Abstract
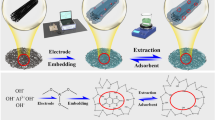
The synthesis of highly stable metallic organic–inorganic hybrid nanocomposites from the non-toxic and biodegradable organic–inorganic metallic precursors, which are the natural polymer products of organic molecules, carbohydrates, and amino acids, has engrossed the recent researchers. In this work, a simple potentiostatic approach has been designed for the electrochemical preparation of gold nanoparticle-decorated copper cross-linked pectin (CuCP–AuNPs). Here, CuCP act as a scaffold or stabilizing agent for the preparation of uniform AuNPs. The studies for surface morphology and crystal structure of our composite were carried out using field emission scanning electron microscopy (FESEM) and X-ray diffraction spectroscopy (XRD). Energy-dispersive X-ray spectroscopy (EDX) was employed for the elemental analysis of the composite. A remarkable cathodic peak current response was obtained by our modified electrode for the presence of H2O2. CuCP–AuNP-modified glassy carbon electrode (GCE) possesses the values for limits of detection (LOD) and sensitivity of 0.22 μΜ and 6800 μA mM cm−2, respectively, in a linear range from 1 to 2110 μΜ. The detection of H2O2 in presence of other biologically interfering molecules represents the high selectivity of our fabricated electrode. The examination for the sensitive determination of H2O2 conducted in commercially available lens cleaning solutions validates the practical feasibility of the proposed modified electrode.






Similar content being viewed by others
References
Rani KK, Devasenathipathy R, Wang S-F, Yang C (2017) Simple preparation of birnessite-type MnO2 nanoflakes with multi-walled carbon nanotubes for the sensitive detection of hydrogen peroxide. Ionics 23(11):3219–3226
Cui K, Song Y, Yao Y, Huang Z, Wang L (2008) A novel hydrogen peroxide sensor based on Ag nanoparticles electrodeposited on DNA-networks modified glassy carbon electrode. Electrochem Commun 10(4):663–667
Ksibi M (2006) Chemical oxidation with hydrogen peroxide for domestic wastewater treatment. Chem Eng J 119(2–3):161–165
Mollarasouli F, Asadpour-Zeynali K, Campuzano S, Yáñez-Sedeño P, Pingarrón JM (2017) Non-enzymatic hydrogen peroxide sensor based on graphene quantum dots-chitosan/methylene blue hybrid nanostructures. Electrochim Acta 246:303–314
Sun X, Odyniec ML, Sedgwick AC, Lacina K, Xu S, Qiang T, Bull SD, Marken F, James TD (2017) Reaction-based indicator displacement assay (RIA) for the colorimetric and fluorometric detection of hydrogen peroxide. Org Chem Front 4(6):1058–1062
Hasan SK, Asaduzzaman M, Merkyte V, Morozova K, Scampicchio M (2018) Simultaneous kinetic and thermodynamic-based assay to determine the hydrogen peroxide (H2O2) scavenging activity of berry extracts by using reaction calorimetry. Food Anal Methods 11(2):432–439
Song M, Wang J, Chen B, Wang L (2017) A facile, nonreactive hydrogen peroxide (H2O2) detection method enabled by ion chromatography with UV detector. Anal Chem 89(21):11537–11544
Seven O, Sozmen F, Turan IS (2017) Self immolative dioxetane based chemiluminescent probe for H2O2 detection. Sensors Actuators B Chem 239:1318–1324
Tang B, Wang Y, Liang H, Chen Z, He X, Shen H (2006) Studies on the oxidation reaction of tyrosine (Tyr) with H2O2 catalyzed by horseradish peroxidase (HRP) in alcohol–water medium by spectrofluorimetry and differential spectrophotometry. Spectrochim Acta A Mol Biomol Spectrosc 63(3):609–613
Klassen NV, Marchington D, McGowan HC (1994) H2O2 determination by the I3-method and by KMnO4 titration. Anal Chem 66(18):2921–2925
Asif SAB, Khan SB, Asiri AM (2017) Assessment of graphene oxide/MgAl oxide nanocomposite as a non-enzymatic sensor for electrochemical quantification of hydrogen peroxide. J Taiwan Inst Chem Eng 74:255–262
Sherino B, Mohamad S, Halim SNA, Manan NSA (2018) Electrochemical detection of hydrogen peroxide on a new microporous Ni–metal organic framework material-carbon paste electrode. Sensors Actuators B Chem 254:1148–1156
Devasenathipathy R, Kohilarani K, Chen S-M, Wang S-F, Wang S-C, Chen C-K (2016) Electrochemical preparation of biomolecule stabilized copper nanoparticles decorated reduced graphene oxide for the sensitive and selective determination of hydrogen peroxide. Electrochim Acta 191:55–61
Zhang C, Jiang H, Ma R, Zhang Y, Chen Q (2017) Simple non-enzymatic electrochemical sensor for hydrogen peroxide based on nafion/platinum nanoparticles/reduced graphene oxide nanocomposite modified glassy carbon electrode. Ionics 23(5):1309–1317
Bai W, Zheng J, Sheng Q (2013) A non-enzymatic hydrogen peroxide sensor based on Ag/MnOOH nanocomposites. Electroanalysis 25(10):2305–2311
Ensafi AA, Zandi-Atashbar N, Ghiaci M, Taghizadeh M, Rezaei B (2015) Synthesis of new copper nanoparticle-decorated anchored type ligands: applications as non-enzymatic electrochemical sensors for hydrogen peroxide. Mater Sci Eng C 47:290–297
Janyasupab M, Liu C-W, Zhang Y, Wang K-W, Liu C-C (2013) Bimetallic Pt–M (M= Cu, Ni, Pd, and Rh) nanoporous for H2O2 based amperometric biosensors. Sensors Actuators B Chem 179:209–214
Chen L, Fujita T, Ding Y, Chen M (2010) A three-dimensional gold-decorated nanoporous copper core–shell composite for electrocatalysis and nonenzymatic biosensing. Adv Funct Mater 20(14):2279–2285
Liu L, Huang Z, Wang D, Scholz R, Pippel E (2011) The fabrication of nanoporous Pt-based multimetallic alloy nanowires and their improved electrochemical durability. Nanotechnology 22(10):105604
Noh H-B, Lee K-S, Chandra P, Won M-S, Shim Y-B (2012) Application of a Cu–Co alloy dendrite on glucose and hydrogen peroxide sensors. Electrochim Acta 61:36–43
Upadhyay S, Rao GR, Sharma MK, Bhattacharya BK, Rao VK, Vijayaraghavan R (2009) Immobilization of acetylcholineesterase–choline oxidase on a gold–platinum bimetallic nanoparticles modified glassy carbon electrode for the sensitive detection of organophosphate pesticides, carbamates and nerve agents. Biosens Bioelectron 25(4):832–838
Easow JS, Selvaraju T (2013) Unzipped catalytic activity of copper in realizing bimetallic Ag@ Cu nanowires as a better amperometric H2O2 sensor. Electrochim Acta 112:648–654
Gholivand MB, Azadbakht A (2011) A novel hydrazine electrochemical sensor based on a zirconium hexacyanoferrate film-bimetallic Au–Pt inorganic–organic hybrid nanocomposite onto glassy carbon-modified electrode. Electrochim Acta 56(27):10044–10054
Ni Y, Song H, Kokot S (2013) A novel electrochemical method for the analysis of hydrogen peroxide with the use of a glassy carbon electrode modified by a Prussian blue/copper-gold bimetallic nanoparticles hybrid film. Electroanalysis 25(9):2211–2220
Li X, Du X (2017) Molybdenum disulfide nanosheets supported Au-Pd bimetallic nanoparticles for non-enzymatic electrochemical sensing of hydrogen peroxide and glucose. Sensors Actuators B Chem 239:536–543
Uzunoglu A, Scherbarth AD, Stanciu LA (2015) Bimetallic PdCu/SPCE non-enzymatic hydrogen peroxide sensors. Sensors Actuators B Chem 220:968–976
Li L, Niu R, Zhang Y (2018) Ag–Au bimetallic nanocomposites stabilized with organic–inorganic hybrid microgels: synthesis and their regulated optical and catalytic properties. RSC Adv 8(22):12428–12438
Zhou Q, Lin Y, Zhang K, Li M, Tang D (2018) Reduced graphene oxide/BiFeO3 nanohybrids-based signal-on photoelectrochemical sensing system for prostate-specific antigen detection coupling with magnetic microfluidic device. Biosens Bioelectron 101:146–152
Zhang K, Lv S, Lin Z, Li M, Tang D (2018) Bio-bar-code-based photoelectrochemical immunoassay for sensitive detection of prostate-specific antigen using rolling circle amplification and enzymatic biocatalytic precipitation. Biosens Bioelectron 101:159–166
Qiu Z, Shu J, Tang D (2017) Near-infrared-to-ultraviolet light-mediated photoelectrochemical aptasensing platform for cancer biomarker based on core–shell NaYF4: Yb, Tm@ TiO2 upconversion microrods. Anal Chem 90(1):1021–1028
Ahrabi SF, Madsen G, Dyrstad K, Sande SA, Graffner C (2000) Development of pectin matrix tablets for colonic delivery of model drug ropivacaine. Eur J Pharm Sci 10(1):43–52
Gong J-L, Wang X-Y, Zeng G-M, Chen L, Deng J-H, Zhang X-R, Niu Q-Y (2012) Copper (II) removal by pectin–iron oxide magnetic nanocomposite adsorbent. Chem Eng J 185:100–107
Devasenathipathy R, Karuppiah C, Chen S-M, Mani V, Vasantha VS, Ramaraj S (2015) Highly selective determination of cysteine using a composite prepared from multiwalled carbon nanotubes and gold nanoparticles stabilized with calcium crosslinked pectin. Microchim Acta 182(3–4):727–735
Seslija S, Veljovic D, Krusic MK, Stevanovic J, Velickovic S, Popovic I (2016) Cross-linking of highly methoxylated pectin with copper: the specific anion influence. New J Chem 40(2):1618–1625
Venkatakrishnan S, Veerappan G, Elamparuthi E, Veerappan A (2014) Aerobic synthesis of biocompatible copper nanoparticles: promising antibacterial agent and catalyst for nitroaromatic reduction and C–N cross coupling reaction. RSC Adv 4(29):15003–15006
Khazaei A, Khazaei M, Rahmati S (2015) A green method for the synthesis of gelatin/pectin stabilized palladium nano-particles as efficient heterogeneous catalyst for solvent-free Mizoroki–Heck reaction. J Mol Catal A Chem 398:241–247
Chen D, Yang K, Wang H, Zhou J, Zhang H (2015) Cr (VI) removal by combined redox reactions and adsorption using pectin-stabilized nanoscale zero-valent iron for simulated chromium contaminated water. RSC Adv 5(80):65068–65073
Devasenathipathy R, Mani V, Chen S-M, Arulraj D, Vasantha V (2014) Highly stable and sensitive amperometric sensor for the determination of trace level hydrazine at cross linked pectin stabilized gold nanoparticles decorated graphene nanosheets. Electrochim Acta 135:260–269
Das S, Chaudhury A, Ng K-Y (2011) Preparation and evaluation of zinc–pectin–chitosan composite particles for drug delivery to the colon: role of chitosan in modifying in vitro and in vivo drug release. Int J Pharm 406(1–2):11–20
Vilhena C, Goncalves M, Mota A (2004) Binding of copper (II) to pectins by electrochemical methods. Electroanalysis 16(24):2065–2072
Jia N, Huang B, Chen L, Tan L, Yao S (2014) A simple non-enzymatic hydrogen peroxide sensor using gold nanoparticles-graphene-chitosan modified electrode. Sensors Actuators B Chem 195:165–170
Kamyabi MA, Hajari N, Babaei N, Moharramnezhad M, Yahiro H (2017) Silica template electrodeposition of copper oxide nanostructures on Ni foam as an ultrasensitive non-enzymatic glucose sensor. J Taiwan Inst Chem Eng 81:21–30
Tian L, Zhong X, Hu W, Liu B, Li Y (2014) Fabrication of cubic PtCu nanocages and their enhanced electrocatalytic activity towards hydrogen peroxide. Nanoscale Res Lett 9(1):68
Wang J, Gao H, Sun F, Xu C (2014) Nanoporous PtAu alloy as an electrochemical sensor for glucose and hydrogen peroxide. Sensors Actuators B Chem 191:612–618
Xu J, Wei X-W, Song X-J, Lu X-J, Ji C-C, Ni Y-H, Zhao G-C (2007) Synthesis and electrocatalytic activity of multi-walled carbon nanotubes/Cu–Ag nanocomposites. J Mater Sci 42(16):6972–6976
Lin D, Li Y, Zhang P, Zhang W, Ding J, Li J, Wei G, Su Z (2016) Fast preparation of MoS 2 nanoflowers decorated with platinum nanoparticles for electrochemical detection of hydrogen peroxide. RSC Adv 6(58):52739–52745
Xu C, Liu Y, Su F, Liu A, Qiu H (2011) Nanoporous PtAg and PtCu alloys with hollow ligaments for enhanced electrocatalysis and glucose biosensing. Biosens Bioelectron 27(1):160–166
Huang B, Wang Y, Lu Z, Du H, Ye J (2017) One pot synthesis of palladium-cobalt nanoparticles over carbon nanotubes as a sensitive non-enzymatic sensor for glucose and hydrogen peroxide detection. Sensors Actuators B Chem 252:1016–1025
Zhou J, Zhao Y, Bao J, Huo D, Fa H, Shen X, Hou C (2017) One-step electrodeposition of au-Pt bimetallic nanoparticles on MoS2 nanoflowers for hydrogen peroxide enzyme-free electrochemical sensor. Electrochim Acta 250:152–158
Acknowledgements
Dr. Rajkumar Devasenathipathy gratefully acknowledges National Taipei University of Technology, Taiwan, for the postdoctoral fellowship.
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
ESM 1
(PDF 53 kb)
Rights and permissions
About this article
Cite this article
Kohila rani, K., Liu, YX., Devasenathipathy, R. et al. Simple preparation of gold nanoparticle-decorated copper cross-linked pectin for the sensitive determination of hydrogen peroxide. Ionics 25, 309–317 (2019). https://doi.org/10.1007/s11581-018-2573-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11581-018-2573-8