Abstract
The processing of speech information from various sensory modalities is crucial for human communication. Both left posterior superior temporal gyrus (pSTG) and motor cortex importantly involve in the multisensory speech perception. However, the dynamic integration of primary sensory regions to pSTG and the motor cortex remain unclear. Here, we implemented a behavioral experiment of classical McGurk effect paradigm and acquired the task functional magnetic resonance imaging (fMRI) data during synchronized audiovisual syllabic perception from 63 normal adults. We conducted dynamic causal modeling (DCM) analysis to explore the cross-modal interactions among the left pSTG, left precentral gyrus (PrG), left middle superior temporal gyrus (mSTG), and left fusiform gyrus (FuG). Bayesian model selection favored a winning model that included modulations of connections to PrG (mSTG → PrG, FuG → PrG), from PrG (PrG → mSTG, PrG → FuG), and to pSTG (mSTG → pSTG, FuG → pSTG). Moreover, the coupling strength of the above connections correlated with behavioral McGurk susceptibility. In addition, significant differences were found in the coupling strength of these connections between strong and weak McGurk perceivers. Strong perceivers modulated less inhibitory visual influence, allowed less excitatory auditory information flowing into PrG, but integrated more audiovisual information in pSTG. Taken together, our findings show that the PrG and pSTG interact dynamically with primary cortices during audiovisual speech, and support the motor cortex plays a specifically functional role in modulating the gain and salience between auditory and visual modalities.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Humans communicate everyday by processing multimodal sensory information, especially integrating the auditory and visual inputs. The McGurk effect is well known in the multisensory integration underlying human speech perception. Listeners often perceive a McGurk illusion when incongruent auditory and visual signals are combined together (McGurk and MacDonald 1976). When an auditory syllable /pa/ was presented with a visual syllable /ka/, a different auditory syllable /ta/ will be perceived. Although the McGurk effect is robust and prevalent, the susceptibility of illusion varies among different individuals (Barutchu et al. 2019; Feng et al. 2019; Mallick et al. 2015). The individual McGurk susceptibility is correlated with ability of multisensory integration (Beauchamp et al. 2010; Benoit et al. 2010; Marques et al. 2016; Nath and Beauchamp 2012) and the ability of coordinated audiovisual modulation (Li et al. 2021).
Investigating the neural substrates that contribute to the McGurk illusion is of importance in helping understand the mechanisms underlying the multisensory information processing. Previous studies have suggested that the parts of Wernicke’s area (BA 42/22) in the posterior superior temporal cortex, especially the superior temporal gyrus (STG) and sulcus are involved in the multisensory speech processing (Friederici et al. 2017; Friederici 2011). Sensitivity to different phonetic features has been demonstrated in the middle and posterior superior temporal gyrus (mSTG/ pSTG) by using data-mining algorithms to identify patterns of activity in functional magnetic resonance imaging (fMRI) (Campbell 2011; Kilian-Hütten et al. 2011). Functional brain neuroimaging studies have demonstrated that the posterior superior temporal gyrus/sulcus (pSTG/S) is critical in the multisensory integration of audiovisual speech information (Beauchamp 2016; Beauchamp et al. 2004, 2008; Park et al. 2018) and the processing of short-timescale patterns (i.e., phonemes)-related activation is in the left mid-superior temporal gyrus (mSTG) (DeWitt and Rauschecker 2012). In addition to STG, motor structures play an important role in the multisensory speech perception (Benoit et al. 2010; Callan et al. 2014; D'Ausilio et al. 2009; Liebenthal and Möttönen 2018; Pulvermuller et al. 2006; Wilson et al. 2004). A study, by combining the fMRI and transcranial magnetic stimulation (TMS), directly proves that the TMS of the motor cortex lip areas weakened the McGurk effect greatly, suggesting that the motor network contributes to the illusion avoidance in the multisensory speech processing (Murakami et al. 2018). Moreover, a recent study reveals the redundant and synergistic cross-modal interactions in the left pSTG/S and motor cortex, respectively, during audiovisual speech processing (Park et al. 2018). Therefore, multiple neural mechanisms may support the audiovisual speech perception (Meijer et al. 2019). However, these studies typically investigated the STG/S and motor cortex activity related to the audiovisual speech stimuli and failed to focus on the dynamic interaction between audiovisual stimuli and brain signals.
The dynamic causal modeling (DCM) has been extended for causal inferences about the mechanism for an experimental condition to modulate the connections in a hypothesized neuronal network (Stephan et al. 2007; Zhang and Du, 2022). Previously, DCM can be applied to acquire the fMRI data during the audiovisual speech perception. For instance, both intrinsic STG and left to right STG connections are crucial in identifying the self-voice error and sensorimotor integration (Parkinson et al. 2013). A DCM study has shown that bidirectional connection between premotor cortex and STS and that between planum temporal and premotor cortex are significant during the speech perception, supporting the involvement of premotor cortex (Osnes et al. 2011). Besides, a study about the STS effective connectivity signature has suggested that the integration outcome of audiovisual speech primarily depends on whether the STS converges onto a multimodal syllable representation (Bouton et al. 2020). In general, the available evidence suggests both the effects of indirect and bidirectional influences of the STG/S and motor cortex on sensory processes during speech perception. However, they failed to explore the different modulatory influences of the pSTG/S and motor cortex either on auditory and visual processing areas and the effects of brain connections on the McGurk effect task performance. Studying the potential distinct functional roles of pSTG/S and motor cortex in processing streams of face and voice speech information will substantially advance the distinct mechanisms to support the audiovisual speech comprehension.
In the present study, behavioral measures and fMRI data were combined to explore the dynamic interactions of motor cortex and pSTG with primary sensory inputs during the audiovisual speech perception. We hypothesized that the motor cortex and pSTG involve in the distinct processes to support multisensory speech perception. Based on our previous study that focused mainly on brain activation instead of connectivity (Li et al. 2021), four brain regions from the left cerebral hemisphere were chosen for the DCM models: precentral gyrus (PrG, motor cortex), pSTG (multisensory region), mSTG (primary auditory speech processing), and FuG (visual lip movements processing). Forty-four models were selected to verify the effectiveness of network connectivity by these four regions of interest (ROIs). Random-effects Bayesian Model Selection (BMS) and Model Averaging (BMA) were applied to confirm the model fitting the observed data best and to estimate the subject-specific connectivity parameters. Subsequently, we employed the Spearman correlation analysis to explore the relation of model parameters to individual behavioral performance of McGurk effect. Finally, we further explored the connection strength differences between strong and weak McGurk perceivers.
Materials and methods
Participants
Sixty-three healthy volunteers (27 females, 21.7 years old in average (18–28 years)) who completed both behavioral and fMRI experiment were included in the study, as described in our previous study (Li et al. 2021). All participants were Chinese native speakers and right-handed, had normal or corrected-to-normal vision, and no any hearing disorders or psychiatric illnesses. They all had written informed consents on participating and understanding all experiments. This study was approved by the ethical committee of School of Life Science and Technology at University of Electronic Science and Technology of China.
Stimuli and procedure
The stimuli and procedure was described in (Li et al. 2021). In brief, a behavioral experiment outside scanning was conducted to examine individual McGurk susceptibility. A task fMRI experiment was performed to examine the brain activity and time course in response to non-McGurk congruent audiovisual syllables.
In the behavioral McGurk experiment, we used the McGurk incongruent audiovisual syllable pairs consisted of an auditory recording of the syllable /pa/ and a digital video of a female speaker pronouncing the syllable [ka]. The combination of auditory track (/pa/) and visual track ([ka]) resulted in 29 stimuli onset asynchronies (SOAs), including 0 ms, ± 33 ms, ± 67 ms, ± 100 ms, ± 133 ms, ± 167 ms, ± 200 ms, ± 233 ms, ± 267 ms, ± 300 ms, ± 333 ms, ± 367 ms, ± 400 ms, ± 433 ms, and ± 467 ms (positive for visual-leading and negative for audio-leading). Stimuli for each SOA were presented 10 trails, and the 290 trails were randomly presented. When a trial was begun, a fixation cross was displayed at center of the visual field with jittered duration (random selection from 500/750/1000/1250/1500 ms), followed by stimuli presentation and response (3000 ms, Fig. 1A). The E-Prime version 2.0 (Psychology Software Tools, https://pstnet.com/) was employed for stimuli presentation. Visual stimuli were displayed by a Samsung Sync monitor with 1024 × 768 pixels, and participants were approximately 50 cm away. Auditory stimuli were given by a Sennheiser headphone at a comfortable and fixed level. Participants should fixate the center of monitor screen and to observe lip movements of the speaker. In addition, we informed participants to listen carefully and report the syllable that they perceived (/pa/, /ka/, or /ta/). Participants performed behavioral responses by pressing the corresponding keys on a standard terminal keyboard with their right hand.
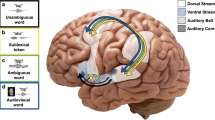
Behavioral and fMRI experimental paradigm. A In behavioral task, all participants were instructed to observe the lip movements and listen carefully, and then report what syllable they percept. B In the fMRI task, all participants were allowed to do the same actions with that in the behavioral task excluding reporting. SOA, stimulus onset asynchrony
The fMRI syllabic perception experiment was performed using the synchronous audiovisual-matched syllables, which included three conditions as follows: /pa/ + [pa], /ka/ + [ka], and /ta/ + [ta] (/auditory stimuli/ + [visual stimuli], Fig. 1B). Their order was balanced in three sessions (run1: /pa/, /ka/, and /ta/; run2: /ka/, /ta/, and /pa/; run3: /ta/, /pa/, and /ka/). A fixation cross was displayed at the visual field center with 20 s at the beginning of each session. After that, there were 18 blocks (6 blocks × 3 conditions) per session, and 7 trails were presented continuously in sequence in each block. Each trial consisted of audiovisual synchronous stimuli with 2000 ms and a fixation cross stimulus with 1000 ms. During fMRI data acquisition, a monitor viewed through a mirror equipped to the MRI head-coil was employed to present the visual stimuli and was about 60 cm from the participants. The projector was a 25.00 cm × 18.75 cm Avotec projector (SV6011) with 1024 × 768 pixels. Auditory stimuli were played using full-coverage Avotec headphones (SS3300) at 120 dB. Although the threshold of 120 dB marks the onset of pain during daily environment, the threshold was a comfort degree of our audio stimulus, allowing the participants (wearing earplugs and headphones) to hear the stimuli clearly under a noisy fMRI scanning environment. Participants were required to just observe the lip movements and listen carefully without reporting.
fMRI data acquisition
All MRI data were collected using a 3.0 T GE 750 scanner (General Electric, Fairfield, Connecticut, USA) with high-speed gradients. Before scanning, participants were allowed to be familiar with the environment and sounds. The participants were required to wear eight-channel prototype quadrature birdcage head coils fitted with foam padding for the purpose of minimizing the head motion. Besides, the foam pads and earplugs were arranged for reducing the head movement and scanner noise to the maximal content. Functional images were captured by using a gradient-recalled echo-planar imaging (EPI) sequence. The repetition time = 2000 ms, echo time = 30 ms, flip angle = 90°, bandwidth = 250 Hz / pixel, 43 axial slices, slice thickness = 3.2 mm without gap, matrix = 64 × 64, and field of view (FOV) = 240 mm. Finally, we obtained 199 volumes for each participant.
Behavioral data analysis
To quantify the individual McGurk susceptibility, we calculated the mean proportion of behavioral responses to 10 trails for 29 SOAs across all participants. When an auditory stimuli /pa/ and a visual stimuli [ka] were presented, participants may perceive McGurk fusion and report the syllable /ta/. The mean proportion of behavioral responses of /ta/ reached the peak at SOA of + 133 ms, so it was selected as an individual McGurk susceptibility for each participant (see (Li et al. 2021) for more details).
fMRI preprocessing
In this present study, we selected the Pre-processing for Task fMRI Data module of Data Processing and Analysis of Brain Imaging (DPABI) version 6.1 (http://rfmri.org/dpabi) (Yan et al. 2016) to preprocess functional images. The first 10 functional images per session of each participant were discarded to ensure steady-state longitudinal magnetization. 189 remaining images of each session were slice-time corrected and realigned to the middle volume of every session for correction of inter-scan head motion. In addition, the mean frame-wise displacement (FD) was calculated for subsequent group comparisons (He et al. 2016; Li et al. 2018; Lu et al. 2020). Further, all images were spatially normalized to Montreal Neurological Institute (MNI) reference space by using EPI template and resampled to 3 × 3 × 3 mm3 voxels. The normalized functional images were finally smoothed with a full width half maximum (FWHM) Gaussian kernel of 6 mm.
General linear model analysis
Task-dependent activation was estimated by performing a single-subject analysis with a general linear model (GLM) in Statistical Parametric Mapping (SPM) version 12 (release 6225, https://www.fil.ion.ucl.ac.uk/spm). Prior to this process, the analyzed data were high-pass filtered with a cut-off period of 128 s to exclude the slow signal drifts. In the present study, the data were modeled as a parametric design. Three syllable stimuli (/pa/ + [pa], /ka/ + [ka], and /ta/ + [ta]) were modeled together as a regressor according to the absence of significant main effect of three syllables (Li et al. 2021). We calculated six realignment parameters (three translations and three rotations) for each volume when the motion was corrected, and they were added as nuisance covariates. Each block was convolved by a canonical hemodynamic response function with a duration of 21 s and a stimulus onset interval of 21 s. A T-statistical contrast was specified for task-dependent effects. The finally obtained images were underwent a second-level analysis. One-sample t test was used to infer significantly different activations on the group-level (n = 63; P < 0.05, FDR-corrected, Fig. 2A). Furthermore, we specified an F-statistical contrast for subsequent volumes of interest (VOI) extraction when it was adjusted for effects of interest (Torrisi et al. 2013).
A Brain regions with significant task-dependent activation in the group-level statistic (n = 63; one-sample t-test, P < 0.05, FDR-corrected). Brain map was generated with BrainNet Viewer version 1.61 (http://www.nitrc.org/projects/bnv/); B ROIs. The anatomy map (n = 63) was employed to determine node peak selection, in which four peaks were showed in red. We only explored regions from the left hemisphere. The anatomy map was visualized with the MRIcron version 4 (https://www.nitrc.org/projects/mricron). MNI, Montreal Neurological Institute; FuG, fusiform gyrus; pSTG, posterior superior temporal gyrus; mSTG, mid-superior temporal gyrus; PrG, precentral gyrus
Dynamic causal modeling analysis
DCM can be adopted to explain the changes in regional activity modulating the connectivity among different brain regions (Friston et al. 2003). In present study, DCM and BMA analyses were applied to investigate the dynamic interactions between unimodal and multimodal audiovisual systems during the perception of synchronized and matched audiovisual syllables, which were conducted within DCM12 as implemented in SPM12 (https://www.fil.ion.ucl.ac.uk/spm).
ROIs selection
We focused on two unimodal sensory areas for DCM, including the primary auditory and visual speech cortex and two multisensory speech processing areas. Previous studies have supported the role of the mSTG as primary auditory cortex (DeWitt and Rauschecker 2012; Friederici et al. 2017). Visual speech activations have been reported in the FuG (Bernstein and Liebenthal 2014; Campbell 2011; Capek et al. 2008). In terms of multisensory processing, it has been converged that the pSTG/S is very critical for the cross-modal integration of audiovisual speech information (Beauchamp 2016; Beauchamp et al. 2004, 2008; Park et al. 2018). In addition to incorporating these three brain regions into the DCM model, the motor cortex was also included as an important multisensory speech processing region (Benoit et al. 2010; Callan et al. 2014; D'Ausilio et al. 2009; Liebenthal and Möttönen 2018; Pulvermuller et al. 2006; Wilson et al. 2004). To be specific, as regions showing significantly different activations on the group-level (Table 1) as well as between strong and weak perceivers (Supplementary Table S1), these four ROIs were identified with peak MNI coordinates of group-level task activation maps: left PrG (motor cortex: − 54, 0, 48), left pSTG (multisensory region: − 54, − 39, 18), left mSTG (auditory speech processing: − 54, − 18, 3), and left FuG (visual lip movements processing: − 30, − 78, − 18) (Table 1 and Fig. 2B). Locations of the ROIs could be determined by limiting the four seed regions by an 8 mm sphere around the peak voxel (MNI coordinates of the highest T value). Subsequently, the ROIs were overlapped with the corresponding Automated Anatomical Labeling atlas (Tzourio-Mazoyer et al. 2002) to ensure the brain voxels within each ROI fell inside the corresponding specific brain regions.
Time series extraction
After the age, gender, and years of education of all participants were matched, a total of 46 normal subjects (excluding the medial McGurk perceivers) were enrolled into the following analyses. Of note, one subgroup included the strong McGurk perceivers (with a susceptibility > 50%, n = 26), and the other subgroup included the weak McGurk perceivers (with a susceptibility < 50%, n = 20) by referring to (Li et al. 2021). We calculated the first eigenvariates from all voxels in the four ROIs to extract the time series of each participant. Region-specific time series was concatenated over the three sessions (Noppeney et al. 2008) and adjusted to the participant’s F-statistical contrast (effects of interest). In addition, ROIs were activated at the individual level so that the time course could be extracted (P < 0.1, uncorrected). As suggested by (Zeidman et al. 2019a), if subjects with no voxel survived in an ROI, we increased the threshold with the step of 0.05 until P < 0.5. One participant was excluded because some activations were missed on the single-subject level in ROIs above the predefined threshold. Finally, we included 25 strong and 20 weak perceivers (45 participants in total) for the following analyses. Demographic characteristics are listed in Table 2.
Model space construction
-
A.
Endogenous connection (factor 1)
Bidirectional endogenous connections among mSTG, pSTG, and FuG were based on the following findings: (1) the left pSTG is proved to be connected to left mSTG (Friederici et al. 2017) and left FuG (Kreifelts et al. 2007) by some structural data and effective connectivity data; (2) The ROI approach further confirmed that effective connectivity between pSTG and ipsilateral FuG and the effective connectivity between pSTG and mSTG was greatly enhanced during audiovisual integration (Kreifelts et al. 2007); (3) A framework has been established to demonstrate bidirectional connectivity between visual, auditory and multisensory area (Keil and Senkowski, 2018).
Given that the motor cortex was also play an important role in multisensory speech processing (Benoit et al. 2010; Callan et al. 2014; D'Ausilio et al. 2009; Liebenthal and Möttönen 2018; Pulvermuller et al. 2006; Wilson et al. 2004), we further studied the multisensory speech perception processing DCMs that with or without bidirectional connections between PrG and mSTG/pSTG/FuG. To investigate the connection strength differences between groups of strong and weak perceivers, we put forward our hypothesis refer to previous studies (David et al. 2011; Straube et al. 2018). Similar to (Friston et al. 2016), extrinsic connections were modified among different models but respected basic feature and seven families were formed (Supplementary Figure S1 and Table 3): (1) Family 1: PrG bidirectional connected to other three regions; (2) Family 2: PrG bidirectional connected to mSTG and FuG; (3) Family 3: PrG bidirectional connected to pSTG and FuG; (4) Family 4: PrG bidirectional connected to pSTG and mSTG; (5) Family 5: PrG only bidirectional connected to pSTG; (6) Family 6: PrG only bidirectional connected to mSTG; (7) Family 7: PrG only bidirectional connected to FuG. We grouped all three syllable stimuli (/pa/, /ka/ and /ta/) as driving inputs (C-matrix) that entered the system at mSTG and FuG. The C-matrix was identical in all families.
-
2
Modulatory influence (factor 2)
We used all three speech tasks as modulatory inputs (B-matrix), because the single-modality-sourced information is transferred to integration and modulation regions. After the sensory world was sampled using the motor system, selectively extracting the task-relevant information becomes very important due to limited attentional and processing resources (Wolpert et al. 2011). Speech motor control has been proposed to be related to feedback error detection in sensory cortices, which is then projected back to the motor systems to improve the accuracy (Behroozmand et al. 2015). Therefore, we hypothesized that the unidirectional modulation from mSTG to PrG or from FuG to PrG should exist according to the presence of bidirectional intrinsic connection to PrG in our study. Besides, other modulation between PrG and pSTG/mSTG/FuG might exist or not, so differences among different conditions were further analyzed here by modulating all extrinsic connections in each family. Importantly, the factor 2 introduced here varied as a function of the endogenous connection in factor 1. Therefore, this factor was modeled with 10 alternatives and 44 different models were constructed for each participant. The ten different alternatives were as follows:
-
a.
The presence or absence of modulation between PrG and pSTG:
-
(1)
PrG and pSTG showed no modulation.
-
(2)
A unidirectional modulation from PrG to pSTG was observed.
-
(3)
A unidirectional modulation from pSTG to PrG was observed.
-
(4)
A bidirectional modulation was observed between PrG and pSTG.
-
(1)
-
b.
The presence or absence of modulation between PrG and mSTG:
-
(1)
PrG and mSTG had no modulation.
-
(2)
A unidirectional modulation from mSTG to PrG was observed.
-
(3)
A bidirectional modulation was observed between PrG and mSTG.
-
(1)
-
c.
The presence or absence of modulation between PrG and FuG:
-
(1)
PrG and FuG had no modulation between PrG and FuG.
-
(2)
A unidirectional modulation from FuG to PrG was observed.
-
(3)
A bidirectional modulation was observed between PrG and FuG.
-
(1)
Model space visualization was presented in Supplementary Figure S1. For each model, the connectivity parameters (e.g., endogenous connections, modulatory influences, and driving inputs) were estimated using an expectation maximization algorithm (Dempster et al. 1977; Friston 2002).
Bayesian model comparison
Given that the subjects included in our study were all healthy volunteers and the characteristics of demographic information were relatively consistent, we first involved all the participants for the model selection. Following model specification and parameter estimation, we performed model comparison across 41 participants with a random-effects (RFX) BMS family level inference procedure, which removes uncertainty about the aspects of model structure other than the characteristic of interest (Penny et al. 2010). The exceedance probability for each family of models was calculated so that the most likely generative family of the observed data could be identified. The family of models with the highest exceedance probability was identified as the “best family of models”. After BMS, we performed a RFX BMA within the best family (Penny et al. 2010; Straube et al. 2018). BMA averaged the connectivity parameters within models of the best family for each participant, weighted by their model exceedance probability. The highest model exceedance probability determined the “winning model” being more fit the observed data, based on which the generated subject-specific connectivity parameters were input into parameter statistics (Parker Jones et al. 2013; Straube et al. 2018; Torrisi et al. 2013). To further identify whether the strong and weak McGurk perceivers have different models with distinct modulatory connections, we performed exploratory analysis by putting the two groups into the modeling separately.
Parameter statistics
Classical statistics was used to test the significance of the resulting connection strengths from BMA for both the endogenous connections and modulatory influences (Parker Jones et al. 2013). Prior to classical statistics, we performed multiple linear regression as supported by MATLAB R2018b. We selected age, gender, and years of education to be external regressors to control their effects on connection strengths. Subsequently, we used one-sample t test for calculating the significance of each connection within all participants (n = 45). In order to investigate the relationship between brain effective connectivity and behavioral performance of McGurk effect, we performed Spearman correlation analysis between regressive connection strengths (A- matrix, B- matrix, A- and B- matrix) and individual McGurk susceptibility. Then, we tested the significance of each connection by using one-sample t test within the strong perceivers (n = 25) or weak perceivers (n = 20). To determine why strong and weak McGurk perceivers have similar neural architecture and even similar network interaction pattern but show different behavioral multisensory illusory perceptions, we adopted two-sample t test to evaluate the group differences of each connection. All p values were FDR-corrected by using a threshold of P < 0.05.
Results
DCM model selection
RFX BMS provided evidence for the “best family of models” to be the generative models for the observed data. Family-level inference revealed that the models with bidirectional endogenous connections between the four regions (Family 1) outperformed all families (family exceedance probability = 80.56%, Fig. 3A). Among the 16 “best family of models”, the model with modulatory influences on the connections from PrG to FuG, from FuG to PrG, from PrG to mSTG, from mSTG to PrG, from mSTG to pSTG, from FuG to pSTG (model 13) won over 15 other models (Model Exceedance Probability = 34.92%, Fig. 3B). An overview of the winning model is illustrated in Fig. 3C. Exploratory analysis presented that the strong and weak McGurk have the same best family (family 1) but have different models with several distinct modulatory connections. Specifically, the RFX BMS favored the Model 13 in the strong McGurk perceivers, while Model 11 in the weak McGurk perceivers (Supplementary Figures S2-S3).
A Results of the family level inference in all participants. The best family of models were the DCMs with bidirectional endogenous connections among the four ROIs (Family 1, family exceedance probability = 80.56%). B Results from the BMA within family. RFX BMS favored the model with modulatory influences on the connections from PrG to FuG, FuG to PrG, PrG to mSTG, mSTG to PrG, mSTG to pSTG, FuG to pSTG (Model 13, model exceedance probability = 34.92%). (C) Overview of the winning model (Model 13). FuG, fusiform gyrus; pSTG, posterior superior temporal gyrus; mSTG, mid-superior temporal gyrus; PrG, precentral gyrus
DCM parameter statistics
The DCM parameter statistics are summarized in Table 4 and Fig. 4. Based on the winning model (model 13), the endogenous connections from mSTG to PrG (P = 0.857) and the modulatory connections from FuG to pSTG (0.352) failed to reach the statistical significance level and the remaining connections were all significant (Table 4, < 0.05, FDR-corrected). The averaged coupling strength (A- and B- matrix) showed excitatory connections (shown as red edges in Fig. 4) from mSTG to PrG and pSTG. The remaining coupling connections were inhibitory connections (green edges in Fig. 4). Regarding brain effective connectivity and individual behavioral performance of McGurk effect, we found that the individual McGurk susceptibility is correlated with the coupling connection strengths (FDR corrected, P < 0.05). To further test the coupling strength differences between strong and weak perceivers, we compared these six connections (mSTG → PrG, mSTG → pSTG, PrG → mSTG, PrG → FuG, FuG → PrG, FuG → pSTG) with modulatory influences (B matrix) (see bar plots in Fig. 4).
Winning model with averaged coupling strength (A- and B- matrix) for excitatory (red) and inhibitory (green) connections (*P < 0.05, **P < 0.01, ***P < 0.001, uncorrected). Coupling strength differences between strong and weak perceivers are illustrated using bar plots (solid star: P < 0.05, FDR-corrected). FuG, fusiform gyrus; pSTG, posterior superior temporal gyrus; mSTG, mid-superior temporal gyrus; PrG, precentral gyrus
Connection from PrG to mSTG and that from PrG to FuG
The connection from PrG to mSTG and FuG showed a significant inhibitory effect (P < 0.001). In addition, the greater McGurk susceptibility, the stronger the network coupling including PrG → mSTG (r = 0.54, P < 0.001), PrG → FuG (r = 0.66, P < 0.001). By contrast, strong perceivers showed a significant lower inhibitory effect in both connections (P < 0.001).
Connection from mSTG/FuG to PrG
The connection from mSTG to PrG showed a significant excitatory effect (P < 0.001), and the correlation between that and McGurk susceptibility reached the trend level (r = − 0.30, P = 0.04). The excitatory effect of strong perceivers was significantly lower (P < 0.001). Moreover, an inhibitory connection was observed from FuG to PrG (P < 0.001), which was positive correlated with McGurk susceptibility (r = 0.70, P < 0.001). The inhibitory effect is significantly lower in strong perceivers (P < 0.001).
Connection from mSTG/FuG to pSTG
The connection from mSTG to pSTG showed a significant excitatory effect (P < 0.001), and the connection is positive correlated with McGurk susceptibility (r = 0.67, P < 0.001). The higher coupling was observed in strong perceivers (P < 0.001). In terms of the connection from FuG to pSTG, an inhibitory connection was observed (P = 0.005), which is positive correlated with McGurk susceptibility (r = 0.56, P < 0.001). Besides, the group comparison showed significant less inhibition in strong perceivers (P < 0.001).
Discussion
We investigated how motor cortex and pSTG dynamically interact with the primary sensory inputs during the multisensory speech perception. Behavioral and fMRI audiovisual experiments were carried out, and DCM analysis was applied to reveal the effective connectivity between the cross-modal areas of PrG and pSTG and the unisensory cortices of mSTG and FuG. Our results favored a fully connected model showing that both PrG and pSTG enhanced the auditory signal processing but suppressed the visual signal influence. This observation suggests that multisensory speech perception highly rely on interactions of auditory stream to motor cortex and pSTG. More importantly, the coupling strength of the connections associated with PrG and pSTG was correlated with individual behavioral McGurk susceptibility. Additionally, between-group coupling strength statistics showed that compared with the weak McGurk effect perceivers, the strong perceivers processed less inhibitory visual inputs and excitatory auditory information in PrG and integrated more audiovisual contents in pSTG. Together, these results suggest that the PrG and pSTG interact dynamically with the primary cortices during audiovisual speech and prove that the motor cortex plays a specific role in adjusting the gain and salience between the auditory and visual modalities.
It is helpful to get new mechanistic insights on the functional roles of the PrG and STG/S during the multisensory speech processing from the classical DCM analysis. Our results favored a winning family with a bidirectional endogenous connection from PrG to pSTG, mSTG, and FuG, which are consistent with the previous literature supporting the involvement of motor cortex in audiovisual tasks (Benoit et al. 2010; Callan et al. 2014; D'Ausilio et al. 2009; Liebenthal and Möttönen 2018; Pulvermuller et al. 2006; Skipper et al. 2005; Wilson et al. 2004). Furthermore, we found the winning model in family 1 with the modulations of input on the connections from PrG to mSTG, PrG to FuG, mSTG to PrG, FuG to PrG, mSTG to pSTG, and FuG to pSTG (model 13). The observed dynamic processes suggest that pSTG and motor cortex both serve as important neural substrates in multisensory speech perception. Aside from emphasizing the importance of both mSTG and PrG in multimodal perceptual processes, our winning model show potential different functions between the above two brain areas. In specific, pSTG was only subjected to the influences from bottom-up sensory inputs, while PrG generated top-down feedback modulation while receiving the information flow. Meanwhile, a recent study has proposed that distinct mechanisms may occur in these two regions, in which the STG is likely to exhibit auditory and visual inputs redundantly, while PrG represents the sensory inputs synergistically (Park et al. 2018). As a classic multi-sensory integration area, the pSTG has been proved, in many studies on functional neuroimaging, to be associated with audiovisual speech integration (Daniel et al. 2004; Michael et al. 2004). A recent DCM study has shown that STS could receive and reorder the speech and then determined the multimodal syllable representation (Bouton et al. 2020). In contrast, PrG has been adopted as the top-down modulator to facilitate audiovisual speech comprehension and multisensory integration (Choi et al. 2018; Park et al. 2018). In other words, auditory and visual inputs may be processed at varying degrees of integration in pSTG, and as a modulator, the PrG may require more information from sensory areas. Our results support that the auditory and visual feedforward and feedback interactions may be facilitated by the modulation through PrG, which is helpful to calibrate and characterize the multi-sensory information accurately. The speech perception motor theory has been proved to support the regulation of motor cortex (Corballis 2010), in which the motor cortex involves in audiovisual mapping the sensory inputs onto matching motor representations. Therefore, our DCM findings support the notion that it is possible distinct brain circuits (such as PrG and pSTG) for parallel sensory processing and eventually form a multisensory percept based on their interactive cerebral connections.
In addition, significant positive correlations were found between individual McGurk susceptibility and coupling strengths including PrG → mSTG, PrG → FuG, FuG → pSTG, mSTG → pSTG, and FuG → PrG. Whereas, slight negative correlation (uncorrected) was found between McGurk susceptibility and the coupling strength of connection from mSTG to PrG. In other words, subjects with stronger behavioral McGurk effect were more likely to have higher coupling connectivity from FuG to PrG, or lower coupling connectivity from mSTG to PrG, which provides further evidence that the PrG modulates more visual information relative to auditory information in the population who are more likely to perceive the McGurk effect. These correlation results suggest that the modulatory process in the motor cortex, as well as the audiovisual information integration in the pSTG play important roles in McGurk’s illusory behavior.
Regarding the different behavioral McGurk illusory susceptibility in the healthy population, we further explore the group differences in effective connectivity. Firstly, by putting all the subjects into the modeling, between-group statistical and post-hoc analyses suggested that the mean coupling strength of the winning model (model 13) differed significantly between strong and weak perceivers, including the connection from mSTG to pSTG and that from FuG to pSTG. The connection from mSTG to pSTG is excitatory, whereas that from FuG to pSTG is inhibitory in both groups. In addition, strong perceivers showed more excitatory auditory signal information and less inhibitory visual information flow to pSTG. It seems that the diverse dependency level of inhibitory visual input in pSTG may be the key aspect for varying individual audiovisual susceptibility. Evidence have shown that patients with schizophrenia revealed significantly reduced connectivity in the verbal pathway (from left middle temporal gyrus to left STS) (Wroblewski et al. 2020). Furthermore, the relationship between the McGurk effect and Odd or Eccentric Behaviour associated with schizotypal personality traits was fully mediated by visual accuracy (Muller et al. 2021). These can be considered as a stronger focus on visual information instead of auditory information in the integration process of speech. Interestingly, people relying more on auditory modal in daily (e.g., musicians) are likely to be weak McGurk perceivers (Proverbio et al. 2016). Significant differences were observed in the coupling connectivity from primary sensory areas (mSTG, FuG) to PrG between the McGurk perceiver subgroups. We observed less excitatory auditory streams and inhibitory visual streams flowed into PrG in strong perceivers. Additionally, significant differences were observed in the coupling connectivity from PrG to primary sensory areas (mSTG, FuG) between the two subgroups. The feedback error detection in sensory cortices is involved by the speech motor control, based on which the motor-related areas were activated to adjust (modulate) the parameters during the speech perception (Behroozmand et al. 2015). Prior combined TMS and fMRI study has demonstrated that disruption of the motor cortex lip areas effectively reduced the McGurk effect, indicating that the motor cortex contributes to the detection and resolution of multisensory incompatibility and participates in regulating the speech perception (Murakami et al. 2018). Based on the potential regulation processes in the motor cortex for adjusting the gain and salience between auditory and visual modalities, we speculated that stronger McGurk perceivers may consider that the visual channel is more reliable than the auditory channel. Our such results are consistent with previous findings that incoherent audiovisual context would decrease the weight of visual stream (Nahorna et al. 2012, 2015).
It is worth noting that the strong and weak subgroups have several different modulatory connections when they were put into the modeling separately: the connection from pSTG to PrG in weak group and that from PrG to mSTG in strong group. It is possible that the strong McGurk perceivers showed more downstream from PrG to pSTG. Moreover, the weak McGurk perceivers may be more effective in processing multisensory information, given that the integration area of pSTG would provide feedforward effect to the motor area. Such finding described here is consistent with the ideas in several previous studies, showing that the specificity of phoneme representations and the network connectivity of dorsal stream can be enhanced by lip movement, improving the speech perception (Zhang and Du 2022). It maybe relate to speech processing dorsal stream in sensorimotor integration where the pSTG functions as a sensorimotor interface while the PrG provides articulatory predictions of upcoming speech from other modalities (e.g., lip movements) and feedback to the pSTG for integrating top-down prediction and bottom-up speech (Du et al. 2014; Hickok and Poeppel 2007).
The current study is subjected to several limitations. First, illusion rate of the sample included was 40–60%. To further confirm the findings and accurately follow the brain modifications, researchers should expand the recruitment for large cohorts of participants. Second, mechanisms for processing the McGurk audiovisual pairings differs from that processing the natural audiovisual speech events, but the McGurk pairs were not performed in this study. Therefore, we will design more experiment for congruent and incongruent audiovisual stimuli. Third, whether fMRI signals are conductive to casual inference remain inconclusive, because the accuracy of causal modeling with fMRI data is adversely affected by limited time (Ramsey et al. 2010). Fourth, the individual connections strength is subjected to a certain variability, that is, parameters represented by their covariance matrix are uncertain, so further studies especially in clinical cohorts, might be extended to test the alterations in effective connectivity during multisensory speech perception due to pathological factors (e.g., schizophrenia) (Lu and Pan 2020), or brain lesion (e.g., aphasia) (Krason et al. 2022) by using Parametric Empirical Bayes analyses (PEB) (Bencivenga et al. 2021; Friston et al. 2016; Zeidman et al. 2019a, 2019b). Finally, the current study included non-computer-generated stimuli. In future research, a new informative approach should be employed to investigate the “bistable” support of false audiovisual speech perception induced by audiovisual integration (Thézé et al. 2020).
In summary, our study supports a new perspective on multisensory speech processing, which considers the auditory and visual information integration of pSTG and the characterization of the functional modulation of PrG. Furthermore, we demonstrate that the modulatory process in the motor cortex, as well as the audiovisual information integration in the pSTG play important roles in McGurk’s illusory behavior. Our findings unveil the dynamic interactive processes among cross-modal regions and unisensory cortices during the multisensory speech processing and particularly highlight the specific function of motor cortex in modulating the gain and salience between auditory and visual modalities.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Barutchu A, Toohey S, Shivdasani MN, Fifer JM, Crewther SG, Grayden DB, Paolini AG (2019) Multisensory perception and attention in school-age children. J Exp Child Psychol 180:141–155
Beauchamp MS (2016) Audiovisual speech integration. Science 2:515–526
Beauchamp MS, Lee KE, Argall BD, Martin A (2004) Integration of auditory and visual information about objects in superior temporal sulcus. Neuron 41:809–823
Beauchamp MS, Nath AR, Pasalar S (2010) fMRI-guided transcranial magnetic stimulation reveals that the superior temporal sulcus is a cortical locus of the mcgurk effect. J Neurosci 30:2414–2417
Beauchamp MS, Yasar NE, Frye RE, Ro T (2008) Touch, sound and vision in human superior temporal sulcus. Neuroimage 41:1011–1020
Behroozmand R, Shebek R, Hansen DR, Oya H, Robin DA, Howard MA, Greenlee JDW (2015) Sensory–motor networks involved in speech production and motor control: an fMRI study. Neuroimage 109:418–428
Bencivenga F, Sulpizio V, Tullo M, Galati G (2021) Assessing the effective connectivity of premotor areas during real vs imagined grasping: a DCM-PEB approach. Neuroimage 230:117806
Benoit MM, Raij T, Lin FH, Jaaskelainen IP, Stufflebeam S (2010) Primary and multisensory cortical activity is correlated with audiovisual percepts. Hum Brain Mapp 31:526–538
Bernstein L, Liebenthal E (2014) Neural pathways for visual speech perception. Front Neurosci 8:386
Bouton S, Delgado-Saa J, Olasagasti I, Giraud AL (2020) Audio-visual combination of syllables involves time-sensitive dynamics following from fusion failure. Sci Rep 10:18009
Callan DE, Jones JA, Callan A (2014) Multisensory and modality specific processing of visual speech in different regions of the premotor cortex. Front Psychol 5:389
Campbell R (2011) Speechreading and the Bruce-Young model of face recognition: early findings and recent developments. Br J Psychol 102:704–10
Capek C, Macsweeney M, Woll B, Waters D, McGuire P, David A, Brammer M, Campbell R (2008) Cortical circuits for silent speechreading in deaf and hearing people. Neuropsychologia 46:1233–1241
Choi I, Lee JY, Lee SH (2018) Bottom-up and top-down modulation of multisensory integration. Curr Opin Neurobiol 52:115–122
Corballis MC (2010) Mirror neurons and the evolution of language. Brain Lang 112:25–35
D’Ausilio A, Pulvermuller F, Salmas P, Bufalari I, Begliomini C, Fadiga L (2009) The motor somatotopy of speech perception. Current Biology CB 19:381–385
Daniel ECJAJ, Kevin M, Christian K, Akiko MC, Eric V-B (2004) Multisensory integration sites identified by perception of spatial wavelet filtered visual speech gesture information. J Cogn Neurosci 16:805–816
David O, Maess B, Eckstein K, Friederici AD (2011) Dynamic causal modeling of subcortical connectivity of language. J Neurosci 31:2712–2717
Dempster AP, Laird NM, Rubin DB (1977) Maximum Likelihood from Incomplete Data Via the EM Algorithm. J Roy Stat Soc Ser B (methodol) 39:1–22
DeWitt I, Rauschecker J (2012) Phoneme and word recognition in the auditory ventral stream. Proc Natl Acad Sci USA 109:E505–E514
Du Y, Buchsbaum BR, Grady CL, Alain C (2014) Noise differentially impacts phoneme representations in the auditory and speech motor systems. Proc Natl Acad Sci U S A 111:7126–7131
Feng G, Zhou B, Zhou W, Beauchamp MS, Magnotti JF (2019) A laboratory study of the McGurk effect in 324 Monozygotic and Dizygotic twins. Front Neurosci 13:1029
Friederici A, Chomsky N, Berwick R, Moro A, Bolhuis J (2017) Language, mind and brain. Nat Hum Behav 1:713–722
Friederici AD (2011) The brain basis of language processing: from structure to function. Physiol Rev 91:1357–1392
Friston K, Litvak V, Oswal A, Razi A, Stephan K, van Wijk B, Ziegler G, Zeidman P (2016) Bayesian model reduction and empirical Bayes for group (DCM) studies. Neuroimage 128:413–431
Friston KJ (2002) Bayesian estimation of dynamical systems: an application to fMRI. Neuroimage 16:513–530
Friston KJ, Harrison L, Penny W (2003) Dynamic causal modelling. Neuroimage 19:1273–1302
He Z, Cui Q, Zheng J, Duan X, Pang Y, Gao Q, Han S, Long Z, Wang Y, Li J, Wang X, Zhao J, Chen H (2016) Frequency-specific alterations in functional connectivity in treatment-resistant and -sensitive major depressive disorder. J Psychiatr Res 82:30–39
Hickok G, Poeppel D (2007) The cortical organization of speech processing. Nat Rev Neurosci 8:393–402
Keil J, Senkowski D (2018) Neural oscillations orchestrate multisensory processing. Neuroscientist 24
Kilian-Hütten N, Valente G, Vroomen J, Formisano E (2011) Auditory cortex encodes the perceptual interpretation of ambiguous sound. J Neurosc 31:1715–1720
Krason A, Buxbaum L, Vigliocco G, Mailend M-L, Varley R, Stoll H (2022) Benefit of visual speech information for word comprehension in post-stroke aphasia. Human 6:79
Kreifelts B, Ethofer T, Grodd W, Erb M, Wildgruber D (2007) Audiovisual integration of emotional signals in voice and face: an event-related fMRI study. Neuroimage 37:1445–1456
Li L, Li R, Huang X, Shen F, Wang H, Wang X, Deng C, Wang C, Yang J, Zhang L, Li J, Zou T, Chen H (2021) Motor circuit and superior temporal sulcus activities linked to individual differences in multisensory speech perception. Brain Topogr 34:779–792
Li R, Liao W, Yu Y, Chen H, Guo X, Tang Y-L, Chen H (2018) Differential patterns of dynamic functional connectivity variability of striato-cortical circuitry in children with benign epilepsy with centrotemporal spikes. Hum Brain Mapp 39:1207–1217
Liebenthal E, Möttönen R (2018) An interactive model of auditory-motor speech perception. Brain Lang 187:33–40
Lu F, Cui Q, Huang X, Li L, Duan X, Chen H, Pang Y, He Z, Sheng W, Han S, Chen Y, Yang Y, Luo W, Yu Y, Jia X, Tang Q, Li D, Xie A, Chen H (2020) Anomalous intrinsic connectivity within and between visual and auditory networks in major depressive disorder. Prog Neuropsychopharmacol Biol Psychiatry 100:109889
Lu H, Pan C (2020) The McGurk effect in self-recognition of people with schizophrenia. Soc Behav Personal Int J 48:1–8
Mallick DB, Magnotti JF, Beauchamp MS (2015) Variability and stability in the McGurk effect: contributions of participants, stimuli, time, and response type. Psychon Bull Rev 22:1299–1307
Marques LM, Lapenta OM, Costa TL, Boggio PS (2016) Multisensory integration processes underlying speech perception as revealed by the McGurk illusion. Lang Cog Neurosci 31:1115–1129
McGurk H, MacDonald J (1976) Hearing lips and seeing voices. Nature 264:746–748
Meijer GT, Mertens PEC, Pennartz CMA, Olcese U, Lansink CS (2019) The circuit architecture of cortical multisensory processing: distinct functions jointly operating within a common anatomical network. Prog Neurobiol 174:1–15
Michael S, Beauchamp KEL, Brenna D, Argall AAM (2004) Integration of auditory and visual information about objects in superior temporal sulcus. Neuron 41:809–823
Muller A-M, Dalal TC, Stevenson RA (2021) Schizotypal personality traits and multisensory integration: an investigation using the McGurk effect. Acta Physiol (oxf) 218:103354
Murakami T, Abe M, Wiratman W, Fujiwara J, Okamoto M, Mizuochi-Endo T, Iwabuchi T, Makuuchi M, Yamashita A, Tiksnadi A, Chang FY, Kubo H, Matsuda N, Kobayashi S, Eifuku S, Ugawa Y (2018) The motor network reduces multisensory illusory perception. J Neurosci 38:9679–9688
Nahorna O, Berthommier F, Schwartz JL (2012) Binding and unbinding the auditory and visual streams in the McGurk effect. J Acoust Soc Am 132:1061–1077
Nahorna O, Berthommier F, Schwartz JL (2015) Audio-visual speech scene analysis: characterization of the dynamics of unbinding and rebinding the McGurk effect. J Acoust Soc Am 137:362–377
Nath AR, Beauchamp MS (2012) A neural basis for interindividual differences in the McGurk effect, a multisensory speech illusion. Neuroimage 59:781–787
Noppeney U, Josephs O, Hocking J, Price CJ, Friston KJ (2008) The effect of prior visual information on recognition of speech and sounds. Cereb Cortex 18:598–609
Osnes B, Hugdahl K, Specht K (2011) Effective connectivity analysis demonstrates involvement of premotor cortex during speech perception. Neuroimage 54:2437–2445
Park H, Ince R, Schyns P, Thut G, Gross J (2018) Representational interactions during audiovisual speech entrainment: redundancy in left posterior superior temporal gyrus and synergy in left motor cortex. PLoS Biol 16:e2006558
Parker Jones O, Seghier ML, Kawabata Duncan KJ, Leff AP, Green DW, Price CJ (2013) Auditory-motor interactions for the production of native and non-native speech. J Neurosci 33:2376–2387
Parkinson AL, Korzyukov O, Larson CR, Litvak V, Robin DA (2013) Modulation of effective connectivity during vocalization with perturbed auditory feedback. Neuropsychologia 51:1471–1480
Penny WD, Stephan KE, Daunizeau J, Rosa MJ, Friston KJ, Schofield TM, Leff AP (2010) Comparing families of dynamic causal models. PLoS Comput Biol 6:e1000709
Proverbio AM, Massetti G, Rizzi E, Zani A (2016) Skilled musicians are not subject to the McGurk effect. Sci Rep 6:30423
Pulvermuller F, Huss M, Kherif F, del Prado M, Martin F, Hauk O, Shtyrov Y (2006) Motor cortex maps articulatory features of speech sounds. Proc Natl Acad Sci 103:7865–7870
Ramsey JD, Hanson SJ, Hanson C, Halchenko YO, Poldrack RA, Glymour C (2010) Six problems for causal inference from fMRI. Neuroimage 49:1545–1558
Skipper J, Nusbaum H, Small S (2005) Listening to talking faces: motor cortical activation during speech perception. Neuroimage 25:76–89
Stephan K, Harrison L, Kiebel S, David O, Penny W, Friston K (2007) Dynamic causal models of neural system dynamics:current state and future extensions. J Biosci 32:129–144
Straube B, Wroblewski A, Jansen A, He Y (2018) The connectivity signature of co-speech gesture integration: the superior temporal sulcus modulates connectivity between areas related to visual gesture and auditory speech processing. Neuroimage 181:539–549
Thézé R, Giraud A-L, Mégevand P (2020) The phase of cortical oscillations determines the perceptual fate of visual cues in naturalistic audiovisual speech. Sci Adv 6:6348
Torrisi SJ, Lieberman MD, Bookheimer SY, Altshuler LL (2013) Advancing understanding of affect labeling with dynamic causal modeling. Neuroimage 82:481–488
Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, Mazoyer B, Joliot M (2002) Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage 15:273–289
Wilson SM, Saygin AP, Sereno MI, Iacoboni M (2004) Listening to speech activates motor areas involved in speech production. Nat Neurosci 7:701–702
Wolpert DM, Diedrichsen J, Flanagan JR (2011) Principles of sensorimotor learning. Nat Rev Neurosci 12:739–751
Wroblewski A, He Y, Straube B (2020) Dynamic Causal Modelling suggests impaired effective connectivity in patients with schizophrenia spectrum disorders during gesture-speech integration. Schizophr Res 216:175–183
Yan C-G, Wang X-D, Zuo X-N, Zang Y-F (2016) DPABI: data processing and analysis for (resting-state) brain imaging. Neuroinformatics 14:339–351
Zeidman P, Jafarian A, Corbin N, Seghier M, Razi A, Price C, Friston K (2019a) A guide to group effective connectivity analysis, part 1: first level analysis with DCM for fMRI. Neuroimage 200:174–190
Zeidman P, Jafarian A, Seghier M, Litvak V, Cagnan H, Price C, Friston K (2019b) A guide to group effective connectivity analysis, part 2: second level analysis with PEB. Neuroimage 200:12–25
Zhang L, Du Y (2022) Lip movements enhance speech representations and effective connectivity in auditory dorsal stream. NeuroImage 6:119311
Acknowledgements
We highly appreciate all colleagues for helpful comments on the manuscript and all the selfless volunteers who participated in the study. This work was supported by the National Science and Technology Innovation 2030 Major Program (No. 2022ZD0208900), the National Natural Science Foundation of China (Nos. 82072006, 62036003, 62173070), and the Medical-Engineering Cooperation Funds from University of Electronic Science and Technology of China (No. ZYGX2021YGLH201).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
All authors declare no competing financial interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zou, T., Li, L., Huang, X. et al. Dynamic causal modeling analysis reveals the modulation of motor cortex and integration in superior temporal gyrus during multisensory speech perception. Cogn Neurodyn 18, 931–946 (2024). https://doi.org/10.1007/s11571-023-09945-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11571-023-09945-z