Abstract
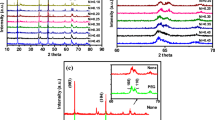
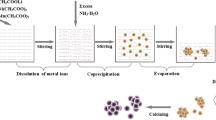
Layered lithium-rich oxide, 0.5Li2MnO3·0.5LiMn1/3Ni1/3Co1/3O2, is synthesized in a mixed molten salt of KCl and LiCl under 750 °C. Its morphology and structure are characterized by X-ray diffraction, scanning electron microscopy, transmission electron microscopy, and nitrogen adsorption and desorption isotherm, and its performances as cathode of lithium-ion battery are investigated by charge–discharge test and electrochemical impedance spectroscopy, with a comparison of the samples synthesized via solid-state reaction. It is found that the resulting product consists of uniform nanoparticles, 50 nm in average, which possesses a well crystallite layered structure although its synthesis temperature is low and thus exhibits excellent cyclic stability and rate capability. The resulting product delivers an initial discharge capacity of 268 mAh g−1 at 0.1 C and has a capacity retention of 82% after 100 cycles at 1 C, compared to the 243 mAh g−1 and 73% for the sample synthesized by solid-state reaction under 900 °C.








Similar content being viewed by others
References
Gong Z-L, Liu H-S, Guo X-J, Zhang Z-R, Yang Y (2004) Effects of preparation methods of LiNi0.8Co0.2O2 cathode materials on their morphology and electrochemical performance. J Power Sources 136(1):139–144. doi:10.1016/j.jpowsour.2004.05.022
Chen D, Li B, Liao Y, Lan H, Lin H, Xing L, Wang Y, Li W (2014) Improved electrochemical performance of LiNi0.5Mn1.5O4 as cathode of lithium ion battery by Co and Cr co-doping. J Solid State Electrochem 18:2027–2033. doi:10.1007/s10008-014-2445-8
Meng YS, Ceder G, Grey CP, Yoon W-S, Jiang M, Breger J, Shao-Horn Y (2005) Cation ordering in layered O3 Li[NixLi1/3-2x/3Mn2/3-x/3]O2(0≤x≤1/2) compounds. Chem Mater 17:2386–2394. doi:10.1021/cm047779m
Sacci Robert L, Black Jennifer M, Nina B, Dudney Nancy J, More Karren L, Unocic Raymond R (2015) Nanoscale imaging of fundamental Li battery chemistry: solid-electrolyte interphase formation and preferential growth of lithium metal nanoclusters. Nano Lett 15:2011–2018. doi:10.1021/nl5048626
Wang D, Huang Y, Huo Z, Chen L (2013) Synthesize and electrochemical characterization of Mg-doped Li-rich layered Li[Li0.2Ni0.2Mn0.6]O2 cathode material. Electrochim Acta 107:461–466. doi:10.1016/j.electacta.2013.05.145
Chen D, Yu Q, Xiang X, Chen M, Chen Z, Song S, Xiong L, Liao Y, Xing L, Li W (2015a) Porous layered lithium-rich oxide nanorods: synthesis and performances as cathode of lithium ion battery. Electrochim Acta 154:83–93. doi:10.1016/j.electacta.2014.12.037
Croy JR, Kang SH, Balasubramanian M, Thackeray MM (2011) Li2MnO3-based composite cathodes for lithium batteries: a novel synthesis approach and new structures. Electrochem Commun 13(10):1063–1066. doi:10.1016/j.elecom.2011.06.037
Gu R-M, Yan S-Y, Sun S, Wang C-Y, Li M-W (2015) Electrochemical behavior of lithium-rich layered oxide Li[Li0.23Ni0.15Mn0.62]O2 cathode material for lithium-ion battery. J Solid State Electrochem 19(6):1659–1669. doi:10.1007/s10008-015-2796-9
Ye D, Ozawa K, Wang B, Hulicova-Jurcakova D, Zou J, Sun C, Wang L (2014) Capacity-controllable Li-rich cathode materials for lithium-ion batteries. Nano Energy 6:92–102. doi:10.1016/j.nanoen.2014.03.013
Yu X, Lyu Y, Gu L, Wu H, Bak S-M, Zhou Y, Amine K, Ehrlich SN, Li H, Nam K-W, Yang X-Q (2014) Understanding the rate capability of high-energy-density Li-rich layered Li1.2Ni0.15Co0.1Mn0.55O2 cathode materials. Adv Energy Mater 4(5):1300950. doi:10.1002/aenm.201300950
Choi NS, Chen Z, Freunberger SA, Ji X, Sun YK, Amine K, Yushin G, Nazar LF, Cho J, Bruce PG (2012) Challenges facing lithium batteries and electrical double-layer capacitors. Angew Chem 51(40):9994–10024. doi:10.1002/anie.201201429
Jarvis KA, Deng Z, Allard LF, Manthiram A, Ferreira PJ (2011) Atomic structure of a lithium-rich layered oxide material for lithium-ion batteries: evidence of a solid solution. Chem Mater 23(16):3614–3621. doi:10.1021/cm200831c
Zou G, Yang X, Wang X, Ge L, Shu H, Bai Y, Wu C, Guo H, Hu L, Yi X, Ju B, Hu H, Wang D, Yu R (2014) Improvement of electrochemical performance for Li-rich spherical Li1.3[Ni0.35Mn0.65]O2+x modified by Al2O3. J Solid State Electrochem 18(7):1789–1797. doi:10.1007/s10008-014-2411-5
Xiang Y, Yin Z, Zhang Y, Li X (2013a) Effects of synthesis conditions on the structural and electrochemical properties of the Li-rich material Li[Li0.2Ni0.17Co0.16Mn0.47]O2 via the solid-state method. Electrochim Acta 91:214–218. doi:10.1016/j.electacta.2012.12.001
Zhao T, Chen S, Li L, Zhang X, Wu H, Wu T, Sun CJ, Chen R, Wu F, Lu J, Amine K (2014) Organic-acid-assisted fabrication of low-cost Li-rich cathode material (Li[Li1/6Fe1/6Ni1/6Mn1/2]O2) for lithium-ion battery. ACS Appl Mater Interfaces 6(24):22305–22315. doi:10.1021/am5062882
Song B, Lai MO, Liu Z, Liu H, Lu L (2013) Graphene-based surface modification on layered Li-rich cathode for high-performance Li-ion batteries. J Mater Chem A 1(34):9954–9965. doi:10.1039/c3ta11580a
Zhu Z, Zhu L (2014) Synthesis of layered cathode material 0.5Li2MnO3·0.5LiMn1/3Ni1/3Co1/3O2 by an improved co-precipitation method for lithium-ion battery. J Power Sources 256:178–182. doi:10.1016/j.jpowsour.2014.01.068
Zhang J, Guo X, Yao S, Zhu W, Qiu X (2013a) Tailored synthesis of Ni0.25Mn0.75CO3 spherical precursors for high capacity Li-rich cathode materials via a urea-based precipitation method. J Power Sources 238:245–250. doi:10.1016/j.jpowsour.2013.03.094
Ito A, Shoda K, Sato Y, Hatano M, Horie H, Ohsawa Y (2011) Direct observation of the partial formation of a framework structure for Li-rich layered cathode material Li[Ni0.17Li0.2Co0.07Mn0.56]O2 upon the first charge and discharge. J Power Sources 196(10):4785–4790. doi:10.1016/j.jpowsour.2010.12.079
Lengyel M, Shen K-Y, Lanigan DM, Martin JM, Zhang X, Axelbaum RL (2016) Trace level doping of lithium-rich cathode materials. J Mater Chem A 4(9):3538–3545. doi:10.1039/c5ta07764h
Chen M, Chen D, Liao Y, Zhong X, Li W, Zhang Y (2016) Layered lithium-rich oxide nanoparticles doped with spinel phase: acidic sucrose-assistant synthesis and excellent performance as cathode of lithium ion battery. ACS Appl Mater Interfaces 8(7):4575–4584. doi:10.1021/acsami.5b10219
Zhang J, Han J, Zhu J, Lin Z, Braga MH, Daemen LL, Wang L, Zhao Y (2014) High pressure-high temperature synthesis of lithium-rich Li3O(Cl, Br) and Li3−xCax/2OCl anti-perovskite halides. Inorg Chem Commun 48:140–143. doi:10.1016/j.inoche.2014.08.036
Li J, Xing L, Zhang R, Chen M, Wang Z, Xu M, Li W (2015) Tris(trimethylsilyl)borate as an electrolyte additive for improving interfacial stability of high voltage layered lithium-rich oxide cathode/carbonate-based electrolyte. J Power Sources 285:360–366. doi:10.1016/j.jpowsour.2015.03.113
Kim JH, Myung ST, Sun YK (2004) Molten salt synthesis of LiNi0.5Mn1.5O4 spinel for 5 V class cathode material of Li-ion secondary battery. Electrochim Acta 49(2):219–227. doi:10.1016/j.electacta.2003.07.003
Chen H, Grey CP (2008) Molten salt synthesis and high rate performance of the “desert-rose” form of LiCoO2. Adv Mater 20(11):2206–2210. doi:10.1002/adma.200702655
Han C-H, Hong Y-S, Park CM, Kim K (2001) Synthesis and electrochemical properties of lithium cobalt oxides prepared by molten-salt synthesis using the eutectic mixture of LiCl–Li2CO3. J Power Sources 92(2001):95–101
Guo Q, Li S, Wang H, Gao Y, Li B (2014) Molten salt synthesis of nano-sized Li4Ti5O12 doped with Fe2O3 for use as anode material in the lithium-ion battery. RSC Adv 4(104):60327–60333. doi:10.1039/c4ra09813g
Zhao X, Cui Y, Xiao L, Liang H, Liu H (2011) Molten salt synthesis of Li1+x (Ni0.5Mn0.5)1−xO2 as cathode material for Li-ion batteries. Solid State Ionics 192(1):321–325. doi:10.1016/j.ssi.2010.04.002
Tang W, Yang X, Liu Z, Kasaishi S, Ooi K (2002) Preparation of fine single crystals of spinel-type lithium manganese oxide by LiCl flux method for rechargeable lithium batteries. Part 1. LiMn2O4. J Mater Chem 12(10):2991–2997. doi:10.1039/b203200g
Zhao Y, Ren W, Wu R, Yue Y, Sun Y (2013) Improved molten salt synthesis and structure evolution upon cycling of 0.5Li2MnO3·0.5LiCoO2 in lithium-ion batteries. J Solid State Electrochem 17(8):2259–2267. doi:10.1007/s10008-013-2089-0
ZhenYao W, Biao L, Jin M, DingGuo X (2014) The enhanced electrochemical performance of nanocrystalline Li[Li0.26Ni0.11Mn0.63]O2 synthesized by the molten-salt method for Li-ion batteries. Electrochim Acta 117:285–291. doi:10.1016/j.electacta.2013.11.124
Zhang T, Li J-T, Liu J, Deng Y-P, Wu Z-G, Yin Z-W, Wu J-H, Huang L, Sun S-G (2016) Improving the electrochemical performance of Li1.14Ni0.18Mn0.62O2 by modulating structure defects via a molten salt method. ChemElectroChem 3(1):98–104. doi:10.1002/celc.201500390
Liu X, Wu Ji, Huang X, Liu Z, Zhang Y, Wang M, Che R (2014a) Predominant orientation growth of Li1.2(Mn0.4Co0.4)O2 cathode materials by NaOH compound molten salt method and their enhanced electrochemical performance. J Mater Chem A 2(36):15200–15208. doi: 10.1039/C4TA02841D
Liu J, Durstock M, Dai L (2014b) Graphene oxide derivatives as hole- and electron-extraction layers for high-performance polymer solar cells. Energy Environ Sci 7(4):1297–1306. doi:10.1039/c3ee42963f
Zhang X, Luo D, Li G, Zheng J, Yu C, Guan X, Fu C, Huang X, Li L (2013b) Self-adjusted oxygen-partial-pressure approach to the improved electrochemical performance of electrode Li[Li0.14Mn0.47Ni0.25Co0.14]O2 for lithium-ion batteries. J Mater Chem A 1:9721–9729. doi:10.1039/C3TA11040K
T-h C, S-m P, Yoshio M (2004) Preparation of layered Li[Ni1⁄3Mn1⁄3Co1⁄3]O2 as a cathode for lithium secondary battery by carbonate coprecipitation method. Chem Lett 33(6):704–705. doi:10.1246/cl.2004.704
Lu Z, MacNeil DD, Dahn JR (2001) Layered cathode materials Li[NixLi(1/3−2x/3)Mn(2/3−x/3)]O2 for lithium-ion batteries. Electrochem Solid-State Lett 4(11):A191. doi:10.1149/1.1407994
Liu G-B, Liu H, Wang Y, Shi Y-F, Zhang Y (2013) The electrochemical properties of Fe- and Ni-cosubstituted Li2MnO3 via combustion method. J Solid State Electrochem 17(9):2437–2444. doi:10.1007/s10008-013-2127-y
Xiang X, Knight JC, Li W, Manthiram A (2014) Understanding the influence of composition and synthesis temperature on oxygen loss, reversible capacity, and electrochemical behavior of xLi2MnO3-(1–x)LiCoO2 cathodes in the first cycle. J Phys Chem C 118(41):23553–23558. doi:10.1021/jp507687h
Shen CH, Wang Q, Fu F, Huang L, Lin Z, Shen SY, Su H, Zheng XM, Xu BB, Li JT, Sun SG (2014) Facile synthesis of the Li-rich layered oxide Li1.23Ni0.09Co0.12Mn0.56O2 with superior lithium storage performance and new insights into structural transformation of the layered oxide material during charge-discharge cycle: in situ XRD characterization. ACS Appl Mater Interfaces 6(8):5516–5524. doi:10.1021/am405844b
Chen M, Xiang X, Chen D, Liao Y, Huang Q, Li W (2015b) Polyethylene glycol-assisted synthesis of hierarchically porous layered lithium-rich oxide as cathode of lithium ion battery. J Power Sources 279:197–204. doi:10.1016/j.jpowsour.2015.01.004
Xia G, Li N, Li D, Liu R, Xiao N, Tian D (2011) Molten-salt decomposition synthesis of SnO2 nanoparticles as anode materials for lithium ion batteries. Mater Lett 65(23–24):3377–3379. doi:10.1016/j.matlet.2011.07.008
Deng ZQ, Manthiram A (2011) Influence of cationic substitutions on the oxygen loss and reversible capacity of lithium-rich layered oxide cathodes. J Phys Chem C 115(14):7097–7103. doi:10.1021/jp200375d
Armstrong AR, Lyness C, Panchmatia PM, Islam MS, Bruce PG (2011) The lithium intercalation process in the low-voltage lithium battery anode Li(1+x)V(1-x)O2. Nat Mater 10(3):223–229. doi:10.1038/nmat2967
Svens P, Eriksson R, Hansson J, Behm M, Gustafsson T, Lindbergh G (2014) Analysis of aging of commercial composite metal oxide—Li4Ti5O12 battery cells. J Power Sources 270:131–141. doi:10.1016/j.jpowsour.2014.07.050
Singh G, West WC, Soler J, Katiyar RS (2012) In situ Raman spectroscopy of layered solid solution Li2MnO3–LiMO2 (M=Ni, Mn, Co). J Power Sources 218:34–38. doi:10.1016/j.jpowsour.2012.06.083
Wei Z, Xia Y, Qiu B, Zhang Q, Han S, Liu Z (2015) Correlation between transition metal ion migration and the voltage ranges of electrochemical process for lithium-rich manganese-based material. J Power Sources 281:7–10. doi:10.1016/j.jpowsour.2015.01.149
Xiang X, Li W (2014) Self-directed chemical synthesis of lithium-rich layered oxide Li[Li0.2Ni0.2Mn0.6]O2 with tightly interconnected particles as cathode of lithium ion batteries with improved rate capability. Electrochim Acta 127:259–265. doi:10.1016/j.electacta.2014.02.037
Takezawa H, Iwamoto K, Ito S, Yoshizawa H (2013) Electrochemical behaviors of nonstoichiometric silicon suboxides (SiOx) film prepared by reactive evaporation for lithium rechargeable batteries. J Power Sources 244:149–157. doi:10.1016/j.jpowsour.2013.02.077
Arias NP, Dávila MT, Giraldo O (2012) Electrical behavior of an octahedral layered OL-1-type manganese oxide material. Ionics 19(2):201–214. doi:10.1007/s11581-012-0725-9
Xiang X, Li X, Li W (2013b) Preparation and characterization of size-uniform Li[Li0.131Ni0.304Mn0.565]O2 particles as cathode materials for high energy lithium ion battery. J Power Sources 230:89–95. doi:10.1016/j.jpowsour.2012.12.050
Hong YJ, Kim JH, Kim MH, Kang YC (2012) Electrochemical properties of 0.3Li2MnO3·0.7LiNi0.5Mn0.5O2 composite cathode powders prepared by large-scale spray pyrolysis. Mater Res Bull 47(8):2022–2026. doi:10.1016/j.materresbull.2012.04.008
Kim D, Kang S-H, Balasubramanian M, Johnson CS (2010) High-energy and high-power Li-rich nickel manganese oxide electrode materials. Electrochem Commun 12(11):1618–1621. doi:10.1016/j.elecom.2010.09.009
Kweon H-J, Park J, Seo J, Kim G, Jung B, Lim HS (2004) Effects of metal oxide coatings on the thermal stability and electrical performance of LiCoCO2 in a Li-ion cell. J Power Sources 126(1–2):156–162. doi:10.1016/j.jpowsour.2003.08.037
Sun Y-K, Kang H-B, Myung S-T, Prakash J (2010) Effect of manganese content on the electrochemical and thermal stabilities of Li[Ni0.58Co0.28−xMn0.14+x]O2 cathode materials for lithium-ion batteries. J Electrochem Soc 157(12):A1335. doi:10.1149/1.3497304
Lee K-S, Myung S-T, Kim D-W, Sun Y-K (2011) AlF3-coated LiCoO2 and Li[Ni1/3Co1/3Mn1/3]O2 blend composite cathode for lithium ion batteries. J Power Sources 196(16):6974–6977. doi:10.1016/j.jpowsour.2010.11.014
Nayak PK, Grinblat J, Levi MD, Haik O, Levi E, Talianker M, Markovsky B, Sun Y-K, Aurbach D (2015) Electrochemical performance of a layered-spinel integrated Li[Ni1/3Mn2/3]O2 as a high capacity cathode material for Li-ion batteries. Chem Mater 27(7):2600–2611. doi:10.1021/acs.chemmater.5b00405
Boulineau A, Simonin L, Colin JF, Bourbon C, Patoux S (2013) First evidence of manganese-nickel segregation and densification upon cycling in Li-rich layered oxides for lithium batteries. Nano Lett 13(8):3857–3863. doi:10.1021/nl4019275
Lu D, Li W, Xiaoxi Z, Yuan Z, Huang Q (2007) Study on electrode kinetics of Li+ insertion in LixMn2O4 (0≤ x ≤ 1) by electrochemical impedance spectroscopy. Journal of Chemical Physics C 111(32):12067–12074. doi:10.1021/jp0732920
He W, Qian J, Cao Y, Ai X, Yang H (2012) Improved electrochemical performances of nanocrystalline Li[Li0.2Mn0.54Ni0.13Co0.13]O2 cathode material for Li-ion batteries. RSC Adv 2(8):3423–3429. doi:10.1039/c2ra20122d
Liu H, Kloepsch R, Wang J, Winter M, Li J (2015) Truncated octahedral LiNi0.5Mn1.5O4 cathode material for ultralong-life lithium-ion battery: positive (100) surfaces in high-voltage spinel system. J Power Sources 300:430–437. doi:10.1016/j.jpowsour.2015.09.066
Acknowledgments
This work is supported by the National Natural Science Foundation of China (Grant No. 21573080), the Natural Science Foundation of Guangdong Province (Grant No. 2014A030313424), the Key Project of Science and Technology in Guangdong Province (Grant No. 2015B010116002), Guangzhou City Project for Cooperation among Industries, Universities and Institutes (Grant No. 201509030005), and the Scientific Research Project of Department of Education of Guangdong Province (Grant No. 2013CXZDA013).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhong, X., Chen, M., Zhu, Y. et al. Layered lithium-rich oxide nanoparticles: low-temperature synthesis in mixed molten salt and excellent performance as cathode of lithium-ion battery. Ionics 23, 1955–1966 (2017). https://doi.org/10.1007/s11581-017-2039-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11581-017-2039-4