Abstract
A novel network version of permutation entropy, the inverted joint permutation entropy (JPEinv), holds potential as non-invasive biomarker of abnormal excitation-inhibition (E-I) ratio in Alzheimer’s disease (AD). In this computational modelling study, we test the hypotheses that this metric, and related measures of signal variability and functional connectivity, are sensitive to altered E-I ratios. The E-I ratio in each neural mass of a whole-brain computational network model was systematically varied. We evaluated whether JPEinv, local signal variability (by permutation entropy) and functional connectivity (by weighted symbolic mutual information (wsMI)) were related to E-I ratio, on whole-brain and regional level. The hub disruption index can identify regions primarily affected in terms of functional connectivity strength (or: degree) by the altered E-I ratios. Analyses were performed for a range of coupling strengths, filter and time-delay settings. On whole-brain level, higher E-I ratios were associated with higher functional connectivity (by JPEinv and wsMI) and lower local signal variability. These relationships were nonlinear and depended on the coupling strength, filter and time-delay settings. On regional level, hub-like regions showed a selective decrease in functional degree (by JPEinv and wsMI) upon a lower E-I ratio, and non-hub-like regions showed a selective increase in degree upon a higher E-I ratio. These results suggest that abnormal functional connectivity and signal variability, as previously reported in patients across the AD continuum, can inform us about altered E-I ratios.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
An increasing number of studies has provided evidence for an imbalance between excitatory and inhibitory (E-I) neuronal activity as key underlying mechanism of neurological disorders, including Alzheimer’s disease (AD), glioma (Numan et al. 2022), epilepsy (Houtman et al. 2021; Pani et al. 2021), and multiple sclerosis (Huiskamp et al. 2022). Especially because of the high failure rate of anti-amyloid clinical trials, there has been increased attention for other disease mechanisms of AD, such as abnormal E-I ratios, that may serve as therapeutic target to stabilize or prevent cognitive decline. AD patients seem to have altered synaptic activity and neuronal function as early as in a presymptomatic disease stage which may contribute to cognitive decline. For example, AD patients have an increased rate of subclinical epileptiform activity compared to controls and the presence of such events was associated with a faster progression in memory decline (Horvath et al. 2021; Vossel et al. 2016). Restoring the E-I ratio, therefore, provides a promising therapeutic (Bakker et al. 2015; Jin et al. 2022; Koch et al. 2022; Menardi et al. 2022; Vossel et al. 2021). Detection of (subclinical) epileptiform activity remains, however, challenging, especially in AD patients. Only a small percentage of AD patients experience seizures throughout the disease course and subclinical epileptiform activity occurs only sporadically. It is, furthermore, primarily reported during sleep and mostly in deeper layers of the cortex (Lam et al. 2017, 2020; Liedorp et al. 2010; Vossel et al. 2013). Besides the development of more accurate automatic spike detection algorithms (Furbass et al. 2020; Wilson and Emerson 2002), there is a need for alternative, quantitative, and more sensitive measures to timely capture abnormal E-I ratios in short noninvasive recordings.
Several neurophysiological measures have potential to identify abnormal E-I ratio. For instance, measures of cortical network hyperexcitability and epileptogenicity (indicating increased E-I ratios) include frequent small sharp spikes, temporal intermittent rhythmic delta activity and paroxysmal slow wave events (Yu et al. 2021). Interregional functional connectivity measures has been proposed as candidate marker of hyperexcitability in several studies (Cuesta et al. 2022; Ranasinghe et al. 2022a, b; Stam et al. 2023). Whereas band-pass power measures may be sensitive to total neuronal activity levels (Luppi et al. 2022; van Nifterick et al. 2022a, b), other neurophysiological measures, such as the steepness of the aperiodic component of the power spectrum (Donoghue et al. 2020; Gao et al. 2017) and the functional E-I (fEI) method (Bruining et al. 2020), serve as more direct indicators of E-I ratio. In a recent study, we proposed a novel network version of permutation entropy, the inverted joint permutation entropy (JPEinv), as a sensitive marker of E-I ratio. This measure integrates local signal variability and interregional connectivity, which both have been related to E-I in previous work (Bruining et al. 2020; Deco et al. 2014; Demirtas et al. 2017; Gao et al. 2017; Garrett et al. 2015; Maestu et al. 2021). The multi-scale character of JPEinv offers a potential advantage in identifying disrupted E-I ratios from large-scale neurophysiological data when compared to methods constrained to a single scale (Scheijbeler et al. 2022). JPEinv distinguished a small group of early-stage AD patients from healthy controls with a slightly higher accuracy than the relative theta power benchmark, which suggests its potentially added value as sensitive marker of E-I (Scheijbeler et al. 2022). However, whether and how JPEinv is related to E-I remained to be investigated.
Investigating how E-I ratio changes translate to neurophysiological measures in human subjects is challenging, because it requires an experimental setup with E-I modulating therapeutics or invasive intracranial recordings. Computational models provide a means to manipulate local E-I ratios, according to pathophysiological mechanisms reported in the literature, and investigate its impact on large-scale neurophysiological activity. The biophysical meaning of neural mass model parameters in particular facilitate the integration of findings from experimental electrophysiology studies, conducted on the micro- and mesoscale, with human electroencephalography (EEG) or magnetoencephalography (MEG) studies, conducted on the macroscale. Furthermore, the realistic large-scale signals generated by these models can be subjected to analyses analogous to those applied to empirical neurophysiological data, and, thus, allowing for the application of the same analytical approaches employed in the prior JPEinv study (Scheijbeler et al. 2022). Previous studies have already demonstrated that neurophysiological abnormalities associated with AD, such as spectral slowing and functional connectivity disruption, can be simulated by disrupting local neuronal activity in computational neural mass models(de Haan et al. 2012; Stefanovski et al. 2019; A. M. van Nifterick et al. 2022a, b). Virtual interventions that re-established E-I balance preserved and recovered the functional connectivity and network integrity (de Haan et al. 2017; Vattikonda et al. 2016). These studies show how computational models may help to better understand the mechanisms underlying abnormal neurophysiological activity in AD patients, and predict treatment effects. In addition, similar models can be used to implement local E-I ratio disruption associated with AD, and study its effect on JPEinv.
This study extends upon prior work with the aim to investigate whether JPEinv is a potential surrogate measure of E-I. Through adapting the excitability of inhibitory interneuron populations in a computational neural mass network model, a potential underlying mechanism of abnormal E-I ratios in AD according to previous literature, we generated scenarios with varying E-I ratios. E-I ratios exist on multiple scales (i.e. synaptic, single neuron or circuit level), but in the current paper we refer to E-I ratios on a whole-brain network level (Sohal and Rubenstein 2019). To characterize to what extent JPEinv and its association with E-I ratio are determined by the underlying symbolic dynamics based measures of local signal variability and functional connectivity between regions, we also performed permutation entropy (PE) and weighted symbolic mutual information (wsMI) analyses. We investigated the relationships between these neurophysiological measures with E-I ratio on whole-brain and regional level. We also computed the hub disruption index (for JPEinv and wsMI) to study whether regions with a high intrinsic level functional connectivity (so-called hubs) were specifically vulnerable to E-I ratio changes. This multi-scale approach will significantly improve our knowledge on how E-I ratio changes translate to local dynamics, interregional functional connectivity, and functional brain network organization.
We here propose a method that could help to identify E-I ratio changes in a non-invasive manner using short term neurophysiological recordings. This could, ultimately, facilitate selection of neurological patients eligible for future clinical trials that target the E-I ratio.
Materials and methods
The computational brain network model
The computational brain network model used in this study consisted of 78 coupled neural masses. The neural mass was originally developed to explain the human resting-state cortical alpha oscillations and involved an excitatory neuronal population and an inhibitory interneuron population (Lopes da Silva et al. 1974). The excitatory and inhibitory neuronal populations were coupled to each other. Each neural mass was considered one of the 78 cortical regions of the Automated Anatomical Labeling (AAL) atlas (Supplementary Table 1) (Gong et al. 2009; Tzourio-Mazoyer et al. 2002). Neural masses were coupled according to an average human diffusion tensor imaging based structural connectivity matrix (Gong et al. 2009) (Fig. 1a, b). Coupling between neural masses was always reciprocal and excitatory. A number of parameters (Table 1) described the average activity of the excitatory and inhibitory neuronal population. Each neuronal population had a membrane potential parameter, determined by Ve(t) for excitatory and Vi(t) for inhibitory populations. The membrane potentials were influenced by impulse responses, which reflected the postsynaptic activity. The impulse responses were taken from (Zetterberg et al. 1978) and can be described as follows:
Flow chart of simulated EEG data generation and analyses. a 78 neural mass models were coupled according to a healthy human structural connectivity matrix (Gong et al. 2009). b Each neural mass consisted of an excitatory pyramidal neuronal population (in red) and inhibitory interneuron population (in blue). The firing threshold potential of the inhibitory interneuron population (Vd2) of each neural mass was adjusted to simulate AD pathology and obtain a network with altered excitation-inhibition (E-I) ratios. c Example traces of simulated cortical activity in three cortical regions in models of varying E-I ratio. d Each simulated time series was transformed into a sequence of symbols. This was accomplished by an ordinal ranking of the amplitudes of each time point in a vector of length n. e–f The variability in the probability distribution of the symbol occurrence per cortical region was evaluated by permutation entropy (PE). The probability of the co-occurrence of symbols between two regions X and Y was calculated, and a volume conduction correction was applied. The variability in co-occurrence probability was measured by the joint permutation entropy (JPE) which was then used to estimate the weighted symbolic mutual information (wsMI). The JPE was inverted to enable an intuitive interpretation, where higher JPEinv indicates stronger connectivity. Vd2: Inhibitory interneuron excitability threshold potential; Front Med Orb L: Frontal Medial Orbital cortex, left hemisphere; Temp Mid L: Temporal medial cortex, left hemisphere; n = number of consecutive data points in the vector; JPEinv: inverted joint permutation entropy
Upon an impulse response, the change in membrane potential was transferred into pulse density (or spike density) by the sigmoid functions S1(x) and S2(x), which were also adapted from Zetterberg et al. (1978):
Within each neural mass, the excitatory to inhibitory coupling within each neural mass was described by C1 and the inhibitory to excitatory coupling by C2. Coupled neural masses received excitatory input from other neural masses with a fixed delay (T) and of strength (S). Each neural mass received additional excitatory input from a thalamic region, with a random fluctuating pulse density P(t).
In this study, we altered the Vd2 parameter, which served as a firing threshold potential for inhibitory interneurons. Higher values of Vd2 indicate that the inhibitory neuronal populations need more excitatory input in order to pulse. Inhibitory interneurons activity is important for the generation of local oscillatory activity and functional connectivity at the large-scale brain level (Deco et al. 2014). The excitability of inhibitory interneurons were specifically altered because of their importance in (early) AD (Martinez-Losa et al. 2018; Palop & Mucke 2016; Scaduto et al. 2023; Verret et al. 2012). Disrupted inhibitory interneuron function has previously been related to increased firing rates of pyramidal neurons and oscillatory slowing, the most robust neurophysiological signature of human AD patients (Engels et al. 2017; Gouw et al. 2017; Luppi et al. 2022), in computational whole-brain network models (Stefanovski et al. 2019; van Nifterick et al. 2022a, b). We, therefore, homogeneously and systematically varied inhibitory interneuron excitability parameter (Vd2) for each neural mass model in the network to alter whole-brain E-I ratio associated with AD (Palop & Mucke 2016; van Nifterick et al. 2022a, b), within a range of 6 to 8 (mV) and with steps of 0.5. E-I ratios were calculated by the excitatory and inhibitory firing rates (E/(E + I)) and averaged across all cortical regions. To confirm that altered excitability of inhibitory interneurons within neural masses indeed changed whole-brain E-I ratio, we plotted the E-I ratio (averaged across all cortical regions) as function of Vd2.
The main output of a neural mass model is the fluctuation of the average membrane potential of the excitatory neuronal population across time (Fig. 1c). These fluctuations simulate the neurophysiological resting-state alpha activity as measured by EEG or MEG and can, thus, also be analyzed as such. Other model output includes the pulse density of the inhibitory and excitatory neuronal populations per unit time, which can be interpreted as a measure of neuronal population firing rate. This is of value as it provides a way to quantify E-I ratios.
Settings of the model parameters are provided in Table 1 and were based on previous studies (Lopes da Silva et al. 1974; Ponten et al. 2010; Stam et al. 1999; Ursino et al. 2007; Zetterberg et al. 1978). The whole-brain coupling parameter (S) determines the strength of the interactions between coupled neural mass models. Previous studies commonly used S-values between 0.5 and 1.5 to create networks that generate dynamics characteristic of being near a phase transition (de Haan et al. 2012; Ponten et al. 2010; Tewarie et al. 2019; van Dellen et al. 2013; A. M. van Nifterick et al. 2022a, b). This is important because the cortex is thought to operate near a critical point, balanced between chaos and order, or between asynchrony and synchrony, which is needed for optimal information processing (Beggs 2008, 2019, 2022; O'Byrne and Jerbi 2022; Xu et al. 2022). Here, S was not restricted to one value but varied between 0 and 2.0, with steps of 0.5. This allows to study how the relationships between E-I ratio and the neurophysiological outcome measures depend on coupling strength.
We ran each model (with a different Vd2) 10 times, using a sample time of 0.002 s, resulting in 40960 samples (or 81.92 s). The model is available from and analyses were performed using an in-house developed software (Brainwave, version 0.9.163.26, available from home.kpn.nl /stam7883/brainwave.html).
Local signal variability
Signal variability of the simulated oscillations per cortical region was quantified by permutation entropy (PE). PE is a robust measure and captures the predictability or regularity of a signal (Fig. 1d–f), see (Bandt and Pompe 2002). Neurophysiological signals that are highly variable, complex, not repetitive, and not predictable have high entropy (close to 1). Neurophysiological signal that are less variable, more simple and repetitive will have low entropy (close to 0). Details about how PE is calculated per region are provided in the Supplementary Material.
Interregional functional connectivity
Information sharing between two brain regions was quantified by the weighted symbolic mutual information (wsMI). The wsMI estimates the extent to which two brain regions present non-random co-occurring symbols (Fig. 1d–f), as explained previously by (Yin et al. 2019). Further details about the mathematical computation of the wsMI can be found in the Supplementary Material. In this modeling study, signal spread and volume conduction are not an issue, but, to allow comparison to previous studies, we performed a similar volume conduction correction as by (King et al. 2013; Scheijbeler et al. 2022). Two brain regions that are functionally connected will have a higher wsMI than regions with a weak or no functional connection.
Network-version of permutation entropy
The JPEinv is a multivariate version of permutation entropy and is a nonlinear coupling measure. JPE measures the variability in the co-occurrence of symbols (Fig. 1d–f). The JPE was inverted (hence JPEinv) to facilitate an intuitive interpretation as a measure of functional connectivity, as was suggested previously (Scheijbeler et al. 2022). Higher functional connectivity between two regions will therefore lead to higher JPEinv values (closer to 1). Further details on JPEinv computation are provided in the Supplementary Material.
The symbolic dynamics parameter settings
Two parameters need to be set for symbolic dynamics analyses: the embedding dimension (n) and the time-delay (τ). We set n to 4 such that the length of n! (24) is much smaller than the length of the investigated time series (4096 samples per iteration), as was recommended previously (Bandt and Pompe 2002). The results could therefore also be compared to the findings of Scheijbeler et al. (2022). Time-delay τ reflects the number of time points between the samples included in the computation of the symbolic dynamics metrics (i.e. PE, wsMI and JPEinv). It therefore determines the time scale under investigation. Previous studies showed the importance of the time-scale to the magnitude and direction of differences found in symbolic dynamics analysis (Costa et al. 2002; Goldberger et al. 2002). Selecting the appropriate time scale for analysis depends on the research question and data type (Kosciessa et al. 2020). The computational model used in this study primarily generates alpha like oscillations. Simulated broadband data will therefore not show full correspondence to physiological broadband data. Previous modeling work did however show that a reduction in inhibitory interneuron excitability could generate slower (theta-like) oscillatory activity (van Nifterick et al. 2022a, b). To analyze both fast and slow events, we first present findings for τ = 1 for broadband (0.5–70 Hz). We also present findings for τ = 1 for (extended) alpha band (6–13 Hz), to capture dynamics on slower time scales. We expected that, since the simulated broadband power spectrum is dominated by power in the alpha band, the results for broadband and extended alpha band would be similar. As alternative to narrow-band pass filtering, broadband data was also analyzed with a higher τ-value. We explored the effect of a range of τ-values on the relationship between JPEinv and E-I ratio (Supplementary Fig. 1). For τ-values > 16 the relationship was independent of τ (Supplementary Fig. 1). We also analyzed broadband data with τ = 50 to capture dynamics on slow time scales.
Whole-brain statistical analyses
To obtain whole-brain average results, PE, wsMI and JPEinv results were averaged across runs (n = 10) and regions (n = 78). The first part of whole-brain level statistical analyses were performed for a subset of models that are believed to best represent the (patho)physiological nature of brain oscillations close to a phase transition: a model with E-I balance (Vd2: 7.0, S: 1.0), a model with low E-I ratio (Vd2: 6.5, S: 1.0) and a model with high E-I ratio (Vd2: 7.5, S: 1.0). We performed independent t-tests to compare PE, wsMI and JPEinv between the model with E-I balance and both high and low E-I ratio models, respectively. Cohen’s d was computed to measure effect sizes. The second part of the whole-brain level statistical analyses were performed for a larger subset of models, that span the full range of E-I ratios (Vd2 range: 6.0–8.0, with steps of 0.5) with a single coupling (S-) value of 1.0. A Pearson’s r correlation coefficient analysis was performed to measure the association between the inhibitory interneuron excitability parameter Vd2 and PE, wsMI and JPEinv, respectively. A Pearson’s r correlation coefficient analysis was also performed to directly measure the association between the E-I ratio and PE, wsMI and JPEinv, respectively. In addition, a Pearson’s r correlation coefficient analyses was performed to study the association between JPEinv and PE and between JPEinv and wsMI. A p-value < 0.05 was considered statistically significant.
Regional statistical analyses and the hub disruption index
For regional analysis, we selected a similar subset of models close to a phase transition: a model with E-I balance (Vd2: 7.0, S: 1.0), a model with a relatively low E-I ratio (Vd2: 6.5, S: 1.0) and a model with a relatively high E-I ratio (Vd2: 7.5, S: 1.0). We first computed the connectivity by both wsMI and JPEinv between each pair of regions (averaged across 10 runs), resulting in a 78 × 78 matrix for each connectivity measure. We then calculated the wsMI- and JPEinv- based functional connectivity strength (or: functional degree) per region, that is, the average functional connectivity of that region compared to all other regions. Regional differences in PE and functional degree based on wsMI and JPEinv between high or low E-I models and the model with E-I balance were tested using multiple non-parametric Mann–Whitney U tests: a comparison of ranks. A False Discovery Rate (FDR) approach was applied using the two-stage linear step-up procedure of Benjamini, Krieger and Yekutielie and using a desired FDR of 1%. To summarize the functional network reorganization after E-I ratio perturbation, the hub disruption index (HDI) was computed (Achard et al. 2012; Termenon et al. 2016). The difference in functional degree of a region in a high or low E-I ratio model compared to the functional degree of that region in the model with E-I balance was plotted as function of the regional degree in the model with E-I balance. A simple linear regression model was fit to the data and the slope defined the HDI. A significantly negative HDI can indicate that the impact of E-I ratio changes is dependent on the initial level of functional degree. Depending on the offset, it indicates that regions with a higher degree in models with balanced E-I ratios are primarily disrupted by altered E-I ratios, or that regions with a lower degree in models with balanced E-I ratios primarily show an increase in degree by altered E-I ratios, or it can be a combination of these two.
All statistical analyses were performed in GraphPad Prism version 9.3.1 for Windows, GraphPad Software, San Diego, California USA, www.graphpad.com.
Results
Inhibitory interneuron excitability alters E-I ratio
To change the model E-I ratio, we altered the Vd2 model parameter homogeneously across the 78 cortical regions. The Vd2 model parameter determined the inhibitory interneurons excitability. Perturbation of the inhibitory interneuron excitability indeed generated changes in firing rates of excitatory and inhibitory neuronal populations and disrupted the E-I ratio on whole-brain level (Fig. 2). Lower inhibitory interneuron excitability (by a higher Vd2) resulted in higher excitatory neuronal firing rates (Fig. 2a), lower inhibitory firing rates (Fig. 2b), and higher E-I ratios (Fig. 2c). It was, therefore, considered an appropriate parameter to simulate AD-related abnormal E-I ratio.
A higher inhibitory interneuron firing threshold potential (Vd2) is associated with higher E-I ratios. In a model with E-I balance, the inhibitory interneuron excitability threshold parameter (Vd2) has a value of 7 (depicted on a white background). a–c A higher Vd2 (depicted on a red background) is associated with a whole-brain average increase in excitatory neuronal population firing activity (E rate) (a), a reduction of inhibitory interneuron population firing rate (I rate) (b), and an increase in the whole-brain average E-I ratio (c). A lower Vd2 resulted in opposite effects, presented in blue. Rates were normalized between 0 and 1, and E-I ratio = E/(E + I). S, coupling strength
Whole-brain level PE, wsMI and JPEinv relate to E-I ratio
On whole-brain level, all three outcome measures (PE, wsMI and JPEinv) showed a nonlinear relationship with E-I ratio (determined by the Vd2 parameter) and coupling strength (S). Statistical comparisons of whole-brain PE, wsMI and JPEinv between a subset of high (Vd2: 6.5, S: 1.0) or low E-I ratio (Vd2: 7.5, S1.0) models and a model with E-I balance (Vd27.0, S: 1.0) confirmed significant differences (Supplementary Table 2). These findings indicate that JPEinv, but also PE and wsMI are markers of E-I. Cohen’s d effect sizes were large for all measures (Supplementary Table 2). The JPEinv showed a larger mean effect size compared to signal variability and functional connectivity by wsMI for 6–13 Hz data specifically. These results suggest that a small change in E-I ratio can cause a large difference in signal variability and functional connectivity measured by both wsMI and JPEinv.
Signal variability was lower (PE values closer to 0) in models with higher E-I ratios (Fig. 3a–c, left column). While the relationship between E-I ratio and signal variability remained consistent, the magnitude of whole brain signal variability and its variation across models with varying E-I ratio differed when examined across various time scales (Fig. 3a–c, left column). Correlation analyses showed a robust correlation between signal variability and Vd2, the control parameter of E-I ratio, across time scales (Supplementary Fig. 2, Supplementary Table 3). Signal variability at whole-brain level was highest for τ = 50 (characterizing slow dynamics), followed by τ = 1 for broadband (capturing mixed dynamics on fast and slow time scales), and for 6–13 Hz (characterizing dynamics in the alpha band specifically) (Fig. 3a–c, left column).
Whole-brain level PE, wsMI and JPEinv relate to E-I ratio and coupling strength. a–c Whole-brain average local signal variability was measured by permutation entropy (PE), and functional connectivity by weighted symbolic mutual information (wsMI) and inverted joint permutation entropy (JPEinv). Results presented for varying levels of E-I ratios, determined by the inhibitory interneuron firing threshold potential (Vd2), and coupling strength (S). Each cell in a heatmap plot presents an average value calculated from data across all 78 regions and over 10 runs. Analyses were repeated for different time scales (by time-delay τ, or narrow band-pass filtering). Yellow/brighter colors mean higher values and blue/darker colors mean lower values
The relationship between functional connectivity (measured by both wsMI and JPEinv) and E-I ratio was in the opposite direction of the relationship between signal variability and E-I ratio: models with higher E-I ratios showed higher connectivity than models with lower E-I ratios (Fig. 3a–c, middle and right column). The magnitude of functional connectivity at whole-brain depended on the time scale under analyses. Functional connectivity by wsMI and JPEinv was highest for 6–13 Hz, followed by τ = 50 and τ = 1 for broadband. Correlation analyses revealed a significant association between the E-I ratio control parameter Vd2 and JPEinv for all investigated time scales (Supplementary Fig. 2, Supplementary Table 3). More diverse patterns appeared for the correlation analyses between E-I and functional connectivity by wsMI across time scales. A significant correlation was found for τ = 50, and τ = 1 for broadband, but not for 6–13 Hz (Supplementary Fig. 2, Supplementary Table 3). This result is probably explained by the inversed U-shape relationship between E-I ratio and functional connectivity by wsMI, which is most evident for 6–13 Hz (Fig. 3b, middle column). Mainly for this time scale, but also for τ = 50, functional connectivity by wsMI was maximal for models that just crossed the transition point (Fig. 3b–c, middle column), at a somewhat high E-I ratio. Maximal functional connectivity by JPEinv could also be observed at a similar (somewhat high) E-I ratio (Fig. 3c, right column), but only for τ = 50 and strong coupling values (S > 1.0). After this point, further increasing the E-I ratio does not further increase the functional connectivity on whole-brain level, but can actually lead to a reduction of connectivity. These findings indicate that the relationship between JPEinv and E-I ratio is more robust than wsMI because it is less influenced by time scale. An additional correlation analyses between the actual E-I ratio (instead of Vd2) and the outcome measures signal variability (PE), functional connectivity by wsMI and JPEinv showed similar results (Supplementary Fig. 3, Supplementary Table 4). These findings support the hypothesis that JPEinv, but also signal variability and functional connectivity by wsMI, are markers of E-I.
Correlation analyses also showed that a higher JPEinv was significantly associated with higher wsMI (Supplementary Fig. 4, Supplementary Table 5) and lower signal variability (Supplementary Fig. 4, Supplementary Table 5). These significant associations were present for all time scales (Supplementary Fig. 4, Supplementary Table 5). These results suggest that JPEinv indeed simultaneously measures both signal variability and functional connectivity. Despite these significant associations, correlation analyses showed a stronger association between JPEinv and signal variability than between JPEinv and wsMI. This would mean that, although JPEinv includes wsMI, JPEinv may also provide information not captured by wsMI under certain conditions.
Region-dependent relationships between PE, wsMI, JPEinv and E-I ratio
Furthermore, we investigated whether the relationship between the E-I ratio and both signal variability and functional connectivity (as measured by wsMI and JPEinv) depended on the region of interest.
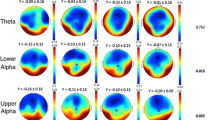
In the selected decreased E-I ratio model (Vd2: 6.5, S: 1.0), signal variability seemed predominantly increased in regions with lower signal variability in the model with E-I balance (Vd2: 7.0, S: 1.0), such as the occipital regions (Fig. 4, Supplementary Fig. 5). In the selected increased E-I ratio model (Vd2: 7.5, S: 1.0) signal variability seemed predominantly decreased in regions with higher signal variability in the model with E-I balance (Vd2: 7.0, S: 1.0), such as the frontal, central and parietal regions (Fig. 4, Supplementary Fig. 5). These findings seemed to be largely independent of the time scale under analysis. Together, these results suggest a region-dependent relationship between signal variability and E-I ratio.
A region-dependent relationship between signal variability and E-I ratio. a–c The brain color plots show the regional level of signal variability (PE). Lower values are depicted in blue/dark colors and higher values in yellow/bright colors. Results are presented for model with varying levels of E-I, as determined by the inhibitory interneuron firing threshold parameter (Vd2). Analyses were repeated for different time scales. PE: permutation entropy; τ: time-delay between samples
Functional connectivity by wsMI was generally increased across the cortex upon an increase in E-I ratio, in particular in regions that showed lower functional connectivity in the model with E-I balance, such as frontal and central regions (Fig. 5, Supplementary Fig. 5–6). For regions with a very high level of functional connectivity by wsMI in the model with E-I balance in 6 -13 Hz and τ = 50 specifically, however, an increase in E-I ratio resulted in a decrease in functional connectivity (Fig. 5, Supplementary Fig. 5b, c, middle column). Upon a decrease in E-I ratio, the functional connectivity measure was decreased across the cortex, particularly in regions with a high functional connectivity in the model with E-I balance, such as the parieto-occipital and temporal regions (Fig. 5, Supplementary Fig. 5–6). Similar effects were found for functional connectivity by JPEinv (Fig. 6, Supplementary Fig. 5, Supplementary Fig. 7). These findings were further quantified by the hub disruption index (HDI) (Fig. 7, Table 2). The HDI analysis confirmed a selective disruption of hub-like regions (i.e. regions with a high average functional connectivity level, or degree), in terms of changes in their functional connectivity to other regions, upon a decreased E-I ratio (Fig. 7). We also found a selective increase of average functional degree in regions with initially low and intermediate degree (or: non-hubs), upon an increased E-I ratio (Fig. 7b–f). The region-specific sensitivity of functional connectivity by wsMI and JPEinv to E-I ratio perturbation was relatively independent of the time scale under analysis. These analyses revealed a specific, degree-dependent disruption of functional connectivity by wsMI and JPEinv upon an altered E-I ratio.
A region-dependent relationship between wsMI and E-I ratio. a–c The brain color plots present weighted symbolic mutual information (wsMI) per region, obtained by averaging the connectivity between that region and all other regions. The results are presented for models with varying levels of E-I, as determined by the inhibitory interneuron firing threshold potential (Vd2). Lower connectivity levels are depicted in blue/dark colors and higher values in yellow/brighter colors. Analyses were repeated for different time scales. wsMI: weighted symbolic mutual information; τ: time-delay between samples
A region-dependent relationship between JPEinv and E-I ratio. a–c The brain color plots present inverted joint permutation entropy (JPEinv) per region, obtained by averaging the connectivity between that region and all other regions. The results are presented for models with varying levels of E-I, as determined by the inhibitory interneuron firing threshold potential (Vd2). Lower connectivity levels are depicted in blue/dark colors and higher values in yellow/brighter colors. Analyses were repeated for different time scales (a–c). JPEinv: inverted joint permutation entropy; τ: time-delay between samples
A degree-dependent impact of abnormal E-I ratio on functional connectivity. a–c The y-axis of the scatter plots show the regional change in functional degree by wsMI and JPEinv after E-I ratio disruption (low E-I ratio (Vd2: 6.5) in blue and high E-I ratio (Vd2: 7.5) in red), compared to the functional degree in that region in a model with E-I balance (Vd2: 7.0). This difference is plotted as function of the regional degree in the model with E-I balance, on the x-axis. Analyses were repeated for different time scales. The (significant) linear regression models with the best fit are shown as solid lines
Discussion
This computational modeling study showed that a network version of permutation entropy, JPEinv, is sensitive to changes in E-I ratio, and may therefore serve as surrogate marker for E-I. Local signal variability (PE) and functional connectivity by wsMI also related to E-I ratio. On whole-brain level, the relationship between signal variability, functional connectivity (by JPEinv or wsMI) and E-I ratio was dependent on the coupling strength, and the filter- and time-delay settings. JPEinv represented both signal variability and functional connectivity by wsMI, with the former exhibiting a stronger relationship. Whole-brain level JPEinv showed the largest change upon alterations in E-I ratio for alpha oscillations, which suggests its additive value in determining E-I ratios compared to the single-scale measures signal variability and functional connectivity by wsMI. On regional level, the ‘hubness’ of a region determined the susceptibility of wsMI- and JPEinv-based functional connectivity disruption to abnormal E-I ratios.
In models with a low E-I ratio, signal variability was high and functional connectivity by wsMI and JPEinv was low. As the E-I ratio increased beyond a certain threshold, signal variability significantly decreased and regions became functionally connected. A schematic representation of the relationships between signal variability, functional connectivity by both wsMI and JPEinv and E-I ratio upon changes in Vd2 is presented in Fig. 8. The transition point was considered representative of a healthy network, balanced between synchrony and asynchrony. Models with higher coupling strength reached this point for lower levels of the E-I determining parameter Vd2. After this point, a further increase in E-I ratio on the outcome measures was depended on the filter and time-delay settings.
A schematic illustration of the relation between signal variability, functional connectivity and E-I ratio in a computational network model. On whole-brain level, in a model with low E-I ratio (the blue part of the plot), signal variability is high (estimated by PE, in green), and functional connectivity, estimated by wsMI (in orange) and JPEinv (in blue), is low. In a model with E-I balance (shown against a white background), a small change in E-I ratio has a large effect on the outcome measures. As the E-I ratio increases (shifting towards the red part of the plot), signal variability decreases and functional connectivity, measured by both wsMI and JPEinv, decreases. Beyond a certain point, a further increase in E-I ratio can lead to a decrease in whole-brain functional connectivity, whereas signal variability remains relatively unchanged. Please note that this plot represents a concept and not actual simulated data
A healthy neuronal network requires a certain level of variability and flexibility in neuronal activity which can be assessed through measures such as PE, among others (Garrett et al. 2013; Waschke et al. 2021; Yin and Kaiser 2021). Previous studies related neuronal signal variability to behavior (Waschke et al. 2021). Its disruption has been linked to neurological disorders with suspected E-I ratio abnormalities, including dementia (Gaubert et al. 2019; Gomez and Hornero 2010; Hohlefeld et al. 2012; Montez et al. 2009; Shumbayawonda et al. 2020; Sorrentino et al. 2021) and epilepsy (Auno et al. 2021; Meisel et al. 2012). Patients in different stages of the AD continuum, for example, showed decreased signal variability in higher frequency bands and faster time scales (Dauwels et al. 2010; Echegoyen et al. 2020; Maturana-Candelas et al. 2019; Stam 2005), and increased signal variability in the lower frequencies and slower time scales (Gaubert et al. 2019; Maturana-Candelas et al. 2019; Mizuno et al. 2010; Scheijbeler et al. 2022). In epilepsy patients, seizures were characterized by a great reduction in signal variability compared to normal EEG (Bruzzo et al. 2008; Nicolaou and Georgiou 2012). In line with these reports, we showed that lower signal variability (indicating more regular and repetitive activity) was associated with an increased E-I ratio. In contrast to previous studies that found maximal variability at E-I balance in neuronal spiking models (Shew and Plenz 2013; Shew et al. 2009, 2011; Yang et al. 2012), we found the highest level of signal variability at the lowest E-I ratios. The characteristics of the model used for signal variability analyses are, thus, important to consider when determining the relation between signal variability and E-I ratios. To infer changes in signal variability as indicator of altered E-I ratio, it is important to better understand whether healthy human neurophysiological data at whole-brain level is characterized by maximal signal variability, and if so, at what time scale.
The level of signal variability in simulated models with E-I balance varied across regions, despite homogeneous Vd2-values. The distribution of signal variability across the brain was comparable to empirical data, showing low signal variability in posterior and high signal variability in anterior regions (Scheijbeler et al. 2022). The heterogeneity in signal variability is likely explained by the regional differences in the number of connections with other regions (and, therefore, a higher level of excitatory input), as determined by the human DTI-based structural connectivity matrix (Gong et al. 2009). The extent of signal variability change upon a shift in E-I ratio was, in turn, also different across regions. An increase in E-I ratio seemed to decrease the signal variability predominantly in those regions with high intrinsic signal variability, whereas regions with low intrinsic signal variability showed minimal or no changes. In contrast, a decrease in E-I ratio seemed to preferentially decrease signal variability in regions with a high intrinsic level of signal variability. Together, these findings point towards a relationship between E-I ratio and local signal variability on whole brain and regional level.
Higher functional connectivity, measured by both wsMI and JPEinv, on whole-brain level related to higher E-I ratios. Maximal functional connectivity was not consistently found for the highest E- I ratios, but was present near a phase transition, at somewhat high E-I ratios, for the extended alpha band oscillations and τ = 50 specifically. A further increase in E-I ratio caused a reduction in functional connectivity. This functional decoupling is possibly explained by the fact that, for these E-I ratios, the neural masses have such high intrinsic activity that it will not be sensitive anymore to input from other regions. Previous studies reported maximal amplitude coupling, phase synchrony and variability in connectivity at E-I balance in a variety of computational network models (Avramiea et al. 2022; Shew et al. 2011; Yang et al. 2012). A deviation from this balance will then, in theory, always result in a reduction of functional connectivity. There are, however, indications from empirical data that healthy brains do not operate exactly at the critical point (characterized by E-I balance), but instead somewhat below that, at a subcritical or quasicritical point (Fosque et al. 2021; Priesemann et al. 2014). This would mean that a relative increase in E-I ratio can increase the functional connectivity until it surpasses the critical point, after which the connectivity will decrease. As for signal variability, it is important to validate this hypothesis in order to use whole-brain average functional connectivity measures as indicators of an abnormal E-I ratio in empirical data.
As suggested here by the hub disruption index results, a change in whole-brain E-I ratio can lead to a region-dependent in- or decrease of functional connectivity. More specifically, we observed a selective disruption of hub-like regions upon a decrease in E-I ratio and a specific increase in functional connectivity by both wsMI and JPEinv in low degree (non-hub) regions as a result of an increased E-I ratio. An increase in E-I ratio could in some scenarios also lead to a decrease in functional connectivity of hub-like regions specifically. The latter functional reorganization has been reported in empirical studies across a number of different diseases with possibly underlying abnormal E-I ratios, including stroke, comatose, and AD patients (Achard et al. 2012; Termenon et al. 2016; Yu et al. 2017). In addition, a dual in- an decrease in functional connectivity have been reported across different studies. For example, EEG and MEG recordings in preclinical and prodromal AD patients have been characterized by increased functional connectivity, in the lower delta and theta band oscillations (Nakamura et al. 2017; Pusil et al. 2019; Ranasinghe et al. 2022a, b), and reduced functional connectivity, usually in higher (alpha, beta, gamma) frequency bands (Briels et al. 2020; Cuesta et al. 2022; Engels et al. 2015; Ranasinghe et al. 2022a, b; Schoonhoven et al. 2022). The differences in connectivity were, compared to controls, also more pronounced in AD patients with than without epileptiform activity (Ranasinghe et al. 2022a, b). As for the whole-brain: after reaching a certain high level of activity, the neuronal populations possibly become insensitive to input from other regions and thereby disrupt the functional connections. Hub-like regions have intrinsically higher activity levels (de Haan et al. 2012) and, therefore, may be the first regions to become functionally decoupled upon an increase in E-I ratio. Whether the combined in- and decrease in functional connectivity as observed in neurological patients represents a whole-brain change in E-I ratio or region-dependent bidirectional changes in E-I ratio remains uncertain. Whereas whole-brain functional connectivity levels already provide information about the underlying E-I balance, analyses of regional functional connectivity changes on different time scales in future studies could provide us with more detailed information about the direction of change in E-I ratio.
To confirm the multi-scale character of JPEinv, we correlated JPEinv to both signal variability and functional connectivity by wsMI. We found a strong negative association between signal variability and JPEinv and a strong positive association between wsMI and JPEinv. These results indicate that JPEinv indeed captures both signal dynamics and interregional connectivity. For 6–13 Hz, interestingly, the correlation between JPEinv and signal variability and functional connectivity by wsMI was weaker compared to the correlation found for 0.5–70 Hz. It is not so trivial why the strength of the relationship between these measures depended on the filter settings. Signal variability and wsMI exhibited the most distinct/specific relationships with the E-I ratio in this frequency range, mainly driven by the differences in relationship between wsMI and E-I ratio across different time scales. For example, the high functional connectivity by wsMI observed in the alpha band was not detectable in 0.5-70 Hz data. Filtering in the alpha band generally decreased the signal variability, and the resulting increased signal-to-noise ratio possibly increased the sensitivity of wsMI (and JPEinv) to capture alpha-band connectivity. Considering that JPEinv combines both measures, it may be expected that JPEinv also shows most distinct relationships to both signal variability and functional connectivity by wsMI for 6–13 Hz.
We hypothesized that the multi-scale character of JPEinv is of potential advantage to identify disrupted E-I ratios from large-scale neurophysiological data (Scheijbeler et al. 2022). The consistently high mean effect sizes for JPEinv, in contrast to PE and wsMI, also pointed towards a potential advantage of combining local and interregional signal dynamics in a single metric to measure changes in E-I ratio.
A natural spatial and temporal heterogeneity in E-I ratios likely exists due to intrinsic differences in neuron subtypes, structural wiring, functional importance and dependence on behavioral state (Dehghani et al. 2016; Misic et al. 2010). Previous neuronal population modeling studies have revealed that pathological differences in E-I ratio across brain regions, determined by amyloid- and tau-protein distribution patterns, can explain AD-like neurophysiological signatures, such as spectral slowing (Alexandersen et al. 2023; Ranasinghe et al. 2022; Stefanovski et al. 2019). We here simply applied equal E-I ratio changes across all brain regions, a potential limitation of the study. Due to the interregional differences in number of structural connections, however, we found a similar spatial distribution of signal variability (and functional connectivity by JPEinv) in models with E-I balance as observeded in empirical data (Scheijbeler et al. 2022). The regional specificity of E-I ratio changes on the outcome measures could also be explained by the regional difference in connectivity. Overall, these findings suggest that the observed regional signal variability changes and abnormal functional network organization in early stage AD patients, for example, could be explained by abnormal E-I ratio on whole-brain level.
The findings of the study should be considered with the following strengths and limitations in mind. The biophysical nature of the model parameters allowed us to provide meaningful explanations of changes in large-scale dynamics and integrate findings from experimental studies. The realistic model output also allowed us to take a similar analyses approach as other (empirical) studies and therefore facilitates comparison of results. We reported the level of E-I ratio changes that were investigated, which is important for a correct interpretation and translation of findings (Ahmad et al. 2022). We studied the time scale dependency of functional dynamics using multiple approaches and measured simulated brain dynamics on both a regional and whole-brain level. This multi-scale approach provided substantial insight into how E-I ratio changes are reflected in local dynamics, interregional functional connectivity, and functional brain network organization. As any other model, the computational neural mass network model did not capture the full complexity of the brain and, therefore, the translation of the findings to individuals in the clinic remains uncertain. This is especially true for the study of empirical oscillations in other frequencies than the (extended) alpha band. It is important to replicate these findings using alternative models in future studies. Future studies should investigate whether JPEinv is specific to the mechanisms underlying E-I ratio disruption, and if the results of the current study extend to all disorders characterized by changes in E-I ratio such as epilepsy (Houtman et al. 2021; Pani et al. 2021), glioma (Numan et al. 2022), multiple sclerosis (Huiskamp et al. 2022), autism (Bruining et al. 2020; Manyukhina et al. 2022) and schizophrenia (Molina et al. 2020). We also aim to validate the results using empirical data, by comparing JPEinv and related measures between AD patients with and without subclinical epileptiform activity. We used similar outcome measures and symbolic dynamics parameters as in a previous study, and, therefore, volume conduction correction was applied. This is, however, not an issue in ‘model space’ and may have biased our results.
This study proposes a novel nonlinear functional connectivity measure that can help identify E-I ratio changes from short resting-state brain recordings. The metric holds great potential to do so because it captures information about the local dynamics, in terms of variability in symbolic patterns, and the interaction of these dynamics between regions. Previous research has already identified changes in JPEinv in patients at an early stage of AD. Our study extended upon this work by showing that these changes may indeed reflect an abnormal E-I ratio. The identification of a novel E-I measure helps to better understand the mechanisms underlying neurophysiological alterations in AD patients across different stages and, ultimately, provides useful insights for both prognostic and therapeutic purposes.
Data availability
The datasets generated and analyzed during the current study will be publicly available at time of publication at https://github.com/annevannifterick/JPEinv_EI_model. The model is available from and analyses were performed using an in-house developed software (Brainwave, version 0.9.163.26, available from home.kpn.nl /stam7883/brainwave.html).
References
Achard S, Delon-Martin C, Vertes PE, Renard F, Schenck M, Schneider F, Heinrich C, Kremer S, Bullmore ET (2012) Hubs of brain functional networks are radically reorganized in comatose patients. Proc Natl Acad Sci U S A 109(50):20608–20613. https://doi.org/10.1073/pnas.1208933109
Ahmad J, Ellis C, Leech R, Voytek B, Garces P, Jones E, Buitelaar J, Loth E, Dos Santos FP, Amil AF, Verschure P, Murphy D, McAlonan G (2022) From mechanisms to markers: novel noninvasive EEG proxy markers of the neural excitation and inhibition system in humans. Transl Psychiatry 12(1):467. https://doi.org/10.1038/s41398-022-02218-z
Alexandersen CG, de Haan W, Bick C, Goriely A (2023) A multi-scale model explains oscillatory slowing and neuronal hyperactivity in Alzheimer’s disease. J R Soc Interface 20(198):20220607. https://doi.org/10.1098/rsif.2022.0607
Auno S, Lauronen L, Wilenius J, Peltola M, Vanhatalo S, Palva JM (2021) Detrended fluctuation analysis in the presurgical evaluation of parietal lobe epilepsy patients. Clin Neurophysiol 132(7):1515–1525. https://doi.org/10.1016/j.clinph.2021.03.041
Avramiea AE, Masood A, Mansvelder HD, Linkenkaer-Hansen K (2022) Long-range amplitude coupling is optimized for brain networks that function at criticality. J Neurosci 42(11):2221–2233. https://doi.org/10.1523/JNEUROSCI.1095-21.2022
Bakker A, Albert MS, Krauss G, Speck CL, Gallagher M (2015) Response of the medial temporal lobe network in amnestic mild cognitive impairment to therapeutic intervention assessed by fMRI and memory task performance. Neuroimage Clin 7:688–698. https://doi.org/10.1016/j.nicl.2015.02.009
Bandt C, Pompe B (2002) Permutation entropy: a natural complexity measure for time series. Phys Rev Lett 88(17):174102. https://doi.org/10.1103/PhysRevLett.88.174102
Beggs JM (2008) The criticality hypothesis: how local cortical networks might optimize information processing. Philos Trans A Math Phys Eng Sci 366(1864):329–343. https://doi.org/10.1098/rsta.2007.2092
Beggs JM (2019) The critically tuned cortex. Neuron 104(4):623–624. https://doi.org/10.1016/j.neuron.2019.10.039
Beggs JM (2022) The cortex and the critical point: understanding the power of emergence. MIT Press, Cambridge
Briels CT, Schoonhoven DN, Stam CJ, de Waal H, Scheltens P, Gouw AA (2020) Reproducibility of EEG functional connectivity in Alzheimer’s disease. Alzheimers Res Ther 12(1):68. https://doi.org/10.1186/s13195-020-00632-3
Bruining H, Hardstone R, Juarez-Martinez EL, Sprengers J, Avramiea AE, Simpraga S, Houtman SJ, Poil SS, Dallares E, Palva S, Oranje B, Matias Palva J, Mansvelder HD, Linkenkaer-Hansen K (2020) Measurement of excitation-inhibition ratio in autism spectrum disorder using critical brain dynamics. Sci Rep 10(1):9195. https://doi.org/10.1038/s41598-020-65500-4
Bruzzo AA, Gesierich B, Santi M, Tassinari CA, Birbaumer N, Rubboli G (2008) Permutation entropy to detect vigilance changes and preictal states from scalp EEG in epileptic patients. A Prelim Study Neurol Sci 29(1):3–9. https://doi.org/10.1007/s10072-008-0851-3
Costa M, Goldberger AL, Peng CK (2002) Multiscale entropy analysis of complex physiologic time series. Phys Rev Lett 89(6):068102. https://doi.org/10.1103/PhysRevLett.89.068102
Cuesta P, Ochoa-Urrea M, Funke M, Hasan O, Zhu P, Marcos A, Lopez ME, Schulz PE, Lhatoo S, Pantazis D, Mosher JC, Maestu F (2022) Gamma band functional connectivity reduction in patients with amnestic mild cognitive impairment and epileptiform activity. Brain Commun 4(2):012. https://doi.org/10.1093/braincomms/fcac012
Dauwels J, Vialatte F, Cichocki A (2010) Diagnosis of Alzheimer’s disease from EEG signals: Where are we standing? Curr Alzheimer Res 7(6):487–505. https://doi.org/10.2174/156720510792231720
de Haan W, Mott K, van Straaten EC, Scheltens P, Stam CJ (2012) Activity dependent degeneration explains hub vulnerability in Alzheimer’s disease. PLoS Comput Biol 8(8):e1002582. https://doi.org/10.1371/journal.pcbi.1002582
de Haan W, van Straaten ECW, Gouw AA, Stam CJ (2017) Altering neuronal excitability to preserve network connectivity in a computational model of Alzheimer’s disease. PLoS Comput Biol 13(9):e1005707. https://doi.org/10.1371/journal.pcbi.1005707
Deco G, Ponce-Alvarez A, Hagmann P, Romani GL, Mantini D, Corbetta M (2014) How local excitation-inhibition ratio impacts the whole brain dynamics. J Neurosci 34(23):7886–7898. https://doi.org/10.1523/Jneurosci.5068-13.2014
Dehghani N, Peyrache A, Telenczuk B, Le Van Quyen M, Halgren E, Cash SS, Hatsopoulos NG, Destexhe A (2016) Dynamic balance of excitation and inhibition in human and monkey neocortex. Sci Rep 6:23176. https://doi.org/10.1038/srep23176
Demirtas M, Falcon C, Tucholka A, Gispert JD, Molinuevo JL, Deco G (2017) A whole-brain computational modeling approach to explain the alterations in resting-state functional connectivity during progression of Alzheimer’s disease. Neuroimage Clin 16:343–354. https://doi.org/10.1016/j.nicl.2017.08.006
Donoghue T, Haller M, Peterson EJ, Varma P, Sebastian P, Gao R, Noto T, Lara AH, Wallis JD, Knight RT, Shestyuk A, Voytek B (2020) Parameterizing neural power spectra into periodic and aperiodic components. Nat Neurosci 23(12):1655–1665. https://doi.org/10.1038/s41593-020-00744-x
Echegoyen I, Lopez-Sanz D, Martinez JH, Maestu F, Buldu JM (2020) Permutation entropy and statistical complexity in mild cognitive impairment and Alzheimer’s disease: an analysis based on frequency bands. Entropy (basel). https://doi.org/10.3390/e22010116
Engels MM, Stam CJ, van der Flier WM, Scheltens P, de Waal H, van Straaten EC (2015) Declining functional connectivity and changing hub locations in Alzheimer’s disease: an EEG study. BMC Neurol 15:145. https://doi.org/10.1186/s12883-015-0400-7
Engels MMA, van der Flier WM, Stam CJ, Hillebrand A, Scheltens P, van Straaten ECW (2017) Alzheimer’s disease: The state of the art in resting-state magnetoencephalography. Clin Neurophysiol 128(8):1426–1437. https://doi.org/10.1016/j.clinph.2017.05.012
Fosque LJ, Williams-Garcia RV, Beggs JM, Ortiz G (2021) Evidence for quasicritical brain dynamics. Phys Rev Lett 126(9):098101. https://doi.org/10.1103/PhysRevLett.126.098101
Furbass F, Kural MA, Gritsch G, Hartmann M, Kluge T, Beniczky S (2020) An artificial intelligence-based EEG algorithm for detection of epileptiform EEG discharges: validation against the diagnostic gold standard. Clin Neurophysiol 131(6):1174–1179. https://doi.org/10.1016/j.clinph.2020.02.032
Gao R, Peterson EJ, Voytek B (2017) Inferring synaptic excitation/inhibition balance from field potentials. Neuroimage 158:70–78. https://doi.org/10.1016/j.neuroimage.2017.06.078
Garrett DD, Samanez-Larkin GR, MacDonald SW, Lindenberger U, McIntosh AR, Grady CL (2013) Moment-to-moment brain signal variability: A next frontier in human brain mapping? Neurosci Biobehav Rev 37(4):610–624. https://doi.org/10.1016/j.neubiorev.2013.02.015
Garrett DD, Nagel IE, Preuschhof C, Burzynska AZ, Marchner J, Wiegert S, Jungehulsing GJ, Nyberg L, Villringer A, Li SC, Heekeren HR, Backman L, Lindenberger U (2015) Amphetamine modulates brain signal variability and working memory in younger and older adults. Proc Natl Acad Sci U S A 112(24):7593–7598. https://doi.org/10.1073/pnas.1504090112
Gaubert S, Raimondo F, Houot M, Corsi MC, Naccache L, Diego Sitt J, Hermann B, Oudiette D, Gagliardi G, Habert MO, Dubois B, De Vico Fallani F, Bakardjian H, Epelbaum S, Alzheimer's Disease Neuroimaging I (2019) EEG evidence of compensatory mechanisms in preclinical Alzheimer’s disease. Brain 142(7):2096–2112. https://doi.org/10.1093/brain/awz150
Goldberger AL, Peng CK, Lipsitz LA (2002) What is physiologic complexity and how does it change with aging and disease? Neurobiol Aging 23(1):23–26. https://doi.org/10.1016/s0197-4580(01)00266-4
Gomez C, Hornero R (2010) Entropy and complexity analyses in Alzheimer’s disease: an MEG study. Open Biomed Eng J 4:223–235. https://doi.org/10.2174/1874120701004010223
Gong G, He Y, Concha L, Lebel C, Gross DW, Evans AC, Beaulieu C (2009) Mapping anatomical connectivity patterns of human cerebral cortex using in vivo diffusion tensor imaging tractography. Cereb Cortex 19(3):524–536. https://doi.org/10.1093/cercor/bhn102
Gouw AA, Alsema AM, Tijms BM, Borta A, Scheltens P, Stam CJ, van der Flier WM (2017) EEG spectral analysis as a putative early prognostic biomarker in nondemented, amyloid positive subjects. Neurobiol Aging 57:133–142. https://doi.org/10.1016/j.neurobiolaging.2017.05.017
Hohlefeld FU, Huebl J, Huchzermeyer C, Schneider GH, Schonecker T, Kuhn AA, Curio G, Nikulin VV (2012) Long-range temporal correlations in the subthalamic nucleus of patients with Parkinson’s disease. Eur J Neurosci 36(6):2812–2821. https://doi.org/10.1111/j.1460-9568.2012.08198.x
Horvath AA, Papp A, Zsuffa J, Szucs A, Luckl J, Radai F, Nagy F, Hidasi Z, Csukly G, Barcs G, Kamondi A (2021) Subclinical epileptiform activity accelerates the progression of Alzheimer’s disease: a long-term EEG study. Clin Neurophysiol 132(8):1982–1989. https://doi.org/10.1016/j.clinph.2021.03.050
Houtman SJ, Lammertse HCA, van Berkel AA, Balagura G, Gardella E, Ramautar JR, Reale C, Moller RS, Zara F, Striano P, Misra-Isrie M, van Haelst MM, Engelen M, van Zuijen TL, Mansvelder HD, Verhage M, Bruining H, Linkenkaer-Hansen K (2021) STXBP1 syndrome is characterized by inhibition-dominated dynamics of resting-state EEG. Front Physiol 12:775172. https://doi.org/10.3389/fphys.2021.775172
Huiskamp M, Kiljan S, Kulik S, Witte ME, Jonkman LE, Gjm Bol J, Schenk GJ, Hulst HE, Tewarie P, Schoonheim MM, Geurts JJ (2022) Inhibitory synaptic loss drives network changes in multiple sclerosis: an ex vivo to in silico translational study. Mult Scler 28(13):2010–2019. https://doi.org/10.1177/13524585221125381
Jin N, Gureviciene I, Atalay AN, Hakli S, Ziyatdinova S, Tanila H (2022) Preclinical evaluation of drug treatment options for sleep-related epileptiform spiking in Alzheimer’s disease. Alzheimers Dement (n y) 8(1):e12291. https://doi.org/10.1002/trc2.12291
King JR, Sitt JD, Faugeras F, Rohaut B, El Karoui I, Cohen L, Naccache L, Dehaene S (2013) Information sharing in the brain indexes consciousness in noncommunicative patients. Curr Biol 23(19):1914–1919. https://doi.org/10.1016/j.cub.2013.07.075
Koch G, Casula EP, Bonni S, Borghi I, Assogna M, Minei M, Pellicciari MC, Motta C, D’Acunto A, Porrazzini F, Maiella M, Ferrari C, Caltagirone C, Santarnecchi E, Bozzali M, Martorana A (2022) Precuneus magnetic stimulation for Alzheimer’s disease: a randomized, sham-controlled trial. Brain 145(11):3776–3786. https://doi.org/10.1093/brain/awac285
Kosciessa JQ, Kloosterman NA, Garrett DD (2020) Standard multiscale entropy reflects neural dynamics at mismatched temporal scales: What’s signal irregularity got to do with it? PLoS Comput Biol 16(5):e1007885. https://doi.org/10.1371/journal.pcbi.1007885
Lam AD, Deck G, Goldman A, Eskandar EN, Noebels J, Cole AJ (2017) Silent hippocampal seizures and spikes identified by foramen ovale electrodes in Alzheimer’s disease. Nat Med 23(6):678–680. https://doi.org/10.1038/nm.4330
Lam AD, Sarkis RA, Pellerin KR, Jing J, Dworetzky BA, Hoch DB, Jacobs CS, Lee JW, Weisholtz DS, Zepeda R, Westover MB, Cole AJ, Cash SS (2020) Association of epileptiform abnormalities and seizures in Alzheimer disease. Neurology 95(16):e2259–e2270. https://doi.org/10.1212/WNL.0000000000010612
Liedorp M, Stam CJ, van der Flier WM, Pijnenburg YA, Scheltens P (2010) Prevalence and clinical significance of epileptiform EEG discharges in a large memory clinic cohort. Dement Geriatr Cogn Disord 29(5):432–437. https://doi.org/10.1159/000278620
Lopes da Silva FH, Hoeks A, Smits H, Zetterberg LH (1974) Model of brain rhythmic activity. The Alpha-Rhythm of the Thalamus Kybernetik 15(1):27–37. https://doi.org/10.1007/bf00270757
Luppi JJ, Schoonhoven DN, van Nifterick AM, Gouw AA, Hillebrand A, Scheltens P, Stam CJ, de Haan W (2022) Oscillatory activity of the hippocampus in Prodromal Alzheimer’s disease: a source-space magnetoencephalography study. J Alzheimers Dis 87(1):317–333. https://doi.org/10.3233/JAD-215464
Maestu F, de Haan W, Busche MA, DeFelipe J (2021) Neuronal excitation/inhibition imbalance: core element of a translational perspective on Alzheimer pathophysiology. Ageing Res Rev 69:101372. https://doi.org/10.1016/j.arr.2021.101372
Manyukhina VO, Prokofyev AO, Galuta IA et al (2022) Globally elevated excitation–inhibition ratio in children with autism spectrum disorder and below-average intelligence. Mol Autism 13:20. https://doi.org/10.1186/s13229-022-00498-2
Martinez-Losa M, Tracy TE, Ma K, Verret L, Clemente-Perez A, Khan AS, Cobos I, Ho K, Gan L, Mucke L, Alvarez-Dolado M, & Palop JJ (2018) Nav1.1-overexpressing interneuron transplants restore brain rhythms and cognition in a mouse model of Alzheimer's disease. Neuron, 98(1): 75–89 e75. https://doi.org/10.1016/j.neuron.2018.02.029
Maturana-Candelas A, Gomez C, Poza J, Pinto N, Hornero R (2019) EEG characterization of the Alzheimer’s disease continuum by means of multiscale entropies. Entropy (basel). https://doi.org/10.3390/e21060544
Meisel C, Storch A, Hallmeyer-Elgner S, Bullmore E, Gross T (2012) Failure of adaptive self-organized criticality during epileptic seizure attacks. PLoS Comput Biol 8(1):e1002312. https://doi.org/10.1371/journal.pcbi.1002312
Menardi A, Rossi S, Koch G, Hampel H, Vergallo A, Nitsche MA, Stern Y, Borroni B, Cappa SF, Cotelli M, Ruffini G, El-Fakhri G, Rossini PM, Dickerson B, Antal A, Babiloni C, Lefaucheur JP, Dubois B, Deco G, Santarnecchi E (2022) Toward noninvasive brain stimulation 20 in Alzheimer’s disease. Ageing Res Rev 75:101555. https://doi.org/10.1016/j.arr.2021.101555
Misic B, Mills T, Taylor MJ, McIntosh AR (2010) Brain noise is task dependent and region specific. J Neurophysiol 104(5):2667–2676. https://doi.org/10.1152/jn.00648.2010
Mizuno T, Takahashi T, Cho RY, Kikuchi M, Murata T, Takahashi K, Wada Y (2010) Assessment of EEG dynamical complexity in Alzheimer’s disease using multiscale entropy. Clin Neurophysiol 121(9):1438–1446. https://doi.org/10.1016/j.clinph.2010.03.025
Montez T, Poil SS, Jones BF, Manshanden I, Verbunt JP, van Dijk BW, Brussaard AB, van Ooyen A, Stam CJ, Scheltens P, Linkenkaer-Hansen K (2009) Altered temporal correlations in parietal alpha and prefrontal theta oscillations in early-stage Alzheimer disease. Proc Natl Acad Sci U S A 106(5):1614–1619. https://doi.org/10.1073/pnas.0811699106
Nakamura A, Cuesta P, Kato T, Arahata Y, Iwata K, Yamagishi M, Kuratsubo I, Kato K, Bundo M, Diers K, Fernandez A, Maestu F, Ito K (2017) Early functional network alterations in asymptomatic elders at risk for Alzheimer’s disease. Sci Rep 7(1):6517. https://doi.org/10.1038/s41598-017-06876-8
Nicolaou N, Georgiou J (2012) Detection of epileptic electroencephalogram based on permutation entropy and support vector machines. Expert Syst Appl 39(1):202–209. https://doi.org/10.1016/j.eswa.2011.07.008
Numan T, Breedt LC, Maciel B, Kulik SD, Derks J, Schoonheim MM, Klein M, de Witt Hamer PC, Miller JJ, Gerstner ER, Stufflebeam SM, Hillebrand A, Stam CJ, Geurts JJG, Reijneveld JC, Douw L (2022) Regional healthy brain activity, glioma occurrence and symptomatology. Brain 145(10):3654–3665. https://doi.org/10.1093/brain/awac180
O’Byrne J, Jerbi K (2022) How critical is brain criticality? Trends Neurosci 45(11):820–837. https://doi.org/10.1016/j.tins.2022.08.007
Palop JJ, Mucke L (2016) Network abnormalities and interneuron dysfunction in Alzheimer disease. Nat Rev Neurosci 17(12):777–792. https://doi.org/10.1038/nrn.2016.141
Pani SM, Fraschini M, Figorilli M, Tamburrino L, Ferri R, Puligheddu M (2021) Sleep-related hypermotor epilepsy and non-rapid eye movement parasomnias: Differences in the periodic and aperiodic component of the electroencephalographic power spectra. J Sleep Res 30(5):e13339. https://doi.org/10.1111/jsr.13339
Ponten SC, Daffertshofer A, Hillebrand A, Stam CJ (2010) The relationship between structural and functional connectivity: graph theoretical analysis of an EEG neural mass model. Neuroimage 52(3):985–994. https://doi.org/10.1016/j.neuroimage.2009.10.049
Priesemann V, Wibral M, Valderrama M, Propper R, Le Van Quyen M, Geisel T, Triesch J, Nikolic D, Munk MH (2014) Spike avalanches in vivo suggest a driven, slightly subcritical brain state. Front Syst Neurosci 8:108. https://doi.org/10.3389/fnsys.2014.00108
Pusil S, Lopez ME, Cuesta P, Bruna R, Pereda E, Maestu F (2019) Hypersynchronization in mild cognitive impairment: the “X” model. Brain 142(12):3936–3950. https://doi.org/10.1093/brain/awz320
Ranasinghe KG, Kudo K, Hinkley L, Beagle A, Lerner H, Mizuiri D, Findlay A, Miller BL, Kramer JH, Gorno-Tempini ML, Rabinovici GD, Rankin KP, Garcia PA, Kirsch HE, Vossel K, Nagarajan SS (2022a) Neuronal synchrony abnormalities associated with subclinical epileptiform activity in early-onset Alzheimer’s disease. Brain 145(2):744–753. https://doi.org/10.1093/brain/awab442
Ranasinghe KG, Verma P, Cai C, Xie X, Kudo K, Gao X, Lerner H, Mizuiri D, Strom A, Iaccarino L, La Joie R, Miller BL, Gorno-Tempini ML, Rankin KP, Jagust WJ, Vossel K, Rabinovici GD, Raj A, Nagarajan SS (2022b) Altered excitatory and inhibitory neuronal subpopulation parameters are distinctly associated with tau and amyloid in Alzheimer’s disease. Elife. https://doi.org/10.7554/eLife.77850
Scaduto P, Lauterborn JC, Cox CD, Fracassi A, Zeppillo T, Gutierrez BA, Keene CD, Crane PK, Mukherjee S, Russell WK, Taglialatela G, Limon A (2023) Functional excitatory to inhibitory synaptic imbalance and loss of cognitive performance in people with Alzheimer’s disease neuropathologic change. Acta Neuropathol 145(3):303–324. https://doi.org/10.1007/s00401-022-02526-0
Scheijbeler EP, van Nifterick AM, Stam CJ, Hillebrand A, Gouw AA, de Haan W (2022) Network-level permutation entropy of resting-state MEG recordings: A novel biomarker for early-stage Alzheimer’s disease? Netw Neurosci. https://doi.org/10.1162/netn_a_00224
Schoonhoven DN, Briels CT, Hillebrand A, Scheltens P, Stam CJ, Gouw AA (2022) Sensitive and reproducible MEG resting-state metrics of functional connectivity in Alzheimer’s disease. Alzheimers Res Ther 14(1):38. https://doi.org/10.1186/s13195-022-00970-4
Shew WL, Plenz D (2013) The functional benefits of criticality in the cortex. Neuroscientist 19(1):88–100. https://doi.org/10.1177/1073858412445487
Shew WL, Yang H, Petermann T, Roy R, Plenz D (2009) Neuronal avalanches imply maximum dynamic range in cortical networks at criticality. J Neurosci 29(49):15595–15600. https://doi.org/10.1523/JNEUROSCI.3864-09.2009
Shew WL, Yang H, Yu S, Roy R, Plenz D (2011) Information capacity and transmission are maximized in balanced cortical networks with neuronal avalanches. J Neurosci 31(1):55–63. https://doi.org/10.1523/JNEUROSCI.4637-10.2011
Shumbayawonda E, Lopez-Sanz D, Bruna R, Serrano N, Fernandez A, Maestu F, Abasolo D (2020) Complexity changes in preclinical Alzheimer’s disease: an MEG study of subjective cognitive decline and mild cognitive impairment. Clin Neurophysiol 131(2):437–445. https://doi.org/10.1016/j.clinph.2019.11.023
Sohal VS, Rubenstein JLR (2019) Excitation-inhibition balance as a framework for investigating mechanisms in neuropsychiatric disorders. Mol Psychiatry 24(9):1248–1257. https://doi.org/10.1038/s41380-019-0426-0
Sorrentino P, Rucco R, Baselice F, De Micco R, Tessitore A, Hillebrand A, Mandolesi L, Breakspear M, Gollo LL, Sorrentino G (2021) Flexible brain dynamics underpins complex behaviours as observed in Parkinson’s disease. Sci Rep 11(1):4051. https://doi.org/10.1038/s41598-021-83425-4
Stam CJ (2005) Nonlinear dynamical analysis of EEG and MEG: review of an emerging field. Clin Neurophysiol 116(10):2266–2301. https://doi.org/10.1016/j.clinph.2005.06.011
Stam CJ, Pijn JP, Suffczynski P, Lopes da Silva FH (1999) Dynamics of the human alpha rhythm: evidence for non-linearity? Clin Neurophysiol 110(10):1801–1813. https://doi.org/10.1016/s1388-2457(99)00099-1
Stam CJ, van Nifterick AM, de Haan W, Gouw AA (2023) Network hyperexcitability in early Alzheimer’s Disease: Is functional connectivity a potential biomarker? Brain Topogr. https://doi.org/10.1007/s10548-023-00968-7
Stefanovski L, Triebkorn P, Spiegler A, Diaz-Cortes MA, Solodkin A, Jirsa V, McIntosh AR, Ritter P, Alzheimer's Disease Neuroimaging I (2019) Linking molecular pathways and large-scale computational modeling to assess candidate disease mechanisms and pharmacodynamics in Alzheimer’s disease. Front Comput Neurosci 13:54. https://doi.org/10.3389/fncom.2019.00054
Termenon M, Achard S, Jaillard A, Delon-Martin C (2016) The “Hub Disruption index,” a reliable index sensitive to the brain networks reorganization. A study of the contralesional hemisphere in stroke. Front Comput Neurosci 10:84. https://doi.org/10.3389/fncom.2016.00084
Tewarie P, Liuzzi L, O’Neill GC, Quinn AJ, Griffa A, Woolrich MW, Stam CJ, Hillebrand A, Brookes MJ (2019) Tracking dynamic brain networks using high temporal resolution MEG measures of functional connectivity. Neuroimage 200:38–50. https://doi.org/10.1016/j.neuroimage.2019.06.006
Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, Mazoyer B, Joliot M (2002) Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage 15(1):273–289. https://doi.org/10.1006/nimg.2001.0978
Ursino M, Zavaglia M, Astolfi L, Babiloni F (2007) Use of a neural mass model for the analysis of effective connectivity among cortical regions based on high resolution EEG recordings. Biol Cybern 96(3):351–365. https://doi.org/10.1007/s00422-006-0122-4
van Dellen E, Hillebrand A, Douw L, Heimans JJ, Reijneveld JC, Stam CJ (2013) Local polymorphic delta activity in cortical lesions causes global decreases in functional connectivity. Neuroimage 83:524–532. https://doi.org/10.1016/j.neuroimage.2013.06.009
van Nifterick AM, Gouw AA, van Kesteren RE, Scheltens P, Stam CJ, de Haan W (2022a) A multiscale brain network model links Alzheimer’s disease-mediated neuronal hyperactivity to large-scale oscillatory slowing. Alzheimers Res Ther 14(1):101. https://doi.org/10.1186/s13195-022-01041-4
van Nifterick AM, Gouw AA, van Kesteren RE, Scheltens P, Stam CJ, de Haan W (2022b) A multiscale brain network model links Alzheimer’s disease-mediated neuronal hyperactivity to large-scale oscillatory slowing. Alzheimer’s Res Therapy 14(1):101. https://doi.org/10.1186/s13195-022-01041-4
Vattikonda A, Surampudi BR, Banerjee A, Deco G, Roy D (2016) Does the regulation of local excitation-inhibition balance aid in recovery of functional connectivity? A computational account. Neuroimage 136:57–67. https://doi.org/10.1016/j.neuroimage.2016.05.002
Verret L, Mann EO, Hang GB, Barth AM, Cobos I, Ho K, Devidze N, Masliah E, Kreitzer AC, Mody I, Mucke L, Palop JJ (2012) Inhibitory interneuron deficit links altered network activity and cognitive dysfunction in Alzheimer model. Cell 149(3):708–721. https://doi.org/10.1016/j.cell.2012.02.046
Vossel KA, Beagle AJ, Rabinovici GD, Shu H, Lee SE, Naasan G, Hegde M, Cornes SB, Henry ML, Nelson AB, Seeley WW, Geschwind MD, Gorno-Tempini ML, Shih T, Kirsch HE, Garcia PA, Miller BL, Mucke L (2013) Seizures and epileptiform activity in the early stages of Alzheimer disease. JAMA Neurol 70(9):1158–1166. https://doi.org/10.1001/jamaneurol.2013.136
Vossel KA, Ranasinghe KG, Beagle AJ, Mizuiri D, Honma SM, Dowling AF, Darwish SM, Van Berlo V, Barnes DE, Mantle M, Karydas AM, Coppola G, Roberson ED, Miller BL, Garcia PA, Kirsch HE, Mucke L, Nagarajan SS (2016) Incidence and impact of subclinical epileptiform activity in Alzheimer’s disease. Ann Neurol 80(6):858–870. https://doi.org/10.1002/ana.24794
Vossel K, Ranasinghe KG, Beagle AJ, La A, Ah Pook K, Castro M, Mizuiri D, Honma SM, Venkateswaran N, Koestler M, Zhang W, Mucke L, Howell MJ, Possin KL, Kramer JH, Boxer AL, Miller BL, Nagarajan SS, Kirsch HE (2021) Effect of levetiracetam on cognition in patients with Alzheimer disease with and without epileptiform activity: a randomized clinical trial. JAMA Neurol 78(11):1345–1354. https://doi.org/10.1001/jamaneurol.2021.3310
Waschke L, Kloosterman NA, Obleser J, Garrett DD (2021) Behavior needs neural variability. Neuron 109(5):751–766. https://doi.org/10.1016/j.neuron.2021.01.023
Wilson SB, Emerson R (2002) Spike detection: a review and comparison of algorithms. Clin Neurophysiol 113(12):1873–1881. https://doi.org/10.1016/s1388-2457(02)00297-3
Xu L, Feng J, Yu L (2022) Avalanche criticality in individuals, fluid intelligence, and working memory. Hum Brain Mapp 43(8):2534–2553. https://doi.org/10.1002/hbm.25802
Yang H, Shew WL, Roy R, Plenz D (2012) Maximal variability of phase synchrony in cortical networks with neuronal avalanches. J Neurosci 32(3):1061–1072. https://doi.org/10.1523/JNEUROSCI.2771-11.2012
Yin D, Kaiser M (2021) Understanding neural flexibility from a multifaceted definition. Neuroimage 235:118027. https://doi.org/10.1016/j.neuroimage.2021.118027
Yin Y, Shang PJ, Ahn AC, Peng CK (2019) Multiscale joint permutation entropy for complex time series. Phys Stati Mech Appl 515:388–402. https://doi.org/10.1016/j.physa.2018.09.179
Yu M, Engels MMA, Hillebrand A, van Straaten ECW, Gouw AA, Teunissen C, van der Flier WM, Scheltens P, Stam CJ (2017) Selective impairment of hippocampus and posterior hub areas in Alzheimer’s disease: an MEG-based multiplex network study. Brain 140(5):1466–1485. https://doi.org/10.1093/brain/awx050
Yu T, Liu X, Wu J, Wang Q (2021) Electrophysiological biomarkers of epileptogenicity in Alzheimer’s disease. Front Hum Neurosci 15:747077. https://doi.org/10.3389/fnhum.2021.747077
Zetterberg LH, Kristiansson L, Mossberg K (1978) Performance of a model for a local neuron population. Biol Cybern 31(1):15–26. https://doi.org/10.1007/BF00337367
Acknowledgements
The authors would like to thank the researchers of the Clinical Neurophysiology and MEG Center Amsterdam for their valuable suggestions and fruitful discussions.
Funding
This work was funded by the Netherlands Organization for Health Research and Development (ZonMW; Grant #91218018, Grant #733050812). Research of Amsterdam Alzheimer Center is part of the neurodegeneration program of Amsterdam Neuroscience. The Amsterdam Alzheimer Center is supported by Alzheimer Nederland and Stichting VUmc funds.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Data collection and analysis were performed by AMN. CJS wrote the Brainwave software. The first draft of the manuscript was written by AMN and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
WH and AAG are founders of the EEGlab at Amsterdam UMC, location VUmc, The Netherlands. ES is part of the EEGlab research team. The EEGlab performs central EEG analysis for clinical trials funded by Vivoryon Therapeutics, EIP-Pharma, Fujifilm Toyoma, Immunobrain and Treeway. This funding does only involve financial support for AAG. The other authors have no competing interests to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
van Nifterick, A.M., Scheijbeler, E.P., Gouw, A.A. et al. Local signal variability and functional connectivity: Sensitive measures of the excitation-inhibition ratio?. Cogn Neurodyn 18, 519–537 (2024). https://doi.org/10.1007/s11571-023-10003-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11571-023-10003-x