Abstract
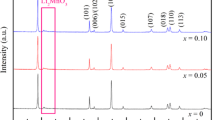
Li-rich Li1 + x (Mn0.675Ni0.1625Co0.1625)1 − x O2 (x = 0.1, 0.2, 0.3, and 0.4) materials were prepared using MCO3 precursors through hydrothermal treatment with using urea as precipitator. X-ray diffraction (XRD), scanning electron microscopy (SEM), energy-dispersive X-ray spectroscopy (EDS), transmission electron microscope (TEM), X-ray photoelectron spectroscopy (XPS), cyclic voltammetry (CV), electrochemical impedance spectroscopy (EIS), and charge/discharge test are conducted to evaluate the physical and electrochemical properties of the spherical mesocrystal and resulting materials. Results show that the c/a ratio for the cathode material decreases and the layered structure deteriorates with increasing Li content. Li1.2Mn0.54Ni0.13Co0.13O2 (x = 0.2) exhibits the excellent electrochemical performance with an initial discharge capacity of 339 mAh g−1 at 20 mA g−1 and a capacity of 115 mAh g−1 at 400 mA g−1 after 50 cycles.








Similar content being viewed by others
References
Stoyanova R, Zhecheva E, Zarkoba L (1994) Effect of Mn-substitution for Co on the crystal structure and acid delithiation of LiMnyCo1-yO2 solid solutions. Solid State Ionics 73:233–240
Ohzuku T, Ueda A, Nagayama M (1993) Electrochemistry and structural chemistry of LiNiO2(R3m) for 4 volt secondary lithium cells. J Electrochem Soc 140:1862–1870
Shi SJ, Lou ZR, Xia TF, Wang XL, Gu CD, Tu JP (2014) Hollow Li1.2Mn0.5Co0.25Ni0.05O2 microcube prepared by binary template as a cathode material for lithium ion batteries. J Power Sources 257:198–204
Yia TF, Tao W, Chen B, Zhu YR, Yang SY, Xie Y (2016) High-performance xLi2MnO3(1-x)LiMn1/3Co1/3Ni1/3O2 (0.1≤ x ≤0.5) as Cathode Material for Lithium-ion Battery. Electrochim Acta 188:686–695
He ZJ, Wang ZX, Chen H, Huang ZM, Li XH, Guo HJ, Wang RH (2015) Electrochemical performance of zirconium doped lithium rich layered Li1.2Mn0.54Ni0.13Co0.13O2 oxide with porous hollow structure. J Power Sources 299:334–341
Zhang Y, Li Y, Xia X, Wang X, Gu C, Tu J (2015) High-energy cathode materials for Li-ion batteries: a review of recent developments. Science China Technol Sci. Science China Technol Sci 58:1809–1828
Martha SK, Nanda J, Veith GM, Dudney NJ (2012) Electrochemical and rate performance study of high voltage lithium-rich composition: Li1.2Mn0.525Ni0.175Co0.1O2. J Power Sources 199:220–226
Shin S-S, Sun Y-K, Amine K (2002) Synthesis and electrochemical properties of Li [Li(1−2x)/3NixMn(2−x)/3]O2 as cathode materials for lithium secondary batteries. J Power Sources 112:634–638
Shi SJ, Tu JP, Tang YY, Yu YX, Zhang YQ, Wang XL (2013) Synthesis and electrochemical performance of Li1.131Mn0.504Ni0.243Co0.122O2 cathode materials for lithium ion batteries via freeze drying. J Power Sources 221:300–307
Zheng Z, Liao S-X, Xu B-B, Zhong B-H (2015) The roles of nickel/manganese in electrochemical cycling of lithium-rich Mn-based nickel cathode materials. Ionics 21:3295–3300
Zhang L, Jin K, Wang L, Zhang Y, Li X, Song Y (2015) High capacity Li1.2Mn0.54Ni0.13Co0.13O4 cathode materials synthesized using mesocrystal precursors for lithium-ion batteries. J Alloys Compd 638:298–304
Cölfen H, Antonietti M (2005) Mesocrystals: inorganic superstructures made by highly parallel crystallization and controlled alignment. Angew Chem Int Ed 44:5576–5591
Zhou L, O’Brien P (2012) Mesocrystals-Properties and Applications. J Phys Chem Lett 3:620–628
Song RQ, Cölfen H (2010) Mesocrystals—Ordered nanoparticle superstructures. Adv Mater 22:1301–1330
Fang J, Ding B, Gleiter H (2011) Mesocrystals: syntheses in metals and applications. Chem Soc Rev 40:5347–5360
Tang Z, Wang Z, Li X, Peng W (2012) Influence of lithium content on the electrochemical performance of Li1+x(Mn0.533Ni0.233Co0.233)1−xO2 cathode materials. J Power Sources 208:237–241
Gao Y, Yakovleva M (1998) Novel LiNi1−xTix/2Mgx/2O2 Compounds as Cathode Materials for Safer Lithium‐Ion Batteries. W. Ebner. Electrochem Solid-State Lett 1:117–119
Ohzuku T, Takeda S, Iwanaga M (1999) Solid-state redox potentials for Li[Me1/2Mn3/2]O2 (Me: 3dtransition metal) having spinel-framework structures: a series of 5 volt materials for advanced lithium-ion batteries. J Power Sources 81:90–94
Liu Y, Liu S (2013) Effect of cooling method on the electrochemical performance of 0.5Li2MnO3·0.5LiNi0.5Mn0.5O2 cathodes. Ionics 19:477–481
Kang SH, Kim J, Stoll ME, Abraham D, Sun YK, Amine K (2002) Layered Li(Ni0.5−xMn0.5−xM2x)O2(M′=Co, Al, Ti; x=0, 0.025) cathode materials for Li-ion rechargeable batteries. J Power Sources 112:41–48
Hattori Y, Konishi T, Kaneko K (2002) XAFS and XPS studies on the enhancement of methane adsorption by NiO dispersed ACF with the relevance to structural change of NiO. Chem Phys Lett 355:37–42
Moses AW, Flores HGG, Kim J-G, Langell MA (2007) Surface properties of LiCoO2, LiNiO2 and LiNi1−xCoxO2. Appl Surf Sci 253:4782–4791
Casella IG, Guascito MR (1999) Anodic electrodeposition of conducting cobalt oxyhydroxide films on a gold surface. XPS study and electrochemical behaviour in neutral and alkaline solution. J Electroanal Chem 476:54–63
Sivaprakash S, Majumder S, Katiyar R (2009) Investigations on 0.5Li(Ni0.8Co0.15Zr0.05)O2 –0.5Li(Li1⁄3Mn2⁄3)O2 Cathode for Li Rechargeable Batteries. J Electrochem Soc 156:A328–A333
Paulsen J, Thomas C, Dahn J (2000) O2 Structure Li2/3[Ni1/3Mn2/3]O2: A New Layered Cathode Material for Rechargeable Lithium Batteries. J Electrochem Soc 147:861–868
Sivaprakash S, Majumder S (2010) Spectroscopic analyses of 0.5Li[Ni0.8Co0.15Zr0.05O2 -0.5Li[Li1/3Mn2/3]O2 composite cathodes for lithium rechargeable batteries. Solid State Ionics 181:730–739
Park S-H, Kang S-H, Johnson C, Amine K, Thackeray M (2007) Lithium–manganese–nickel-oxide electrodes with integrated layered–spinel structures for lithium batteries. Electrochem Commun 9:262–268
Lu Z, Dahn JR (2002) Understanding the anomalous capacity of Li/Li[NixLi(1/3−2x/3)Mn(2/3−x/3)]O2 cells using in situ X-ray diffraction and electrochemical studies. J Electrochem Soc 149:A815–A822
Zhang L, Borong W, Ning L, Feng W (2014) Hierarchically porous micro-rod lithium-rich cathode material Li1.2Ni0.13Mn0.54Co0.13O2 for high performance lithium-ion batteries. Electrochim Acta 118:67–74
Li L, Zhang X, Chen R, Zhao T, Lu J, Wu F, Amine K (2014) Synthesis and electrochemical performance of cathode material Li1.2Co0.13Ni0.13Mn0.54O2 from spent lithium-ion batteries. J Power Sources 249:28–34
Bettge M, Li Y, Sankaran B, Rago ND, Spila T, Haasch RT, Petrov I, Abraham DP (2013) Improving highcapacity Li1.2Ni0.15Mn0.55Co0.1O2 -based lithium-ion cells by modifiying the positive electrode with alumina. J Power Sources 233:346–357
Zhang YD, Li Y, Niu XQ, Wang DH, Zhou D, Wang XL, Gu CD, Tu JP (2015) A peanut-like hierarchical micro/nano-Li1.2Mn0.54Ni0.18Co0.08O2 cathode material for lithium-ion batteries with enhanced electrochemical performance. J Mater Chem A 3:14291–14297
Du CQ, Zhang F, Ma CX, Wu JW, Tang ZY, Zhang XH, Qu DY (2016) Synthesis and electrochemical properties of Li1.2Mn0.54Ni0.13Co0.13O2 cathode material for lithium-ion battery. Ionics 22:209–218
Acknowledgments
This work is supported by Foundation of Henan Educational Committee (No. 13A530366), Foundation for Young Teachers of 2012 Henan Province Colleges and Universities (GGJS-116), Foundation for Young Teachers of Zhengzhou University of Light Industry (2011XGGJS005), and Scientific Research Foundation of Zhengzhou University of Light Industry in 2015 (No. 2015XJJZ036).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhang, L., Wang, H. & Wang, L. Effect of Li content on the electrochemical performance of Li1 + x (Mn0.675Ni0.1625Co0.1625)1 − x O2 cathode materials for high-power Li-ion batteries. Ionics 23, 829–835 (2017). https://doi.org/10.1007/s11581-016-1882-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11581-016-1882-z