Abstract
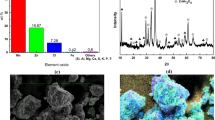
In this study, manganese recycled from cathodes of spent Zn–MnO2 batteries was used for the sol–gel synthesis of a manganese oxide pseudocapacitor with high specific capacitance. The recycled material is a mixture of α-MnO2, Mn3O4, and ZnMn2O4, according to X-ray diffraction, energy dispersive X-ray, Raman spectra, and chemical analysis. Manganese oxide has smaller particle size when synthesized at 700 °C for 3 h (M1) than when synthesized at 700 °C for 12 h (M2), as evidenced by scanning electron microscopy and transmission energy microscopy. The specific capacitances of M1 and M2 after 1000 cyclic voltammetry cycles at 50 mV s−1 are 350 F g−1 and 125 F g−1, respectively. After 500 galvanostatic cycles at 1.0 A g−1, M1 and M2 exhibit specific capacitances of 275 F g−1 and 150 F g−1, respectively. Electrochemical impedance spectra revealed that M1 has lower charge transfer resistance and diffusion resistance than M2.










Similar content being viewed by others
References
Linden D, Reddy TB (2002) Handbook of batteries, 3rd edn. McGraw-Hill, New York
Carvalho BB, Pegoretti VCB, Celante VG, Dixini PVM, Gastelois PL, Macedo WAA, Freitas MBJG (2017) Effect of temperature on the electrochemical synthesis of MnO2 recycled from spent Zn–MnO2 alkaline batteries and application of recycled MnO2 as electrochemical pseudocapacitors. Mater Chem Phys 196:126–136
ABRELPE (2017) Panorama dos Resíduos Sólidos no Brasil. In: Assoc. Bras. Empres. Limp. Pública e Resíduos Especiais. http://www.abrelpe.org.br/Panorama/panorama2016.pdf. Accessed 19 Mar 2018
Comission E (2018) Batteries & accumulators. http://ec.europa.eu/environment/waste/batteries/. Accessed 5 Oct 2018
Espinosa DCR, Bernardes AM, Tenório JAS (2004) An overview on the current processes for the recycling of batteries. J Power Sources 135:311–319
Bernardes AM, Espinosa DCR, Tenório JAS (2004) Recycling of batteries: a review of current processes and technologies. J Power Sources 130:291–298
Sayilgan E, Kukrer T, Civelekoglu G, Ferella F, Akcil A, Veglio F, Kitis M (2009) A review of technologies for the recovery of metals from spent alkaline and zinc-carbon batteries. Hydrometallurgy 97:158–166
Dixini PVM, Celante VG, Lelis MFF, Freitas MBJG (2014) Recycling of the anode from spent Ni-MH batteries for synthesis of the lanthanide oxysulfide/oxysulfate compounds used in an oxygen storage and release system. J Power Sources 260:163–168
Dixini PVM, Pegoretti VCB, Celante VG, Betim FS, Freitas MBJG (2017) Electrodeposition study of simulated and dissolution solutions of the positive electrode of a spent Ni-MH battery using the electrochemical quartz crystal microbalance and inductively coupled plasma optical emission spectrometry. Ionics 23:3235–3243
Da Silva PS, Schmitz EPS, Spinelli A, Garcia JR (2012) Electrodeposition of Zn and Zn-Mn alloy coatings from an electrolytic bath prepared by recovery of exhausted zinc-carbon batteries. J Power Sources 210:116–121
Xi G, Xi Y, Xu H, Wang L (2016) Study of the preparation of NI-Mn-Zn ferrite using spent NI-MH and alkaline Zn-Mn batteries. J Magn Magn Mater 398:196–199
Li L, Dunn JB, Zhang XX, Gaines L, Chen RJ, Wu F, Amine K (2013) Recovery of metals from spent lithium-ion batteries with organic acids as leaching reagents and environmental assessment. J Power Sources 233:180–189
Li L, Qu W, Zhang X, Lu J, Chen R, Wu F, Amine K (2015) Succinic acid-based leaching system: a sustainable process for recovery of valuable metals from spent Li-ion batteries. J Power Sources 282:544–551
Winter M, Brodd RJ (2004) What are batteries, fuel cells, and supercapacitors? Chem Rev 104:4245–4269
Zhang Y, Feng H, Wu X, Wang L, Zhang A, Xia T, Dong H, Li X, Zhang L (2009) Progress of electrochemical capacitor electrode materials: a review. Int J Hydrog Energy 34:4889–4899
Sarac FE, Unal U (2015) Electrochemical-hydrothermal synthesis of manganese oxide films as electrodes for electrochemical capacitors. Electrochim Acta 178:199–208
Wang G, Zhang L, Zhang J (2012) A review of electrode materials for electrochemical supercapacitors. Chem Soc Rev 41:797–828
Toupin M, Brousse T, Bélanger D (2004) Charge storage mechanism of MnO2 electrode used in aqueous electrochemical capacitor. Chem Mater 16:3183–3190
Liu E-H, Li W, Li J, Meng XY, Ding R, Tan ST (2009) Preparation and characterization of nanostructured NiO/MnO2 composite electrode for electrochemical supercapacitors. Mater Res Bull 44:1122–1126
Hashemzadeh F, Mehdi Kashani Motlagh M, Maghsoudipour A (2009) A comparative study of hydrothermal and sol-gel methods in the synthesis of MnO2 nanostructures. J Sol-Gel Sci Technol 51:169–174
Tang W, Shan X, Li S, Liu H, Wu X, Chen Y (2014) Sol–gel process for the synthesis of ultrafine MnO2 nanowires and nanorods. Mater Lett 132:317–321
Yang X-H, Wang Y-G, Xiong H-M, Xia Y-Y (2007) Interfacial synthesis of porous MnO2 and its application in electrochemical capacitor. Electrochim Acta 53:752–757
Wang C, Zhan Y, Wu L, Li Y, Liu J (2014) High-voltage and high-rate symmetric supercapacitor based on MnO2-polypyrrole hybrid nanofilm. Nanotechnology 25:305401
Buzatu M, Sǎceanu S, Ghica VG et al (2013) Simultaneous recovery of Zn and MnO2 from used batteries, as raw materials, by electrolysis. Waste Manag 33:1764–1769
Ali GAM, Tan LL, Jose R, Yusoff MM, Chong KF (2014) Electrochemical performance studies of MnO2 nanoflowers recovered from spent battery. Mater Res Bull 60:5–9
Wei F, Cui X, Chen W, Ivey DG (2008) Phase-controlled synthesis of MnO2 nanocrystals by anodic electrodeposition: implications for high-rate capability electrochemical supercapacitors. J Phys Chem C 112:15075–15083
Rodrigues S, Munichandraiah N, Shukla AK (1998) A cyclic voltammetric study of the kinetics and mechanism of electrodeposition of manganese dioxide. J Appl Electrochem 28:1235–1241
Hussain S, Amade R, Jover E, Bertran E (2013) Water plasma functionalized CNTs/MnO2 composites for supercapacitors. Sci World J 2013:1–9
Devaraj S, Munichandraiah N (2009) EQCM investigation of the electrodeposition of MnO2 and its capacitance behavior. Electrochem Solid-State Lett 12:21–25
Devaraj S, Munichandraiah N (2008) Effect of crystallographic structure of MnO2 on its electrochemical capacitance properties. J Phys Chem C 112:4406–4417
Clarke CJ, Browning GJ, Donne SW (2006) An RDE and RRDE study into the electrodeposition of manganese dioxide. Electrochim Acta 51:5773–5784
Barik SP, Prabaharan G, Kumar B (2016) An innovative approach to recover the metal values from spent lithium-ion batteries. Waste Manag 51:222–226
Li L, Bian Y, Zhang X, Guan Y, Fan E, Wu F, Chen R (2018) Process for recycling mixed-cathode materials from spent lithium-ion batteries and kinetics of leaching. Waste Manag 71:362–371
Yang Y, Xu S, He Y (2017) Lithium recycling and cathode material regeneration from acid leach liquor of spent lithium-ion battery via facile co-extraction and co-precipitation processes. Waste Manag 64:219–227
Zhang L-H, Wu S-S, Wan Y, Huo YF, Luo YC, Yang MY, Li MC, Lu ZG (2017) Mn3O4/carbon nanotube nanocomposites recycled from waste alkaline Zn–MnO2 batteries as high-performance energy materials. Rare Metals 36:0–6
APHA (2012) Standard methods for the examination of water and wastewater, 2nd edn. American Public Health Association, Washington
Brunauer S, Emmet PH, Teller E (1936) Adsorption of gases in multimolecular layers, vol 407, pp 309–319
Webb P, Orr C (1997) Analytical methods in fine particle technology. Micromeritics Instrument Corp., Norcross
Vinje K (1995) Characterization of porous solids III. Appl Catal A Gen 121:N23–N24
Gherbi R, Bessekhouad Y, Trari M (2016) Optical and transport properties of Sn-doped ZnMn2O4 prepared by sol–gel method. J Phys Chem Solids 89:69–77
Sing KSW (1995) Physisorption of nitrogen by porous materials. J Porous Mater 2:5–8
Huang T, Zhao C, Qiu Z, Luo J, Hu Z (2017) Hierarchical porous ZnMn2O4 synthesized by the sucrose-assisted combustion method for high-rate supercapacitors. Ionics 23:139–146
Nádherný L, Marysko M, David Sedmidubský CM (2016) Structural and magnetic properties of ZnxMn3−xO4 spinels. J Magn Magn Mater 413:89–96
Gao T, Fjellvåg H, Norby P (2009) A comparison study on Raman scattering properties of α- and β-MnO2. Anal Chim Acta 648:235–239
Gao T, Glerup M, Krumeich F, Nesper R, Fjellvåg H, Norby P (2008) Microstructures and spectroscopic properties of cryptomelane-type manganese dioxide nanofibers. J Phys Chem C 112:13134–13140
Yang L, Cheng S, Ji X, Jiang Y, Zhou J, Liu M (2015) Investigation into the origin of pseudocapacitive behavior of Mn3O4 electrodes using operando Raman spectroscopy. J Mater Chem A 3:7338–7344
Acknowledgments
The authors thank CAPES, NCQP-UFES, FAPES, and CNPq for their financial support; IFES/Aracruz and LPT/LMC for the XRD, SEM/EDX, and BET analyses; and LUCCAR for the TEM analysis.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(PDF 106 kb)
Rights and permissions
About this article
Cite this article
Dixini, P.V.M., Carvalho, B.B., Gonçalves, G.R. et al. Sol–gel synthesis of manganese oxide supercapacitor from manganese recycled from spent Zn–MnO2 batteries using organic acid as a leaching agent. Ionics 25, 4381–4392 (2019). https://doi.org/10.1007/s11581-019-02995-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11581-019-02995-6