Abstract
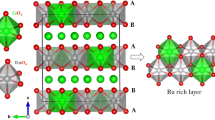
Density functional theory study of Li2NiTiO4 and vanadium-doped Li2NiTiO4 are performed for understanding their structural and electrochemical phenomena, viz., structural geometry like lattice parameters, change in lattice volume with Li+ extraction, Li+ de-intercalation voltage, electrochemical capacity etc. Li2NiTiO4 has cubic symmetry with space group Fm3m (space group number 225). De-intercalated structure of LiNiTiO4 is obtained by removing one Li atom from Li2NiTiO4 unit cell while shifting another Li atom from octahedral site 4b to tetrahedral lattice site 8c. Due to Li+ extraction, the change in unit cell volume is ~ 5.9%. Li+ de-intercalation voltage is calculated by subtracting total energy of the unit cell of LiNiTiO4 and bcc Li from Li2NiTiO4. The voltage comes out to be 4.84 V which is nearly at the threshold for electrochemical stability of used electrolytes. The redox couples in this case are Ni+2/Ni2 + δ and O−2/O−2+γ. The presence of redox couple O−2/O−2+γ leads to the probability of evolution of oxygen during charging. However, partial (50% in this case) vanadium doping at Ti site reduces the redox voltage to 4.64 V considering the de-intercalation reaction forming LiNiTi0.5V0.5O4 from Li2NiTi0.5V0.5O4, by activating the redox couple V+3/V+4 in Li2NiTi0.5V0.5O4. This also reduces the possibility of evolution of oxygen during de-intercalation reaction by shifting the main redox couple from O−2/O−2+γ to V+3/V+4, which leads to greater structural stability of electrode materials during charge-discharge cycles.





Similar content being viewed by others
References
Shukla AK, Kumar TP (2008) Materials for next generation lithium batteries. Curr Sci 94:314–331
Chakrabarti S, Thakur AK, Biswas K (2016) Density functional theory study of LiFeTiO4. J Power Sources 313:81–90
Chakrabarti S, Thakur AK, Biswas K (2013) DFT analysis of lithium de-intercalation in Li2FeVO4. Ionics 19:1515–1526
Zin EM, Zin B, Jeon Y-S, Park K-H, Gu H-B (2009) Electrochemical properties of LiMnO2 for lithium polymer battery. J Power Sources 189:620–623
Sheu SP, Shih IC, Yao CY, Chen JM, Hurng WM (1997) Studies of LiNiO2 in lithium-ion batteries. J Power Sources 68:558–560
Kalyani P, Kalaiselvi N (2005) Various aspects of LiNiO2 chemistry: A review. Sci Technol Adv Mater 6:689–703
Cheruku R, Vijayan L, Govindaraj G (2013) Structural and electrical conductivity studies of nanocrystalline Li2NiTiO4 material. Indian J Pure and Appl Physics 51:343–345
Trócoli R, Cruz-Yusta M, Morales J, Santos-Peña J (2013) On the limited electroactivity of Li2NiTiO4 nanoparticles in lithium batteries. Electrochim Acta 100:93–100
Prabaharana SRS, Michael MS, Ikuta H, Uchimotob Y, Wakihara M (2004) Li2NiTiO4 – a new positive electrode for lithium batteries: soft-chemistry synthesis and electrochemical characterization. Solid State Ionics 172:39–45
Wang Y, Wangand Y, Wang F (2014) Facile molten salts synthesis of Li2NiTiO4 cathode. Mater Li-ion Batter Nano Scale Res Lett 9:197–201
Kawano Y, Kitajou A, Okada S (2013) Synthesis and cathode properties of a cubic rock-salt type Si-doped Li2NiTiO4 for lithium-ion batteries. J Power Sour 242:768–774
Küzma M, Dominko R, Meden A, Makovec D, Bele M, Jamnik J, Gaberscek M (2009) Electrochemical activity of Li2FeTiO4 and Li2MnTiO4 as potential active materials for Li ion batteries: a comparison with Li2NiTiO4. J Power Sour 189:81–88
Wu J, Wang H, Quan J, Ma Z, Li D (2015) Enhanced electrochemical performance of Li2NiTiO4 with micro-structural rearrangement via urea treatment. RSC Adv 5:2844–2850
Hamaguchi M, Momida H, Oguchi T (2018) First-principles study on cathode properties of Li2MTiO4 (M =V, Cr, Mn, Fe, Co, and Ni) with oxygen deficiency for Li-ion batteries. J Phys Soc Jpn 87:044805-1–044805-8
Kresse G, Marsman M and Furthmuller J, Computational Materials Physics, Faculty of Physics, Universitat Wien, Sensengasse 8/12, A-1090 Wien, Austria, https://www.vasp.at/
Blaha P, Schwarz K, Madsen GKH, Kvasnicka D and Luitz J, WIEN2k, An Augmented Plane Wave + Local Orbitals Programme for Calculating Crystal Properties (Karlheinz Schwarz, Techn. Universitat Wien, Austria), 2001.ISBN 3-9501031-1-2,
Perdew JP, Burke K, Ernzerhof M (1996) Generalized gradient approximation made simple. Phys Rev Lett 77(18):3865–3868
Chakrabarti S, Biswas K (2017) DFT study of Mg2TiO4 and Ni doped Mg1.5Ni0.5TiO4 as electrode material for Mg ion battery application. J Mater Sci 52:10972–10980
Aydinol MK, Kohan AF, Ceder G (1997) Ab initio calculation of the intercalation voltage of lithium-transition-metal oxide electrodes for rechargeable batteries. J Power Sour 68:664–668
Chakrabarti S, Thakur AK, Biswas K (2017) Effect of Ti modification on Structural, Electronic and Electrochemical properties of Li2FeSiO4—A DFT study using FPLAPW approach. Electrochim Acta 236:288–296
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Chakrabarti, S., Biswas, K. DFT study of Li2NiTiO4 and vanadium-doped Li2NiTiO4. Ionics 26, 1357–1363 (2020). https://doi.org/10.1007/s11581-019-03289-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11581-019-03289-7