimian SK, Rayman S, White RE (2013) Extension of physics-based single particle model for higher charge–discharge rates. J Power Sources 224:180–194. https://doi.org/10.1016/j.jpowsour.2012.09.084
Guo M, White RE (2012) An approximate solution for solid-phase diffusion in a spherical particle in physics-based Li-ion cell models. J Power Sources 198:322–328. https://doi.org/10.1016/j.jpowsour.2011.08.096
Santhanagopalan S, Guo Q, Ramadass P, White RE (2006) Review of models for predicting the cycling performance of lithium ion batteries. J Power Sources 156:620–628. https://doi.org/10.1016/j.jpowsour.2005.05.070
Subramanian VR, Tapriyal D, White RE (2004) A boundary condition for porous electrodes. Electrochem Solid-State Lett 7:A259–A263. https://doi.org/10.1149/1.1773751
Guduru A, Northrop PW, Jain S, Crothers AC, Marchant TR, Subramanian VR (2012) Analytical solution for electrolyte concentration distribution in lithium-ion batteries. J Appl Electrochem 42:189–199. https://doi.org/10.1007/s10800-012-0394-4
Johan MR, Arof AK (2007) Modeling of electrochemical intercalation of lithium into a LiMn2O4 electrode using Green function. J Power Sources 170:490–494. https://doi.org/10.1016/j.jpowsour.2007.03.069
Johan MR, Arof AK (2004) Analytical solution to the material balance equation by integral transform for different cathode geometries. Ionics. 10:405–414. https://doi.org/10.1007/bf02378001
Ali SH, Hussin A, Arof A (2002) Short- and long-time solutions for material balance equation in lithium-ion batteries by Laplace transform. J Power Sources 112:435–442. https://doi.org/10.1016/s0378-7753(02)00420-2
Smith KA, Rahn CD, Wang C-Y (2008) Model Order Reduction of 1D diffusion systems via residue grouping. J Dyn Syst Meas Control 130(011012):1–8. https://doi.org/10.1115/1.2807068
Subramanian VR, Ritter JA, White RE (2001) Approximate solutions for galvanostatic discharge of spherical particles I. Constant diffusion coefficient. J Electrochem Soc 148. https://doi.org/10.1149/1.1409397.
Luo W, Lyu C, Wang L, Zhang L (2013) An approximate solution for electrolyte concentration distribution in physics-based lithium-ion cell models. Microelectron Reliab 53:797–804. https://doi.org/10.1016/j.microrel.2012.11.002
Cai L, White RE et al (2009) J Electrochem Soc 156. https://doi.org/10.1149/1.3049347
Carr EJ, Turner IW (2016) A semi-analytical solution for multilayer diffusion in a composite medium consisting of a large number of layers. Appl Math Model 40:7034–7050. https://doi.org/10.1016/j.apm.2016.02.041
Choobineh L, Jain A (2015) An explicit analytical model for rapid computation of temperature field in a three-dimensional integrated circuit (3D IC). Int J Therm Sci 87:103–109. https://doi.org/10.1016/j.ijthermalsci.2014.08.012
Rodrigo MR, Worthy AL (2016) Solution of multilayer diffusion problems via the Laplace transform. Aust J Math Anal Appl 444:475–502. https://doi.org/10.1016/j.jmaa.2016.06.042
Hickson RI, Barry SI, Mercer GN (2009) Critical times in multilayer diffusion. Part 1: Exact solutions. Int J Heat Mass Transf 52:5776–5783. https://doi.org/10.1016/j.ijheatmasstransfer.2009.08.013
Pérez Guerrero JS, Pontedeiro EM, van Genuchten MT, Skaggs TH (2013) Analytical solutions of the one-dimensional advection–dispersion solute transport equation subject to time-dependent boundary conditions. Chem Eng J 221:487–491. https://doi.org/10.1016/j.cej.2013.01.095
Carr EJ, March NG (2018) Semi-analytical solution of multilayer diffusion problems with time-varying boundary conditions and general interface conditions. Appl Math Comput 333:286–303. https://doi.org/10.1016/j.amc.2018.03.095
Parhizi M, Pathak M, Jain A (2020) Analytical model based prediction of state-of-charge (SoC) of a Lithium-ion cell under time-varying charge/discharge currents. J Electrochem Soc 167(120544):1–10. https://doi.org/10.1149/1945-7111/abb34d
Liu S (2006) An analytical solution to Li/Li insertion into a porous electrode. Solid State Ionics 177:53–58. https://doi.org/10.1016/j.ssi.2005.09.053
Parhizi M, Jain A (2021) Analytical modeling of solution phase diffusion in porous composite electrodes under time-dependent flux boundary conditions using Green’s function method. Ionics. 27:213–224. https://doi.org/10.1007/s11581-020-03777-1
Parhizi M, Jain A (2020) Analytical modeling of solid phase diffusion in single-layer and composite electrodes under time-dependent galvanostatic flux boundary condition. J Electrochem Soc 167:1–11. https://doi.org/10.1149/1945-7111/ab847c
Zhou L, Parhizi M, Jain A (2021) Theoretical analysis of transient solution phase concentration field in a porous composite electrode with time-dependent flux boundary condition. J Appl Electrochem 51:1241–1252. https://doi.org/10.1007/s10800-021-01573-x
Krishnan G, Parhizi M, Pathak M, Jain A (2021) Solution phase limited diffusion modeling in a Li-ion cell subject to concentration-dependent pore wall flux. J Electrochem Soc 168:1–9. https://doi.org/10.1149/1945-7111/ac1cfb
Funding
This material is based upon work supported by CAREER Award No. CBET-1554183 from the National Science Foundation. This research was also supported by the Key Project of Science of the Education Bureau of Henan Province (19B460005), the Special Project of Basic Scientific Research Operating Expenses of Henan Polytechnic University (NSFRF180427), and the China Scholarship Council.
Author information
Authors and Affiliations
Contributions
Long Zhou – Methodology, formal analysis, investigation, data curation, visualization; Mohammad Parhizi – Methodology, formal analysis, investigation, data curation, visualization; Manan Pathak – Conceptualization, methodology, visualization, funding acquisition; Ankur Jain – conceptualization, methodology, supervision, project administration, visualization, funding acquisition. All authors contributed towards writing original draft and review/editing.
Corresponding author
Ethics declarations
Conflict of interest/Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zhou, L., Parhizi, M., Pathak, M. et al. Analytical modeling of Li-ion diffusion in a three-layer electrode-separator-electrode stack with time-dependent current. Ionics 28, 1143–1155 (2022). https://doi.org/10.1007/s11581-021-04332-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11581-021-04332-2