Abstract
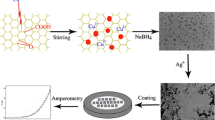
A sensor for the highly sensitive determination of Sudan I based on the amplified electrochemical response of mesoporous TiO2-decorated graphene (GN–TiO2) was fabricated. The nanoparticles of TiO2 arrayed densely and uniformly on the GN sheets, as confirmed by field emission scanning electron microscopy and transmission electron microscopy images. The electrochemical behavior of Sudan I at this sensor was studied in detail, showing that this sensor exhibited electrocatalytic activity for the oxidation of Sudan I because of the significant peak current enhancement and the lowering of oxidation overpotential. Furthermore, the experimental parameters including supporting electrolyte, volume of GN–TiO2 suspension on electrode surface, accumulation potential, and time were optimized and the electrochemical reaction mechanism of Sudan I on this sensor was investigated. The linear range is from 3.3 nM to 0.66 μM, and the limit of detection is estimated to be 0.60 nM. At last, the sensor was used to determine Sudan I in food sample extracts, which are in good agreement with the results obtained by chromatographic method.







Similar content being viewed by others
References
He LM, Su YJ, Fang BH, Shen XG, Zeng ZL, Liu YH (2007) Determination of Sudan dye residues in eggs by liquid chromatography and gas chromatography–mass spectrometry. Anal Chim Acta 594(1):139–146
Baggiani C, Anfossi L, Baravalle P, Giovannoli C, Giraudi G, Barolo C, Viscardi G (2009) Determination of banned Sudan dyes in food samples by molecularly imprinted solid phase extraction—high performance liquid chromatography. J Sep Sci 32(19):3292–3300
Cornet V, Govaert Y, Moens G, Loco JV, Degroodt JM (2006) Development of a fast analytical method for the determination of Sudan dyes in chili—and curry-containing foodstuffs by high performance liquid chromatography—photodiode array detection. J Agric Food Chem 54(3):639–644
Lin HG, Li G, Wu KB (2008) Electrochemical determination of Sudan I using montmorillonite calcium modified carbon paste electrode. Food Chem 107(1):531–536
Yang DX, Zhu LD, Jiang XY (2010) Electrochemical reaction mechanism and determination of Sudan I at a multi wall carbon nanotubes modified glassy carbon electrode. J Electroanal Chem 640(1–2):17–22
Mo ZR, Zhang YF, Zhao FQ, Xiao F, Guo GP, Zeng BZ (2010) Sensitive voltammetric determination of Sudan I in food samples by using gemini surfactant-ionic liquid-multiwalled carbon nanotube composite film modified glassy carbon electrodes. Food Chem 121(1):233–237
Wu YH (2010) Electrocatalysis and sensitive determination of Sudan I at the single-walled carbon nanotubes and iron(III)-porphyrin modified glassy carbon electrodes. Food Chem 121(2):580–584
Chao MY, Ma XY (2012) Electrochemical determination of Sudan I at a silver nanoparticles/poly(aminosulfonic acid) modified glassy carbon electrode. Int J Electrochem Sci 7(7):6331–6342
Yang XF, He DH (2010) Rapid determination of banned Sudan I in foodstuffs using a mesoporous SiO2 modified electrode. J AOAC Int 93(5):1537–1541
Yang DX, Zhu LD, Jiang XY, Guo LP (2009) Sensitive determination of Sudan I at an ordered mesoporous carbon modified glassy carbon electrode. Sensor Actuat B–Chem 141(1):124–129
Yin HS, Zhou YL, Meng XM, Tang TT, Ai SY, Zhu LS (2011) Electrochemical behaviour of Sudan I at Fe3O4 nanoparticles modified glassy carbon electrode and its determination in food samples. Food Chem 127(3):1348–1353
Zhang J, Wang ML, Shentu C, Wang WC, He Y, Chen ZD (2012) Electrochemical detection of Sudan I by using an expanded graphite paste electrode. J Electroanal Chem 685:47–52
Geim AK (2009) Graphene: status and prospects. Science 324(5934):1530–1534
Balandin AA, Ghosh S, Bao W, Calizo I, Teweldebrhan D, Miao F, Lau CN (2008) Superior thermal conductivity of single-layer graphene. Nano Lett 8(3):902–907
Yang JQ, Chen JW, Zhou YK, Wu KB (2011) A nano-copper electrochemical sensor for sensitive detection of chemical oxygen demand. Sensor Actuat B–Chem 153(1):78–82
Cheng Q, Wu C, Chen JW, Zhou YK, Wu KB (2011) Electrochemical tuning the activity of nickel nanoparticle and application in sensitive detection of chemical oxygen demand. J Phys Chem C 115(46):22845–22850
Wang L, Wang DL, Zhu JS, Liang XS (2013) Preparation of Co3O4 nanoplate/graphene sheet composites and their synergistic electrochemical performance. Ionics 19(2):215–220
Gan T, Hu CG, Chen ZL, Hu SS (2011) A disposable electrochemical sensor for the determination of indole-3-acetic acid based on poly(safranine T)-reduced graphene oxide nanocomposite. Talanta 85(1):310–316
Gainsford GJ, Kemmitt T, Lensink C, Milestone NB (1995) Isolation and characterization of anionic titanium tris(glycolate) complexes. Inorg Chem 34(3):746–748
Khushalani D, Ozin GA, Kuperman A (1999) Glycometallate surfactants Part 2: nonaqueous synthesis of mesoporous titanium, zirconium and niobium oxides. J Mater Chem 9(7):1491–1500
Patel R, Ahn SH, Chi WS, Kim JH (2012) Poly(vinyl chloride)-graft-poly(N-vinyl caprolactam) graft copolymer: synthesis and use as template for porous TiO2 thin films in dye-sensitized solar cells. Ionics 18(4):395–402
Low SP, Ahmad A, Rahman MYA (2010) Effect of ethylene carbonate plasticizer and TiO2 nanoparticles on 49 % poly(methyl methacrylate) grafted natural rubber-based polymer electrolyte. Ionics 16(9):821–826
Sreeprasad TS, Samal AK, Pradeep T (2009) Tellurium nanowire-induced room temperature conversion of graphite oxide to leaf-like graphenic structures. J Phys Chem C 113(5):1727–1737
Bourlinos AB, Gournis D, Petridis D, Szabó T, Szeri A, Dékány I (2003) Graphite oxide: chemical reduction to graphite and surface modification with primary aliphatic amines and amino acids. Langmuir 19(15):6050–6055
Li N, Liu G, Zhen C, Li F, Zhang LL, Cheng HM (2011) Battery performance and photocatalytic activity of mesoporous anatase TiO2 nanospheres/graphene composites by template-free self-assembly. Adv Funct Mater 21(9):1717–1722
Acknowledgment
This research is supported by the National Nature Science Foundation of China (No. 61201091), the Natural Science Foundation of He’nan Province of China (No. 132300410060), and the Program for University Innovative Research Team of Henan (No. 2012IRTSHN017).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gan, T., Sun, J., He, M. et al. Highly sensitive electrochemical sensor for Sudan I based on graphene decorated with mesoporous TiO2 . Ionics 20, 89–95 (2014). https://doi.org/10.1007/s11581-013-0951-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11581-013-0951-9