Abstract
Various transition metal oxide materials like MnO2 have been reported as cathode for sodium-ion batteries. However, the large sodium-ion radius and migration barrier cause its poor structural stability and low electrochemical performance. Herein, we present a simple pre-potassiation way to stabilize the nanostructure of amorphous manganese dioxide (a-MnO2). The pre-potassiation amorphous manganese dioxide (K-a-MnO2) heating treatment at 400 °C manifests outstanding electrochemical properties in the aspect of specific capacity and cyclic stabilization, the reversible specific capacity maintains at 180.8 mAh g−1 after 200th cycles under a current density of 0.1 C, and it shows a rate capability of 178.2 mAh g−1, 157.5 mAh g−1, 120.8 mAh g−1, 95.5 mAh g−1, 71.4 mAh g−1, and 47.1 mAh g−1 at 0.1 C, 0.2 C, 0.5 C, 1 C, 2 C and 5 C, respectively. The findings exhibit that the pre-potassiation way can stabilize the structural of a-MnO2 and effectively improve its electrochemical performance.
Graphical abstract

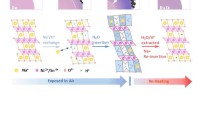
The pre-potassiation amorphous manganese dioxide (K-a-MnO2) heating treatment at 400 °C shows a reversible specific capacity maintains at 180.8 mAh g−1 after 200th cycles under a current density of 0.1 C with a high structure stability.








Similar content being viewed by others
References
Brian LE, Linda FL (2012) Sodium and sodium-ion energy storage batteries. Solid State Mater Sci 16:168–177
Kang B, Ceder G (2009) Battery materials for ultrafast charging and discharging. Nature 458:190–193
Goodenough JB, Kim Y (2010) Challenges for rechargeable Li batteries. Chem Mater 22:587–603
Liu C, Li F, Ma LP, Cheng HM (2010) Advanced materials for energy storage. Adv Mater 22:E28–E62
Palomares V, Serras P, Villaluenga I, Hueso KB, Carretero-González J, Rojo T (2012) Na-ion batteries, recent advances and present challenges to become low cost energy storage systems. Energy Environ Sci 5:5884–5901
Liu J, Xia H, Xue D, Lu L (2009) Double-shelled nanocapsules of V2O5-based composites as high-performance anode and cathode materials for Li ion batteries. J Am Chem Soc 131:12086–12087
Cui DY, Lu P, Yang H, Liu YN, Xue DF (2011) Mild solution route to mixed-phase MnO2 with enhanced electrochemical capacitance. Funct Mater Lett 4:57–60
Armand M, Tarascon JM (2008) Building better batteries. Nature 451:652–657
Ou J, Yang L, Xi X (2016) Flour-assisted simple fabrication of LiCoO2, with enhanced electrochemical performances for lithium ion batteries. J Mater Sci Mater Electron 27:1–7
Simon P, Gogotsi Y (2008) Materials for electrochemical capacitors. Nat Mater 7:845–854
Wang X, Hu P, Niu C, Meng J, Xu X, Wei X, Tang C, Luo W, Zhou L, An Q, Mai L (2015) Novel K3V2(PO4)3/C bundled nanowires as superior sodium-ion battery electrode with ultrahigh cycling stability. Adv Energy Mater 5:1–8
Talaie E, Duffort V, Smith HL, Fultz B, Nazar LF (2015) Structure of the high voltage phase of layered P2-Nax[Mn1/2Fe1/2]O2 and the positive effect of Ni substitution on its electrochemical stability. Energy Environ Sci 8:2512–2523
Kundu D, Talaie E, Duffort V, Nazar LF (2015) The emerging chemistry of sodium ion batteries for electrochemical energy storage. Angew Chem Int Edit 54:3431–3448
Xiong H, Slater MD, Balasubramanian M, Johnson CS, Rajh T (2011) Amorphous TiO2 nanotube anode for rechargeable sodium ion batteries. J Phys Chem Lett 2:2560–2565
Barker J, Saidi MY, Swoyer JL (2003) A sodium-ion cell based on the fluorophosphate compound NaVPO4F. Electrochem Solid State Lett 6:A1–A4
Meins JM, Crosnier-Lopez MP, Hemon-Ribaud A, Courbion G (1999) Phase transitions in the Na3M2(PO4)2F3 family: synthesis, thermal, structural, and magnetic studies. J Solid State Chem 148:260–277
Zhao J, He J, Ding X, Zhou J, Ma Y, Wu S, Huang R (2010) A novel sol-gel synthesis route to NaVPO4F as cathode material for hybrid lithium ion batteries. J Power Sources 195:6854–6859
Shan QY, Guan B, Zhu SJ, Zhang HJ, Zhang YX (2016) Facile synthesis of carbon-doped graphitic C3N4@MnO2 with enhanced electrochemical performance. RSC Adv 6:83209–83216
Sun XX, Wang HJ, Lei ZB, Liu ZH, Wei LL (2014) MnO2 nanoflakes grown on 3D graphite network for enhanced electrocapacitive performance. RSC Adv 4:30233–30240
Liu Y, Xu S, Zhang S, Zhang J, Fan J, Zhou Y (2015) Direct growth of FePO4/reduced graphene oxide nanosheet composites for the sodium-ion battery. J Mater Chem A 3:5501–5508
Devaraj S, Munichadraiah N (2008) A novel sol–gel synthesis route to NaVPO4F as cathode material for hybrid lithium ion batteries. J Phys Chem C 11:4406–4417
Liu R, Duan J, Lee SB (2010) Redox exchange induced MnO2 nano-particle enrichment in poly (3, 4-ethylenedioxythiophene) nano-wires for electrochemical energy storage. ACS Nano 4:4299–4307
Yan J, Fan Z, Wei T, Cheng J, Shao B (2009) Carbon nanotube/MnO2 composites synthesized by microwave-assisted method for supercapacitors with high power and energy densities. J Power Sources 194:1202–1207
Fischer AE, Pettigrew KA, Rolison DR, Stroud RM, Long JW (2007) Incorporation of homogeneous, nanoscale MnO2 within ultraporous carbon structures via self-limiting electroless deposition: implications for electrochemical capacitors. Nano Lett 2:281–286
Mery A, Ghamouss F, Autret C, Farhat D, Tran-Van F (2016) Aqueous ultracapacitors using amorphous MnO2 and reduced graphene oxide. J Power Sources 305:37–45
Yuan A, Zhang Q (2006) A novel hybrid manganese dioxide/activated carbon supercapacitor using lithium hydroxide electrolyte. Electrochem Commun 8:1173–1178
Pang SC, Anderson MA (2000) Novel electrode materials for thin-film ultracapacitors: comparison of electrochemical properties of sol-gel-derived and electrodeposited manganese dioxide. J Electrochem Soc 147:444–450
Devarak S, Munichandraiah N (2008) Effect of crystallographic structure of MnO2 on its electrochemical capacitance properties. J Phys Chem C 112:4406–4417
Tsang C, Kim J, Manthiram A (1998) Synthesis of manganese oxides by reduction of KMnO4 with KBH4 in aqueous solutions. J. Solid State Chem 137:28–32
Wei C, Xu C, Li B, Li H, Du DN, Kang F (2013) Anomalous effect of K ions on electrochemical capacitance of amorphous MnO2. J Power Sources 234:1–7
Liu J, Makwana V, Cai J, Sui SL, Aindow M (2003) Effects of alkali metal and ammonium cation templates on nanofibrous cryptomelane-type manganese oxide octahedral molecular sieves (OMS-2). J Phys Chem B 107:9185–9194
Xu JJ, Yang J (2003) Nanostructured amorphous manganese oxide cryogel as a high-rate lithium intercalation host. Electrochem Commun 5:230–235
Brezesinski T, Wang J, Tolbert SH, Dunn B (2010) Ordered mesoporous [alpha]-MoO3 with iso-oriented nanocrystalline walls for thin-film pseudocapacitors. Nat Mater 9:146–151
Wang J, Polleux J, Lim J, Dunn B (2007) Pseudocapacitive contributions to electrochemical energy storage in TiO2 (Anatase) nanoparticles. J Phys Chem C 111:14925–14931
Gummow RJ, Tiles DC, Thackeray MM (1993) Lithium extraction from orthorhombic lithium manganese oxide and the phase transformation to spinel. Mater Res Bull 28:1249–1256
Reed J, Cedar G, Ven AVD (2004) Layered-to-spinel phase transition in LixMnO2. Electrochem Solid-State Lett 4:A78–A81
Rossouw MH, Thackeray MM (1991) Lithium manganese oxides from Li2MnO3 for rechargeable lithium battery applications. Mater. Res Bull 26:463–473
David WIF, Thackeray MM, Bruce PG, Goodenough JB (1983) Lithium insertion into manganese spinels. Mater Res Bull 18:461–472
Thackeray MM (1997) Manganese oxides for lithium batteries. Prog Solid State Chem 25:1–71
Xu JJ, Ye H, Jain G, Y J (2004) Amorphous manganese oxide remains amorphous upon lithium intercalation and cycling. Electrochem Commun 6:892–897
Feng Q, Kanoh H, Miyai Y, Ooi K (1995) Alkali metal ions insertion/extraction reactions with Hollandite-type manganese oxide in the aqueous phase. Chem Mater 7:148–153
Funding
This work was financially supported by Science and Technology Commission of Shanghai Municipality (19DZ2271100).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
ESM 1
(DOCX 206 kb)
Rights and permissions
About this article
Cite this article
Wang, R., Chen, T., Cao, Y. et al. K+-stabilized nanostructured amorphous manganese dioxide: excellent electrochemical properties as cathode material for sodium-ion batteries. Ionics 27, 1559–1567 (2021). https://doi.org/10.1007/s11581-020-03880-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11581-020-03880-3