Abstract
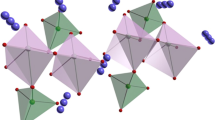
The hierarchical LiMn0.5Fe0.5PO4/C (LMFP) nanorods were first successfully synthesized by rheological phase method using polyethylene glycol 4000 (PEG 4000) as a template reagent. The physical and electrochemical properties of the LiMn0.5Fe0.5PO4/C were characterized by TG-DTG, XRD, FTIR, SEM, TEM, EIS and galvanostatic charge-discharge measurements. The results reveal that the PEG-LMFP/C synthesized with the assistance of PEG 4000 shows unique bundle-type shape assembled of nanorods, while the LMFP/C synthesized without PEG 4000 presents a platelet-like shape with some agglomeration. Besides, a uniformly carbon layer coating on the surface of the PEG-LMFP/C can be seen from TEM images. The PEG-LMFP/C exhibits high specific capacity and superior rate performance with discharge capacities of 162, 133, 108, 95, and 78 mAh · g−1 at 0.1, 1, 5, 10, and 20 C rates, respectively. It is demonstrated that the synthesis of LMFP/C with PEG 4000 can significantly decrease the characteristic sizes of the crystals, resulting in improved electrochemical performance.








Similar content being viewed by others
References
Padhi AK, Nanjundaswamy KS, Goodenough JB (1997) Phospho-olivines as positive-electrode materials for rechargeable lithium batteries. J Electrochem Soc 1444:1188–1194
Hu C, Yi H, Fang H, Yang B, Yao Y, Ma W, Dai Y (2010) Improving the electrochemical activity of LiMnPO4 via Mn-site co-substitution with Fe and Mg. Electrochem Commun 1212:1784–1787
Oh S-M, Myung S-T, Choi YS, Oh KH, Sun Y-K (2011) Co-precipitation synthesis of micro-sized spherical LiMn0.5Fe0.5PO4 cathode material for lithium batteries. J Mater Chem 2148:19368–19374
Akimoto S, Taniguchi I (2013) Synthesis and characterization of LiCo1/3Mn1/3Fe1/3PO4/C nanocomposite cathode of lithium batteries with high rate performance. J Power Sources 242:627–630
Ni J, Gao L (2011) Effect of copper doping on LiMnPO4 prepared via hydrothermal route. J Power Sources 19615:6498–6501
Zhu H-J, Zhai W, Yang M, X-m L, Chen Y-C, Yang H, Shen X-d (2014) Synthesis and characterization of LiMnPO4/C nano-composites from manganese(II) phosphate trihydrate precipitated from a micro-channel reactor approach. RSC Adv 449:25625–25632
Yamada A, Kudo Y, Liu KY (2001) Reaction mechanism of the olivine-type Li x (Mn0.6Fe0.4)PO4 (0 ≤ x ≤ 1). J Electrochem Soc 1487:A747–A754
Chen GY, Wilcox JD, Richardson TJ (2008) Improving the performance of lithium manganese phosphate through divalent cation substitution. Electrochem Solid-State Lett 1111:A190–A194
Liu W, Gao P, Mi Y, Chen J, Zhou H, Zhang X (2013) Fabrication of high tap density LiFe0.6Mn0.4PO4/C microspheres by a double carbon coating-spray drying method for high rate lithium ion batteries. J Mater Chem A 17:2411–2417
Ni J, Han Y, Gao L, Lu L (2013) One-pot synthesis of CNT-wired LiCo0.5Mn0.5PO4 nanocomposites. Electrochem Commun 31:84–87
Hong J, Wang F, Wang X, Graetz J (2011) LiFe x Mn1-x PO4: a cathode for lithium-ion batteries. J Power Sources 1967:3659–3663
Zou Q-Q, Zhu G-N, Xia Y-Y (2012) Preparation of carbon-coated LiFe0.2Mn0.8PO4 cathode material and its application in a novel battery with Li4Ti5O12 anode. J Power Sources 206:222–229
Oh S-M, Jung H-G, Yoon CS, Myung S-T, Chen Z, Amine K, Sun Y-K (2011) Enhanced electrochemical performance of carbon-LiMn1-x Fe x PO4 nanocomposite cathode for lithium-ion batteries. J Power Sources 19616:6924–6928
Saravanan K, Ramar V, Balaya P, Vittal JJ (2011) Li(Mn x Fe1-x )PO4/C (x = 0.5, 0.75 and 1) nanoplates for lithium storage application. J Mater Chem 2138:14925–14935
Zhang B, Wang X, Li H, Huang X (2011) Electrochemical performances of LiFe1-x Mn x PO4 with high Mn content. J Power Sources 19616:6992–6996
Gan Y, Chen C, Liu J, Bian P, Hao H, Yu A (2015) Enhancing the performance of LiMnPO4/C composites through Cr doping. J Alloys Compd 620:350–357
Li BZ, Wang Y, Xue L, Li XP, Li WS (2013) Acetylene black-embedded LiMn0.8Fe0.2PO4/C composite as cathode for lithium ion battery. J Power Sources 232:12–16
Zuo P, Cheng G, Wang L, Ma Y, Du C, Cheng X, Wang Z, Yin G (2013) Ascorbic acid-assisted solvothermal synthesis of LiMn0.9Fe0.1PO4/C nanoplatelets with enhanced electrochemical performance for lithium ion batteries. J Power Sources 243:872–879
Huo Z-Q, Cui Y-T, Wang D, Dong Y, Chen L (2014) The influence of temperature on a nutty-cake structural material: LiMn1-x Fe x PO4 composite with LiFePO4 core and carbon outer layer for lithium-ion battery. J Power Sources 245:331–336
Xiao PF, Ding B, Lai MO, Lu L (2013) High performance LiMn1-x Fe x PO4 (0 ≤ x ≤ 1) synthesized via a facile polymer-assisted mechanical activation. J Electrochem Soc 1606:A918–A926
Li Y, Hong L, Sun J, Wu F, Chen S (2012) Electrochemical performance of Li3V2(PO4)3/C prepared with a novel carbon source, EDTA. Electrochim Acta 85:110–115
Qiao YQ, Wang XL, Zhou JP, Zhang J, Gu CD, Tu JP (2012) Synthesis and electrochemical performance of rod-like LiV3O8 cathode materials for rechargeable lithium batteries. J Power Sources 198:287–293
Yanying W, Yan T, Benhe Z, Heng L, Yanjun Z, Xiaodong G (2014) Facile synthesis of Li 3V 2(PO 4) 3/C nano-flakes with high-rate performance as cathode material for Li-ion battery. J Solid State Electrochem 181:215–221
Kosova NV, Devyatkina ET, Slobodyuk AB, Petrov SA (2012) Submicron LiFe1-y Mn y PO4 solid solutions prepared by mechanochemically assisted carbothermal reduction: the structure and properties. Electrochim Acta 59:404–411
Zhong Y-J, Li J-T, Wu Z-G, Guo X-D, Zhong B-H, Sun S-G (2013) LiMn0.5Fe0.5PO4 solid solution materials synthesized by rheological phase reaction and their excellent electrochemical performances as cathode of lithium ion battery. J Power Sources 234:217–222
Song J, Wang L, Shao G, Shi M, Ma Z, Wang G, Song W, Liu S, Wang C (2014) Controllable synthesis, morphology evolution and electrochemical properties of LiFePO4 cathode materials for Li-ion batteries. Phys Chem Chem Phys 1617:7728–7733
Yang S-L, Ma R-G, Hu M-J, Xi L-J, Lu Z-G, Chung CY (2012) Solvothermal synthesis of nano-LiMnPO4 from Li3PO4 rod-like precursor: reaction mechanism and electrochemical properties. J Mater Chem 2248:25402–25408
Miao X, Yan Y, Wang C, Cui L, Fang J, Yang G (2014) Optimal microwave-assisted hydrothermal synthesis of nanosized xLi2MnO3 · (1 − x) LiNi1/3Co1/3Mn1/3O2 cathode materials for lithium ion battery. J Power Sources 247:219–227
Guo XP, Wang M, Huang XL, Zhao PF, Liu XL, Che RC (2013) Direct evidence of antisite defects in LiFe0.5Mn0.5PO4 via atomic-level HAADF-EELS. J Mater Chem A 131:8775–8781
Saravanan K, Vittal JJ, Reddy MV, Chowdari BVR, Balaya P (2010) Storage performance of LiFe1-x Mn x PO4 nanoplates (x = 0, 0.5, and 1). J Solid State Electrochem 1410:1755–1760
Zong J, Peng Q, Yu J, Liu X (2013) Novel precursor of Mn(PO3(OH))center dot 3H2O for synthesizing LiMn0.5Fe0.5PO4 cathode material. J Power Sources 228:214–219
Dong Y, Zhao Y, Duan H, Liang Z (2014) Enhanced electrochemical performance of LiMnPO4 by Li+-conductive Li3VO4 surface coatings. Electrochim Acta 132:244–250
Ni J, Morishita M, Kawabe Y, Watada M, Takeichi N, Sakai T (2010) Hydrothermal preparation of LiFePO4 nanocrystals mediated by organic acid. J Power Sources 1959:2877–2882
Xiang W, Zhong YJ, Ji JY, Tang Y, Shen HH, Guo XD, Zhong BH, Dou SX, Zhang ZY (2015) Hydrothermal synthesis, evolution, and electrochemical performance of LiMn0.5Fe0.5PO4 nanostructures. Phys Chem Chem Phys 17:18629–18637
Acknowledgments
The authors appreciate the supports of the project funded by the China Postdoctoral Science Foundation (No. 2014M562322) and the AutoCRC Project 1-111 “Development of Advanced Electrode and Electrolytes for LIB.”
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Shen, H., Xiang, W., Shi, X. et al. Hierarchical LiMn0.5Fe0.5PO4/C nanorods with excellent electrochemical performance synthesized by rheological phase method as cathode for lithium ion battery. Ionics 22, 193–200 (2016). https://doi.org/10.1007/s11581-015-1539-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11581-015-1539-3