Abstract
Fungi with prominent hairlike rhizomorphs, aerial habit, sparse small sporophores, and usually saprotrophic biology are commonly known as ‘horsehair or spider fungi’. The earliest descriptions of them, from the mid-late 1800s, were published from Australian material under the names Marasmius crinis-equi and M. equicrinis. For the original ‘horsehair fungus’, we review this early historical material, briefly explore the changes in the species concept over time, and investigate the nomenclatural tangle of potentially competing names. Our analysis of morphological and molecular data for over 60 collections across eastern Australia shows that material labelled as M. crinis-equi forms part of a complex of at least three closely related species and that this name has been misapplied both within Australia and internationally. An epitype is nominated for an updated concept of M. crinis-equi, and the closely related taxa, M. tropicus sp. nov. and M. kabakada sp. nov., are described. Two more distantly related Australian taxa to which the name M. crinis-equi has been misapplied, M. perumbilicatus sp. nov. and M. argillaceus sp. nov., are also described to further stabilise the concept of the authentic horsehair fungus M. crinis-equi.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Marasmius is a genus of around 1000 described species of which Australia has a rich diversity, including some species described from Australian-type material and then considered to be cosmopolitan or at least pantropical. Marasmius crinis-equi F.Muell. ex Kalchbr., the horsehair fungus is one such species. It has been known for 144 years, and until the advent of molecular studies, it was assumed to be a single pantropical species (Desjardin and Horak 1997; Pegler 1965, 1986; Tan et al. 2009; Wannathes et al. 2009). However, Petch (1915, 1923) undertook detailed and methodical research into horsehair blight in Sri Lankan tea plantations. He described three different species of fungi producing aerial rhizomorph tangles in Sri Lanka including Marasmius equicrinis (sic), M. obscuratus (now Gymnopus rigidichordus (Petch) Tkalcec & Mesic)), and M. coronatus (now Crinipellis actinophora (Berk & Broome) Singer). He also distinguished between horsehair blight with black or brown rhizomorphs and thread blight disease with white rhizomorphs. His conclusions were that in Ceylon; horsehair blight was merely epiphytic, not parasitic. However, other researchers disagreed, and M. equicrinis F. Muell. ex Berk. became known as the cause of horsehair blight (a functional pathogen) in tea, coffee, nutmeg, and cocoa plantations across Asia (Dassanayake et al. 2009). Su et al. (2011) noted a serious decline of tea bushes in Taiwan affected by what was thought to be Marasmius crinis-equi. They found experimental evidence of leaf necrosis and drop caused by rhizomorphs producing volatile compounds. These chemicals were identified but not analysed further, and no morphological or molecular confirmation of the causal species of horsehair blight was made. That research has not been repeated. It should be noted that horsehair blight has never been found in commercial crops of tea, coffee, or cocoa in Australia (pers. comm. R. Davis, Department of Agriculture, Fisheries & Forestry (DAFF)).
The name M. crinis-equi was taken up widely after Pegler (1965) redescribed the lectotype, being broadly applied to include any marasmioid fungus with aerial litter trapping rhizomorphs. Tan et al. (2009) and Wannathes et al. (2009) were the first researchers to include sequences (ITS) of M. crinis-equi as part of a broader study of the genus Marasmius sensu stricto from Malaysia and Thailand. They demonstrated that morphologically similar species, (some labelled M. aff crinis-equi) could be quite distinct molecularly, supporting the earlier distinctions made by Petch (1915, 1923). Both provided detailed updated descriptions of what they thought was M. crinis-equi based upon sequenced specimens from Malaysia and Thailand, respectively; none of these collections were from plantations or commercial crops affected by horsehair blight. However, these sequences are unlikely to be the same taxon; thus, a robust species concept for the horsehair fungus remained uncertain.
For the next decade, researchers around the globe continued to apply the name M. crinis-equi broadly to collections that had rhizomorphs, horsehair stipes, and small sporocarps. It is now known that several genera can produce aerial rhizomorphs, including Crinipellis, Gymnopus, and Marasmiellus (Koch et al. 2020; Oliveira et al. 2020). Recognition that the aerial litter trapping habit is more widespread than just within a single taxon caused Oliveira et al. (2024a) to name this functional group the ‘spider fungi guild’, and the name horsehair fungi continued to be specifically applied to Marasmius crinis-equi. As more research was conducted on the diversity of Marasmius species and our understanding of ecological diversity increased, the Tan et al. (2009) and Wannathes et al. (2009) sequences and descriptions were used as indicative of an updated concept of M. crinis-equi (Shay et al. 2017; Grace et al. 2019; Amoako-Attah et al. 2020; Koch et al. 2020; Oliveira et al. 2020). In the recent Oliveira et al. (2024b) revision of the subgenera and sections within Marasmius based on multigene data, a new section/crinis-eques is suggested, encompassing the Malaysian sequences of Tan et al. (2009), Thai sequences of Wannathes et al. (2009), species from Guyana, Cameroon, and Brazil (M. guyanenis, M. madagascariensis, M. neocrinis-equi, and M. arachnotropus), and several un-named species. However, this study still lacked any reference to Australian data. Until original Australian-type material and new collections from type localities were examined morphologically and molecularly, the species concept of the authentic M. crinis-equi remains problematic. In this study, we set out to answer whether this is a single, morphologically variable species or a species complex including different taxa across its purported range in Australia. The results have implications for studies of pathogenicity and species delimitation in other regions.
Historical background
The history of naming the horsehair fungus is full of confusion and ambiguity from the start. Among surviving correspondence between Ferdinand von Mueller, then Government Botanist and Director of the Phytologic Museum of Melbourne (now the National Herbarium of Victoria), and English mycologist, Rev. Miles Berkeley, the horsehair fungus was first mentioned in a letter to Berkeley on April 15, 1875 (Mueller 1875). Mueller noted with excitement that finally ‘fruiting specimens’ had been found of the ‘Horse-hair fungus’, in a collection from Richmond River, New South Wales (NSW), made by Mrs. Mary Hodgkinson, commenting that ‘there are only a few pilei’ and that the ‘pilei were small’. Figure 1 shows that collection (K-M 1435267), viewed in 2023. For full correspondence text, see Supplementary Material. Mueller wrote again to Berkeley on 18 Sep. 1879, asking ‘whether you have published anywhere the Marasmius equicrinis’ and noting that ‘The only fruit-sample is in your possession’ (Mueller 1879).
K-M 1435267 Marasmius equi-crinis Richmond River, Mrs. [Mary] Hodgkinson. One of the original collections sent to Berkeley by Ferdinand von Mueller. a The collection no longer contains sporophores; b closeup of rhizomorphs. Specimen label in Mueller’s handwriting. Images by Isabella Miles-Bunch, 2023 K
Around this time, Mueller either sent a collection directly to the Austro-Hungarian mycologist Rev. Karoly Kalchbrenner, or Kalchbrenner examined material that Mueller had sent to Berkeley. In February 1880, in his publication Fragmenta Phytographiae Australiae, Mueller included a list of names of fungi provided by Kalchbrenner, among which was ‘Marasmius equi-crinis F.v.M.’ (Mueller 1880) the correct orthography of which is equicrinis. While the morphological information associated with the name was minimal, we consider it diagnostic (but see below in relation to requesting a binding decision). In addition to mentioning that the species always grows on tree trunks, Mueller described the mycelium (rhizomorphs) of this ‘remarkable species’ as resembling curled horsehairs. He noted that the distribution included East Gippsland with collections made by N. Taylor and Richmond River with a collection by Maria Hodgkinson. Taylor is presumably Norman Taylor who is known to have collected for Mueller in East Gippsland (George 2009) and Hodgkinson is Mary Hodgkinson (Maroske and Vaughan 2014). Then, in June 1880, Kalchbrenner published the name Marasmius crinis-equi F. Muell. ex Kalchbr. in Grevillea, attributing the name to Mueller and citing the material as ‘Surrounding twigs. (Mueller.)’. The protologue included details of the minute pilei arising from black rhizomorphs similar to horsehair with the comment that ‘The only perfect specimens [i.e. with sporophores] are in the Berkeley Herbarium, Royal Gardens, Kew’(Kalchbrenner 1880) (Fig. 2).
K-M 99658 Agaricus crinisequi Rockingham’s Bay. a This specimen was designated as ‘type’ of M. crinis-equi by Pegler (1965); b closeup of rhizomorphs. Specimen label in Mueller’s handwriting. Images by Isabella Miles-Bunch, 2023 K
Finally, in April 1881, Berkeley included an entry for M. equicrinis F. Muell. ex Berk. in an article on Australian fungi in the Journal of the Linnean Society of Botany (Berkeley 1881). Berkeley described the sporophores as small, milky-white to umber, sparsely sulcate, arising from black fibres resembling the stipe. He cited collections from Dalrymple Creek by Lieutenant Armitage and from Richmond River by Mrs. Armitage. He also cited Grevillea, vol. viii, p.153 (i.e. Kalchbrenner 1880), where the fungus was called M. crinis-equi. Berkeley stated that he followed Mueller’s original name. Cross-checking the names ‘Lieutenant Armitage’ and ‘Mrs Armitage’ against Mueller’s known collectors, and taking into account extant material in Kew Fungarium (K), indicates that the names are errors for William Armit and Mary Hodgkinson (George 2009; Maroske and Vaughan 2014).
According to Mueller’s 1875 letter, all specimens of the horsehair fungus that possessed sporophores had been transferred to Berkeley. Berkeley’s collections were donated in 1879 to become the founding specimens of K. Nine extant early collections have been found in K (pers. comm. L. Davies & I. Miles-Bunch, 2023), seven of which are relevant to this study. As well, there is one early specimen in the National Herbarium of Victoria, Melbourne (MEL).
For over eight decades, the name M. equicrinis was in use following Berkeley (1881). Petch (1915) referred to the fungus as M. equicrinis, and Dennis (1951) treated M. equicrinis as a variety of Marasmius graminum (Lib.) Berk. and mentioned that ‘the types from Richmond River, New South Wales’ are ‘tolerably’ in agreement with Petch’s description of material from Sri Lanka. One decade later, Pegler (1965) in his revision of the types of Australasian Agaricales determined that the name introduced by Kalchbrenner (1880) had priority, and Pegler selected the Rockingham Bay collection (K-M99658) as ‘type’ of M. crinis-equi. It was one of the few historical collections with sporophores (although they are no longer present). Just how this collection came to K (direct, or via Kalchbrenner) is not clear, but it is relevant that at least seven of the other 19 new species described by Kalchbrenner (1880) were located at K by Pegler (1965). The collection is a lectotype as no holotype had been designated by Kalchbrenner (1880). Misplaced typification terms can be corrected under Art. 9.10 of the Code (Turland et al. 2018).
The protologue of M. crinis-equi gave the collection details merely as ‘Surrounding twigs. (Mueller.)’. It was common for Mueller to be misinterpreted as the collector due to his name being included on the printed Melbourne Phytologic Museum labels that accompanied specimens, when in fact he communicated the specimens. Therefore, the original material for the name M. crinis-equi can be considered as sporophores communicated by Mueller prior to the publication of the name. The choice of K-M99658 by Pegler (1965) as type was appropriate as the label is written by Mueller and includes the name Agaricus crinisequi, and Pegler (1965) provided details of spores that indicated there were sporophores present on the collection at the time he examined it.
The details on the label of K-M99658 strongly suggest that it was derived from plant collections made by John Dallachy, a prolific collector for Mueller (Dowe & Maroske 2020). Extant collections of the two host plants (E. dallachiana F.Muell. ex Benth. [now Gossia dallachiana (F.Muell. ex Benth.) N.Snow & Guymer] and E. smithii Poir. [now Syzygium smithii (Poir.) Nied.]) in MEL made in the nineteenth century from Rockingham Bay were all made by Dallachy. Among these is MEL 67165, an 1865 collection that is original material of E. dallachiana, which has a note on the label in Mueller’s hand ‘cum Agarico equicrini’. Indeed, no other collections of E. dallachiana were made until after 1900. We note the use by Mueller of the spellings equicrinis and crinis-equi at different times and also that Mueller in correspondence seemed to indicate that he had only encountered the sporophores in 1875. However, the use of Agaricus (i.e. the generic name of an agaric) on the label of the 1865 collection indicates that sporophores were observed at the time Mueller wrote the label. It is likely that Mueller removed fungal material from one or more collections of Eugenia, and this is the material that makes up K-M99658. There is a very small amount of rhizomorph visible on MEL 67165, but no sporophores. However, because the K material mentions on the label two hosts, the MEL collection is not an isotype. However and whenever the K material was aggregated by Mueller, it was an appropriate selection as lectotype for M. crinis-equi.
Mycologists have continued to follow Pegler (1965) in using the name M. crinis-equi (Singer 1976; Pegler 1986; Grgurinovic 1997; Antonin 2007; Wannathes et al. 2009; Shay et al. 2017). However, May and Wood (1997), in their catalogue of Australian fungi, raised the possibility that M. equicrinis Muell. may have been validly published by Mueller in February 1880, preceding Kalchbrenner’s publication by 4 months; therefore, this name could have priority.
In order to stabilise the modern concept of the species in Australia and internationally, we investigated fungarium material labelled as M. crinis-equi or M. equicrinis, and fresh material collected from 2019 to 2023 in type localities in Eastern Australia. We examined protologues and images of type material (K and MEL) and historical documentation (Mueller correspondence and fungarium labels) to determine the appropriate name, typification, and circumscription for the horsehair fungus originally described from Australia under the names M. crinis-equi and M. equicrinis. A revised description of M. crinis-equi is provided, an epitype selected and several taxa from Australia to which the name M. crinis-equi has been incorrectly applied are described. Sequences of several species of Marasmius that produce aerial leaf litter traps or abundant rhizomorphs from across the globe have been incorporated in the molecular analyses of this study to enable broader geographic comparisons.
Materials and methods
Field collections
Fresh specimens of horsehair fungi were collected from a broad region covering northern New South Wales (NSW) to far north Queensland (FNQ), encompassing geographic regions and habitats that the type material was determined to be sourced from. Collecting was undertaken in National Parks and Reserves in southeast Queensland (SEQ) and FNQ including Natural Bridge, Numinbah, Lamington, Linda Garrett and Bunya Mountains National Parks; Mary Cairncross Reserve, Dilkusha Nature Refuge and Landershute—private property (SEQ); Paluma, Edmund Kennedy and Murray Falls Sections of Girramay, Josephine Falls Section of Wooroonooran and Mt Lewis National Parks (NP); Abergowrie State Forest, Dalrymple Creek, Reserves around Cardwell, Speewah Conservation Park in Barron Gorge NP and Cow Bay Reserve, Julatten and Malanda Conservation Parks; Daintree – private property (FNQ). All collecting was done under Permits Nos. WITK18734918-1 to 2021 and P-PTUKI-100021825 to 2023 (FNQ) and WITK18760918 and WIF418760818 to 2021 and P-PTUKI-100091861–1 to 2024 (SEQ) and with permission from private landowners. Enquiries were made with Department of Agriculture, Fisheries and Forestry (DAFF) extension officers and several tea plantation managers in FNQ; brief surveys were conducted of neglected tea plants adjacent to native rainforest, and specimens of horsehair fungi were collected.
Further collections were made from northern New South Wales in the Border Ranges and Dorrigo National Parks and remnants of The Big Scrub. These surveys and collections were made with permission of the relevant local National Parks Rangers. Additional material consisting only of rhizomorphs was collected in some places for DNA analysis. Habitats surveyed included tropical and subtropical rainforest, wet sclerophyll and coastal mangrove forests, tea plantations, and neglected tea bushes. Collections were made in the wet season, January to April, with the major field trips being February 2021 in FNQ, February 2022 in northern NSW, and a further trip to FNQ in February 2023. All collections were photographed in situ, described fresh then dried on an Ezidri Snackmaker FD500 (Hydraflow Industries Ltd, Upper Hutt, NZ) food dehydrator at lowest setting.
Fungarium collections data
All fungarium material from BRI, MEL, and AD, labelled M. crinis-equi, was examined, and where the collections appeared of good quality and relatively recent (< 20 years old), selected samples were removed for DNA analysis. Images, measurements, and labels of historic Australian collections in K were recorded by K staff and shared with the authors (see Images Supplementary Material), though actual specimens were not physically examined by the authors.
Morphology
Sporophore characters described were pileus diameter range in millimetres, colour using the Flora of British Fungi Colour Identification Chart (Royal Botanic Gardens Edinburgh 1969), shape from juvenile to mature, including whether umbonate or umbilicate, with or without papilla; lamellae (L) number range for up to 10 sporophores, lamellulae (l) number of tiers where present, colour and whether marginate, attachment to stipe and collar; stipe length and diameter range in millimetres, colour from base to apex, surface texture and insertion into substrate; rhizomorphs diameter in millimetres, colour, quantity and an estimate of litter trap size. Spore prints were obtained where possible. Fresh samples of sporophores were taken for DNA analysis and desiccated in silica gel before extraction. Where sporophores were sparse or absent, lengths of clean rhizomorph (~ 6 cm) were cut into 10 mm sections and stored as per sporophores.
Dried specimens were examined microscopically using a Leica dissecting microscope for measuring rhizomorph diameters, and a Prism Optical (Model EX-30 T) compound microscope with a Tucsen GT12 camera (Tucsen Photonics Co., China) with a 100 × objective, for examination of hand-cut sections, rehydrated in 5% potassium hydroxide. Microscopic details were recorded with Mosaic V2.0 software (http://www.tucsen.com). Congo Red, KOH, or Melzer’s stains were used for recording and measuring details of the pileipellis, cheilocystidia, stipe, lamellar and pileal trama, and spore length and width. Spore measurements were made for a minimum of 10 spores per specimen, and up to 50 spores from 3–4 sporophores for new taxa, obtaining a range of length × width in microns, mean length × width, with Q (quotient of length/width) measurements for mean (Qm), minimum and maximum; n = number of spores measured.
Molecular sampling and processing
Samples were first ground with 2 lead balls at high speed for 20 s, twice in a bead mill (Fast Prep-24™ 5G, MP Biomedicals, CA, USA). DNA extraction was performed using the Omega Bio-tek Inc. Norcross, GA, USA, EZNA Forensic Kit following the prescribed protocols for hair, nails, and feathers, apart from substituting 0.8 μL b-mercaptoethanol for 20 μL 1 M DTT and using 50 μL elution buffer twice instead of 100 μL, for a more concentrated extract. The internal transcribed spacer region (ITS1-5.8 s-ITS2), rDNA, was amplified using primers ITS1-F (Gardes and Bruns 1993) and ITS4 (White et al. 1990) and large subunit (LSU) amplified with primers LR7 & LROR (Vilgalys and Hester 1990) in a reaction mixture of 1 μL of each Primer, 12.5 μL My Taq Red Mix (Bioline, NSW, Australia), 0.8 μL 10% bovine serum albumin (BSA), and 7.7 μL sterile water. The thermal cycling conditions included 35 cycles of 95 °C for 1 min, 51 °C for 1 min, and 72 °C for 1 min, with a final extension step of 72 °C for 10 min for the ITS, and 95 °C for 1 min, 48 °C for 1 min, 72 °C for 1 min, with a final extension step of 72 °C for 10 min for LSU. Samples were sent to Macrogen, Seoul, for purification and Sanger sequencing.
GenBank data
BLAST searches were conducted in the GenBank NCBI database < http://www.ncbi.nlm.nih.gov/ > to determine similar species. All sequences named Marasmius crinis-equi in GenBank were compared to sequences generated in this study in an initial alignment and taxa obviously non-Marasmius excluded. Sequences of representative named species showing the characters of subgenus Marasmius, sects. Marasmius, Sanguirotales, Sicciformes, Crinis-eques, and Variabilispori in accordance with Oliveira et al. (2024b) were included. Those producing aerial litter traps (i.e. ‘spider fungi’, Oliveira et al. 2024a), and as yet undescribed, rhizomorph-forming Marasmius species used in bird nests (RAK spp. 1–13, Koch et al. 2020) were also included. As M. crinis-equi has been implicated in thread blight disease, sequences from studies of this disease in Cacao theobroma crops (Ghana and Peru) were also included (Amoako-Attah et al. 2020; Huamán-Pilco et al. 2023). Taxon selection was then further refined for species closely related to our new taxa in a final analysis. Table 1 lists all collections used in the molecular analyses in this study.
Phylogenetic analyses
Sequence editing was performed manually in Geneious Prime 3 version 2023.2.1. (https://www.geneious.com) and initial alignments of ITS and LSU constructed using MAFFT (Katoh and Standley 2013). The ITS alignment was edited to remove some ambiguous bases, and two large indels of > 100 base pairs were removed from M. vigintifolius sequences. Partition Finder was used to choose parameters for RAxML and Bayesian phylogenies. ITS and LSU sequences were analysed separately; due to the lack of comparable LSU data in GenBank for non-Australian material, only the ITS alignment is presented here (The LSU Bayesian phylogenetic tree is in Supplementary material). Maximum likelihood (ML) analyses were conducted using RAxML 8.2.11 (Stamatakis 2014) with the GTR + GAMMA + I model using default parameters for 1500 rapid bootstrap (BS) replicates. Bayesian analysis was performed with MrBayes 3.2.6 (Huelsenbeck and Ronquist 2001) using the substitution model GTR and Metropolis Coupled (MCMCMC) settings, for 1,000,000 iterations. Newly generated sequences were registered with GenBank and new taxa with MycoBank, including the newly epitypified Marasmius crinis-equi.
Results
Over 40 collections of rhizomorph-forming marasmioid fungi were made in FNQ and SEQ in 2021 and a further 24 in 2023. Ten collections were made in northern NSW in 2022. The habitats were tropical and subtropical rainforests. Other habitats including wet sclerophyll and coastal mangrove forests were surveyed, but no horsehair fungi were found. No samples were obtained from tea plantations, but rhizomorph tangles were observed on unpruned tea bushes and surrounding regrowth rainforest; eight collections were made. Both sporophores and rhizomorphs were found in all rainforest locations, almost always in aerial litter traps. The traps consisted of tangles of rhizomorphs attached to understorey tree trunks, saplings, or vines, with varying amounts of trapped debris (dead leaves and twigs) depending on the extent of the rhizomorphs. Most specimens were growing between half to two metres above ground, arising directly from rhizomorphs growing on living understorey plants. Rhizomorphs were also found in abandoned bird nests. Two collections made in Dorrigo NP, NSW (F2021089 in 2021 and F20220033 in 2022) were found on a large metre-high pile of dead wood and litter on the ground. Collections were deliberately made in the wet season, January to April; this coincided with the La Nina events of 2021 and 2022. Persistent heavy rainfall made for ideal rhizomorph-growth and sporophore-producing conditions in Queensland and New South Wales. The distributions of the three species with aerial rhizomorphs, M. crinis-equi, M. kabakada, and M. tropicus, overlap in the core type locality Rockingham Bay region, but only M. crinis-equi also occurs in New South Wales in the Richmond River region.
Phylogenetic analysis
Seventy-six sequences of ITS and 65 of LSU were obtained from either sporophores or rhizomorphs of Australian horsehair fungi. Of these, nine sequences were from genera other than Marasmius—Crinipellis (× 4), Gymnopus (× 4), and Pseudomarasmius (× 1). A number of sequences labelled as M. crinis-equi in GenBank and in herbarium collections were found to belong to other subgenera or sections that were distantly related to authentic M. crinis-equi specimens from Australia and were thus excluded from final analyses.
The outgroups are members of sects. Globulares and Sicci, including Marasmius haematocephalus (Mont.) Fr. (epitype), Marasmius nidus-avis R.A. Koch, N. Siegel & Aime and an undescribed species (sp 9 RAK) from Cameroon (Fig. 3). The whole ingroup (subgenus Marasmius sensu Oliveira et al. (2024b) is strongly supported (BS 95, PP 1); it includes groups with varying support. Section Marasmius, subsect. Sicciformes is paraphyletic (Tan et al. 2009; Wannathes et al. 2009), and species of this subsect. appear in several groups. Two of the lower support groups contain members of sect. Neosessiles and sect. Marasmius, subsect. Marasmius in the sense of traditional classifications (Singer 1986; Antonin 1991; Tan et al. 2009; Wannathes et al. 2009).
Phylogenetic analysis of Marasmius crinis-equi and allied taxa in subgenus Marasmius, sects. Marasmius, Variabilispori, Crinis-eques, Sanguirotales and Sicciformes, including ser. Neosessiles and Sicciformes, inferred from Bayesian and Maximum Likelihood (ML) analyses of ITS region with M. haematocephalus (G/SH) as outgroup. ML bootstrap proportions and Bayesian posterior probabilities are shown as BS/PP, respectively. Nodes receiving support values greater than 95/0.95 are represented by bold branches. Coloured sequences are from this study except MZ219791 from FNQ and EU935555 from Thailand, which fall into the M. tropicus clade. The tree is divided into six groups, representing sections sensu Oliveira et al. (2024b) in coloured boxes
However, in this study, we have used the most recent major re-classification of the genus Marasmius (Oliveira et al. 2024b) which resolves most, but not all, of the issues around paraphyletic sections and subsections in the traditional classifications. Our single gene analysis (ITS) shows broadly similar results to those of Oliveira et al. (2024b) based upon multigene data, with some minor variations. We recovered six groups in subgenus Marasmius Oliveira that correspond to sections /crinis-eques (gp1A and gp1B), /sicciformes (gp2), /marasmius (gp3), /sanguirotales (gp4), and /variabilispori (gp5), and an unresolved group of taxa (gp6) (Fig. 3).
Gp 1A is a well-supported group (BS 92, PP 1) in sect. Crinis-eques including a further strongly supported subclade (BS 98, PP 1) of M. tropicus sp. nov. and sister species, M. arachnotropus Brazil, with low support (BS 52, PP 0.64). Several African species including M. crinis-equi sensu Ghana and undescribed species from Cameroon are more distant in that clade. M. tropicus includes sequences from Thailand and the Solomon Islands. Marasmius crinis-equi s. s. is another strongly supported species (BS 99, PP 1) with sisters M. madagascariensis and M. neocrinis-equi with strong support (BS 90, PP 0.99). The next closest species in Group 1A is M. guyanensis from Thailand, Cameroon, and Principe.
Gp 1B is a subclade with low support (BS 38, PP 0.73) which includes the well-supported species M. perumbilicatus sp. nov. and M. kabakada sp. nov. with its sister, Marasmius sp. 4 RAK with low support (BS 32, PP 0.78). In Oliveira et al. (2024b), this is part of sect. Crinis-eques, but only Marasmius sp. 4 RAK has been included in their analysis. In our maximum likelihood analysis, M. crinis-equi sensu Tan et al. (2009) is included in this group, but in the Bayesian analysis, that species falls in Group 6. This may be due to issues with alignment or that the single gene (ITS) is not informative enough to resolve these relationships.
Gp 2 has low support (BS 44/ PP 0.85) and includes the M. tenuissimus complex, M. infestans and M. neosessiliformis in the revised sect. Sicciformes, ser. Neosessiles (Oliveira et al. 2024b). Sister to that clade with moderate support are species in ser. Sicciformes (BS 76, PP 0.88). Sequences of Australian collections are in orange and are close to or con-specific with described species M. ruforotula from S. Korea and Thailand, M. nigrobrunneus from Thailand, and two of the M. tenuissimus complex in this group.
Gp 3 with high support (BS 100, PP 1) on a long branch are members of what used to be subsects. Marasmius and Sicciformes. They are now placed together in sect. Marasmius, subsects. Marasmius and Guyanensis. M. aff crinis-equi (sensu Wannathes, Thai) is in this subsection.
Gp 4 contains M. argillaceus sp. nov., a well-supported species and M. purpureobrunneolus from Thailand its sister species with moderate support (BS 73, PP 0.85). These are in a broader clade including M. chrysocephalus (Guyana) and M. brevicollus (Thailand), now considered to be part of sect. Sanguirotales sensu Oliveira et al. (2024b) based on multigene analyses.
Gp 5 is sect. Variabilispori with moderately strong support. To date, no Australian species have been found in that section. In our analysis, it includes M. cupressiformis from Cameroon. However, in the Oliveira et al. (2024b) analysis, that species is in the outgroup.
Gp 6 is a polytomy of several small well-supported branches without resolution. Marasmius aff tangerinus is another Australian species close to M. tangerinus Wannathes, Suwanner, Kumla & Lumyong in this group. (See comment on Gp1B. M. crinis-equi sensu Tan is in this group on Bayesian analysis, but not RAxML).
Valid publication and epitypification
Despite mention of horsehair fungi in East Gippsland, no historical or recent specimens from Victoria have been found at K or MEL. The specimen noted in correspondence and annotated by Mueller, from near Sydney collected by Ramsay, is in K (K-M 1435260), but probably never had sporophores. However, the Richmond River (NSW) specimens collected by Hodgkinson are specifically referred to by Mueller and still exist in K (K-M1435267 and K-M1435265).
The historic evidence, including correspondence from Mueller to Berkeley, the lack of documentation of other species of Marasmius with aerial rhizomorphs from that century, the protologues by Mueller, Kalchbrenner, and Berkeley, and images of the earliest extant specimens (seven in K and one in MEL) have been reviewed. It is possible that Ferdinand von Mueller validly published the name Marasmius equi-crinis in Feb. 1880 in reference to the Richmond River collections by Mary Hodgkinson, as noted by May and Wood (1997). While Mueller’s protologue was minimal, referring only to the curled horsehair-like rhizomorphs that clung to tree trunks and the wide distribution of the species from Victoria to Queensland, it is in keeping with the amount of information provided in that era for novel taxa. The additional information in his correspondence with Berkeley had also noted that sporophores were small and sparse.
When publishing Marasmius equicrinis F.Muell. ex Berk., Berkeley (1881) states “see ‘Grevillea,’ vol. viii. p. 153, where it is called M. crinis equi. I, however, follow the original name of Mueller”. Therefore, the name M. equicrinis F.Muell. ex Berk is illegitimate under Art. 52.1 because Berkeley definitely includes the type of the name M. crinis-equi F.Muell. ex Kalchbr., by citing the name itself (as explained by Art. 52.2). If M. equicrinis F.Muell. is validly published, M. equicrinis F.Muell. ex Berk. is also illegitimate under Art. 53.1 as it would be a homonym of the former name. Marasmius equicrinis F.Muell. and M. equicrinis F.Muell. ex Berk. cannot be regarded as isonyms as the type citations differ. Berkeley (1881) cited under M. equicrinis F.Muell. ex Berk. 1881 collections from Dalrymple Ck and Richmond River, corresponding with specimens K-M1435273, K-M1423256, K-M1435267, and K-M1435265. Because the name M. equicrinis F.Muell. ex Berk. is illegitimate, there is no need to typify it with any particular collection.
The authors of this paper will submit a request for a binding decision as to the adequacy of the descriptive information provided by Mueller (1880) when publishing the name Marasmius equicrinis F. Muell. If the name is ruled validly published, it will need to be typified and epitypified before being taken up—and we would do this in such a way to make M. equicrinis F. Muell. a synonym of M. crinis-equi. Given that there have already been a number of changes over time for the name applied to the species, and that the request for a binding decision will take some time to be processed, until the binding decision is made, we continue to use M. crinis-equi Muell. ex Kalchbr., as published by Kalchbrenner (1880), which has been lectotypified with the collection from Rockingham Bay K-M 99658 by Pegler (1965).
Unfortunately, the type material of M. crinis-equi (K-M 99658, Fig. 2) and all other early collections (K-M 1435265, K-M1435267, K-M 1435273, K-M 1435256, K-M 1435260, K-M 1435269, K-M 1435254, and K-M 1435270 from the Kew Fungarium; and MEL67165, MEL1055152A, and MEL1055153 from the National Herbarium of Victoria) consist only of rhizomorphs and substrate (twigs or leaves). Given that there are several Australian species of Marasmius that produce aerial rhizomorphs (as described below), these historic collections are not identifiable to species. Sporophores had been present originally in some collections, though sparsely, and were gradually destroyed in morphological examinations over the years. Vladimir Antonin (pers. comm.) examined the K collections in 2002 and found there to be no remaining sporophores.
The lectotype material of M. crinis-equi in K is thus ambiguous, and the Fungarium is unwilling for further destructive sampling to be undertaken on the early collections. An epitype therefore needs to be designated for this species (Ariyawansa et al. 2014). Fresh collections were made in the Rockingham Bay area of north Queensland and also in the Richmond River region of New South Wales during this study. Several were morphologically and molecularly appropriate, but sporophores were sparse. The collection nominated below as the epitype had relatively abundant sporophores.
Taxonomy
Marasmius crinis-equi F. Muell. ex Kalchbr., Grevillea 8: 153, 1880, Figs. 4 and 5.
≡Androsaceus crinis-equi (F. Muell. ex Kalchbr.) Overeem, in Heyne, Nutt. Pl. Ned. Ind. 1: 69 (1927).
= Marasmius equicrinis F.Muell. ex Berk., J. Linn. Soc., Bot. 18: 383 (1881), nom. illeg.
≡ Chamaeceras equicrinis (F.Muell. ex Berk.) Kuntze, Revisio Generum Plantarum 3(2): 456 (1898).
≡Marasmius graminum (Lib.) Berk. var. equicrinis (F.Muell. ex Berk.) Dennis, Trans. Brit. Mycol. Soc. 34: 416 (1951).
Holotype: not indicated.
Lectotype: designated by Pegler (1965): K-M99658, ‘Agaricus crinisequi F. Muell. ex Kalchbr. on Eugenia smithii & also on E. dallachyana at Rockingham’s Bay, on twigs’.
Epitype: (designated here, MycoBank: MBT 10019210): Australia, New South Wales, Rocky Creek Dam Road, Big Scrub Loop walking track, S 28° 38′ 12.6″, E 153° 19′ 54.3″, 175 m asl., on fallen branch and Calamus muelleri, 20 Feb 2022, F.E. Guard F2022019, T. Lebel, J. Dearnaley & A.G. Boxshall (BRI AQ1041073.). GenBank No. ITS PP175819, LSU PP175773.
Etymology: Latin for hair (crinis) of the horse (equus).
Original description (translated from Latin): White to fulvous, minute. Pilei rare, membranous, convex, blunt (1–2 mm diameter). Stipe 1 cm or more long, hairlike, stiff, black, glossy, arising from black rhizomorphs, similar to horsehair. Lamellae sparse, distant, paler than pileus. Surrounding twigs. (Mueller). A very curious species. The rhizomorphoid mycelium resembles horsehair and is profusely developed, whilst the pilei are very seldom produced. The stems rise at right angles from the decumbent mycelium. The only perfect specimens are in the Berkeley Herbarium, Royal Gardens, Kew.
Description: Basidiomata tiny, marasmioid. Pileus 2–5(8) mm diam., broadly convex to applanate, umbonate when juvenile, becoming umbilicate with maturity with small dark brown central spot, [other collections have a tiny papilla], surface dry, sulcate, off-white (4D) to buff (52), darkening on drying. Lamellae distant, 7–10(12), adnate to a narrow collar, white, non-marginate, lamellulae absent. Stipe central, filiform, 8–12 × 0.1 mm, black with pale apex, smooth, insititious into rhizomorphs and rarely twigs. Rhizomorphs black, tough, 0.1 mm diam., multiple, branching, arising from dead twigs, forming an aerial tangle in the understorey.
Basidiospores 11–12.5 × 4.5–5 μm, Q = 2.25–2.83, mean 11.5 × 5 μm, Qm = 2.45 (n = 20 from spore print of collection F2021064 /PP175814), ellipsoid to clavate, smooth, thin-walled, hyaline, inamyloid. (See Notes for further comments on spores.) Basidia 4-spored, 20–23 × 7.5–8 μm. Basidioles narrow cylindrical to fusiform 20–22 × 4.5–5 μm. Pleurocystidia absent. Cheilocystidia abundant, Siccus-type broom cells, main body 12–20 × 6.5–11.5 μm, narrow to broadly clavate, sub-globose, occasionally bifurcate, with multiple apical setules 2–4 × 0.5 μm, at times in discrete bunches, thin or thick-walled. Pileipellis a hymeniderm of Siccus-type broom cells, main body 9–24 × 6–23 μm, cylindrical, clavate, sub-globose, broadly oblong, occasionally bifurcate, thin-walled with multiple divergent apical setules, at times in discrete bunches, 2–3 × 0.5–1 μm, sometimes thick-walled, apices blunt. Pileal trama inamyloid to weakly dextrinoid, 3–5 μm diam. Lamellar trama inamyloid, 3.5–6.5 μm diam. Stipe hyphae parallel, cortical hyphae thick-walled, dextrinoid, 4.5–6.5(9) μm diam., medullary hyphae mildly dextrinoid, 4–6.5 μm diam. Caulocystidia absent. Clamp connections present.
Habit, habitat, and distribution: Marasmius crinis-equi is a widely distributed species across eastern Australia, occurring in FNQ, SEQ, and northern NSW. It is almost exclusively a species of the rainforest, both tropical and subtropical. Rhizomorphs are more extensive in wetter, more humid habitats, and sporophores are formed only after several days of rain in the wet season, December to April. They may then be quite profuse but are ephemeral. The rhizomorphs attach to both monocotyledonous plants, including Linospadix monostachya (Walking stick palm) and Calamus muelleri (Lawyer vine), and dicotyledonous plants, including vines Pothos longipes (Candlestick vine) and many understorey saplings. They form aerial litter traps capturing dead leaves and debris, which may consist of only two or three leaves or may be sprawling and extensive, spreading over one metre. Most have no obvious connection to the forest floor. The rhizomorphs are long-lived (unpubl. data).
Notes: Marasmius crinis-equi is distinguished from most other Marasmius species in Australia by the small (less than 6 mm diam.) pale basidiomata, umbilicate with central dark spot or papilla, arising directly from black rhizomorphs in aerial leaf litter traps, distant lamellae attached to a collar, and with a pileipellis consisting of a hymeniderm of Siccus-type broom cells. However, M. crinis-equi belongs to a group of three species that are difficult to differentiate in the field, with very similar pileal dimensions and overlapping spore sizes. It should be noted that spore measurements recorded by Pegler from lectotype material had a wide range of sizes (9–13 × 3.5–5 μm), and we have found that measurements made from spore prints are larger (0.5–2.5 μm longer) than those made from dried tissue in some collections. Although M. tropicus is more likely to have yellowish-brown pilei in the fresh state, off-white and buff pilei are also found (Fig. 9f), making them difficult to separate where the two species’ distribution overlaps in far north Queensland.
While Mueller did not comment on the colour of the pilei, Kalchbrenner (1880) noted them to be ‘white to fulvous’ and Berkeley (1881) described them as ‘umber to milky white’ (1881). Pegler (1965) examined the K collection KM99658, when he designated it as type, but he did not comment on pileal colour of that material. Petch who carried out extensive studies on M. equicrinis (sic), culturing and producing sporophores in Sri Lanka, described them as ‘yellow brown to red brown’ (Petch 1915, 1948), though he noted that in humid conditions the ‘fruitbodies’ were ochraceous to almost white (Petch 1915). Other collections from outside Australia identified as M. crinis-equi are recorded as orange, deep orange, or reddish-brown (Singer 1976; Pegler 1986; Desjardin et al. 2000; Wannathes et al. 2009; Shay et al. 2017). Tan et al. (2009) also commented that the Malaysian species called M. crinis-equi were brownish-orange and lacking a dark central spot or papilla. See Table 2 in the Supplementary material for full details comparing morphological character variation. Collections made in this study were white, off-white to buff, darkening to fulvous (12) on ageing or drying.
Small, subtle differences in macro- and microscopic characters were noted between Marasmius crinis-equi and M. kabakada sp. nov. (See Notes under M. kabakada). Molecular analyses (ITS and LSU) show that the cryptic taxa form three distinct, strongly supported clades. The sister species of M. crinis-equi are M. madagascariensis and M. neocrinis-equi with strong support (BS 90, PP 0.99) (Gp1A, Fig. 3). These taxa differ morphologically in pileus and lamellar colour, stipe length, substrate, and rhizomorphs.
We have chosen this widespread Australian taxon to represent the species concept for the authentic horsehair fungus, Marasmius crinis-equi, because (i) this is the only taxon whose distribution includes the core type locality Rockingham Bay region (QLD) and the Richmond River region (NSW) mentioned by Mueller (1880) and Kalchbrenner (1880); (ii) elements of the macro- and micro-morphology do not disagree with protologues of Mueller (1880) and Kalchbrenner (1880) nor Pegler (1965) lectotype details; (iii) the pileal colour more closely aligns to Kalchbrenner’s description of this species than M. tropicus; (iv) we felt that pathogenicity is a later character (post-Petch) applied to a very broad concept of horsehair blight fungi and should not be considered in defining the species.
Synonyms: According to May and Wood (1997), various other species names have been placed in synonymy with M. crinis-equi, including M. repens Henn. from Cameroon and Alectoria australiensis C. Knight. In addition, van Overeem (1927) placed ‘Cassutha cornea’ Rumph. and Androsaceus ramentaceus Pat. under M. crinis-equi. Without being able to examine the types, and recognizing the number of different taxa revealed from molecular analyses, it is beyond the scope of this study to resolve the identity of those names, but they should not be included as synonyms of M. crinis-equi without further study. M. trichorhizus Speg. from Paraguay (Killermann 1928, under M. equicrinis) was another synonym which was recently re-described by Oliveira et al. (2020) and demonstrated to be distinct from M. crinis-equi.
Additional specimens examined: Australia, NSW, Dorrigo National Park, The Glades in subtropical rainforest understorey, 26 Feb 2022, L. Elder, [F.E. Guard F2022038](BRI AQ1041071, GenBank ITS PP175824, LSU PP175824); QLD, Balmoral Ridge, Dilkusha Nature Refuge, in riparian rainforest in Calamus muelleri, 24 Mar 2021, F.E. Guard F2021094 & R.S. Philpot (BRI AQ1045317, GenBank ITS PP17582, LSU PP175770 and F2021095 (BRI AQ1045318, GenBank ITS PP175823, LSU PP175772), Barron Gorge National Park, Speewah Walking Track, wet tropical rainforest understorey, 12 Feb 2021, F.E. Guard F2021052, T. Lebel & O. Albert-Mitchell (BRI AQ1045322, GenBank ITS PP175812, LSU PP175774), Bunya Mts National Park, Paradise Falls Track, in rainforest, F.E. Guard F2012041 & P.L. Leonard (BRI AQ798623; GenBank ITS OP562725, LSU OP562714), Maleny, Mary Cairncross Scenic Reserve, in coppicing shoots of Pouteria australis, 5 Mar 2021, F.E. Guard F2021083 & R.S. Philpot (BRI AQ1045321, GenBank ITS PP175817, LSU PP175802), Mt Lewis National Park, walking track to bowerbird bower in wet tropical rainforest, 16 Feb 2021, F.E. Guard F2021064, T. Lebel & J. Dearnaley (BRI AQ1045320, GenBank ITS PP175814, LSU PP175777); Paluma National Park, H Track, in tropical rainforest on aerial leaves and twigs, 3 Feb 2021, F.E. Guard F2021010 (BRI AQ1045319, GenBank ITS PP175821); and F.E. Guard F2021009, T. Lebel, D. Garvie, P. Sheridan & R.S. Philpot (BRI AQ1045316, GenBank ITS PP175825, LSU PP175799).
Other historic specimens examined from K and MEL: See Supplementary material 3.
Marasmius kabakada F.E. Guard, Albert-Mitchell, Lebel, Dearnaley sp. nov. Figs. 6 and 7.
MycoBank: MB 853126.
Holotype: Australia, QLD, Daintree region, Diwan, Cow Bay, S 16° 12′ 15.7″, E 145° 24′ 23.5″, 44 m asl., in tropical rainforest understorey, 14 Feb 2021, F.E. Guard F2021060, T. Lebel & M.D. Barrett (BRI AQ1045328; GenBank Nos. ITS PP175850, LSU PP175850).
Etymology. This Marasmius is named kabakada, the Jabalbina Yalanji name for a rainy place, to honour the First Nations people on whose land in the Daintree wet tropics it was collected. The epithet is a noun in apposition.
Description: Basidiomata tiny, marasmioid. Pileus 1.5–3 mm diam., convex to broadly convex to almost applanate, surface dry, deeply sulcate, umbilicate usually without central spot, off-white to buff (52), becoming orange-brown with age or on drying. Flesh papery thin, almost translucent. Lamellae distant, 6–8, adnate to narrow collar, white, non-marginate, lamellulae absent. Stipe central, filiform, 4–8(15) × 0.1 mm, usually black, occasionally reddish-brown with pale apex, smooth, insititious into black rhizomorphs and rarely dead leaves. Rhizomorphs black, tough 0.1–0.15 mm diam., branching to form aerial tangles.
Basidiospores 10–12 × 3.5–4 μm, mean 10.5 × 4 μm, Q = 2.54–3.02, Qm = 2.76, n = 10 from tissue, narrowly clavate, smooth-walled, inamyloid. Basidia not seen. Basidioles fusiform to clavate, 18–22 × 6–8 μm. Pleurocystidia absent. Cheilocystidia abundant forming a sterile edge, Siccus-type broom cells, clavate, cylindric, sub-globose, occasionally lobed, main body 6–13 × 5–7 μm, with blunt terminal setules, usually crowded, but occasionally sparse and rarely branched, 2–5 × 1–2 μm. Pileipellis a hymeniderm of Siccus-type broom cells, cylindric, clavate, broadly oblong, occasionally lobed, main body 6.5–11(14) × 5–10(14) μm with blunt terminal setules 1.5–3.5 × 0.5–1 μm, sometimes thick-walled and in bunches. Pileal trama inamyloid to faintly dextrinoid, hyphae 3.5–5.5 μm diam., occasionally to 11.5 μm diam., thin-walled. Lamellar trama inamyloid, hyphae 3–5 (7) μm diam., thin-walled. Stipe hyphae parallel, cortical hyphae dextrinoid, thick-walled, 4–5.5 μm diam., medullary hyphae inamyloid, thin-walled, 5–8 μm diam. Caulocystidia absent. Clamp connections present.
Habit, habitat, and distribution: Marasmius kabakada may form extensive aerial tangles in lawyer canes (Calamus australis) and other vines with dead leaves, but often is small and fruits very sparsely. It usually occurs in lowland, wet tropical rainforest understorey, but has been found once in a bird nest at 400 m above sea level (the rhizomorphs were used in the wall structure of the nest). It has been found from Murray Falls National Park, near Cardwell to Iron Range on Cape York (Fig. 7).
Notes: Marasmius kabakada is very similar in appearance to M. crinis-equi. However, subtle differences can help to separate them. These include overlapping but slightly smaller pileus size (1.5–3 mm cf. 2–5(8) mm.); overlapping but slightly fewer lamellae (6–8 cf. 7–10(12)); stipe length and colour (4–8 mm, sometimes reddish-brown with pale apex cf. 8–12 mm, always black with pale apex); basidiole width and shape (fusoid 6–8 μm cf. narrow, cylindric 4.5–5 μm); cheilocystidia broom cell bodies slightly smaller (6–13 × 5–7 μm cf. 12–20 × 6.5–11.5 μm), with obtuse apices cf. subacute apices in setules; pileipellis broom cell bodies similarly overlapping but slightly smaller. Molecularly M. kabakada and M. crinis-equi are distantly related. Marasmius kabakada stands as a monophyletic clade with high support within the group (BS 89/PP 0.94). Sister clade with low to moderate support (BS 66/ PP 0.78) is Marasmius sp.4 RAK (Gp 1B, Fig. 3).
The distribution of M. kabakada overlaps with both cryptic morphologic sister species, M. crinis-equi and M. tropicus, though to date it has only been found in far north Queensland.
Additional specimens examined: QLD, Barron Gorge National Park, Speewah Con. Park, in tropical rainforest understorey on saplings, 5 Feb 2023, F.E. Guard F2023020 & O. Albert-Mitchell, (BRI AQ 1045315, GenBank ITS PP175856); Bloomfield, in rainforest clearing among lawyer canes, 1 Feb 2020, O. Albert-Mitchell, OAM67, (BRI AQ, GenBank ITS PP175848, LSU PP175848); Cape York Peninsula, Iron Range Research Station, 1 km east of Lockhart River Airfield, in evergreen, notophyll vine forest, on Alectryon tomentosus sapling, 8 April 2017, D.G. Fell IRRS97 (CNS150057, GenBank ITS and LSU PP335097); and F2021062, (BRI AQ1045329, GenBank ITS PP175849, LSU PP175759); Daintree Ice-cream Co., Daintree, on unpruned tea (Camellia sinensis) bushes, 7 Feb 2023, F.E. Guard F2023037 & R.S. Philpot, (BRI AQ1045953, GenBank ITS PP175855, LSU PP175758); Girramay National Park, Murray Falls Section, on riparian rainforest understorey shrubs, 1 Mar 2021, K. Bransgrove (BRIP72540, GenBank nrITS PP175853, LSU PP175853); Wooroonooran National Park, Josephine Fall section, Mt. Bartle Frere track, in tropical rainforest understorey, 10 Feb 2021, F.E. Guard F2021045 & T. Lebel, (BRI AQ 1045330, GenBank ITS PP175851, LSU PP175756); and 4 Feb 2023, F.E. Guard, F2023016 (BRI AQ1045312, GenBank ITS PP175857, LSU PP175754).
Marasmius tropicus F.E. Guard, T. Lebel & Dearnaley sp. nov. Figs. 8 and 9.
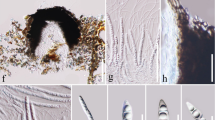
a Habit and b distribution of Marasmius tropicus, which occurs in far north Queensland, Thailand, and the Solomon Islands. Sites of collections in this study are shown with blue dots and historic collection area in red. Insets c–f showing pilei, lamellae and stipe attachment to rhizomorphs. Note colour variation of pilei in M. tropicus. Images by F.E. Guard
MycoBank: MB 853127
Holotype: Australia, QLD, Girramay National Park, Murray Falls Sect., S 18° 09′ 08.6″, E 145° 48′ 54.1″, 84 m asl., in riparian rainforest on saplings, 3 Feb 2023, F.E. Guard, F2023014 (BRI AQ1045310; GenBank Nos. ITS PP175838, LSU PP175763).
Etymology. The Latin word tropicus means tropical and refers to the widespread tropical distribution of this Marasmius.
Description: Basidiomata tiny, marasmioid. Pileus 1.5–6 mm diam., convex to broadly convex, umbilicate with central dark brown spot, usually no central papilla, but it is sometimes seen in juvenile pilei, surface dry and deeply sulcate, off-white, cream (4D) to buff (52), yellowish-brown to ochre (9H), sienna (11), darkening on drying. Lamellae distant, 6–9, adnate to a narrow collar, white, non-marginate, lamellulae absent. Stipe 4–10 × 0.1 mm, central, filiform, black with pale apex, smooth, insititious into aerial, branching, black rhizomorphs of same diameter. Spore print white.
Basidiospores (9.5)10.5–12.5 × 4–5 μm, mean 11.5 × 4 μm, Qm = 2.59, Q = 2.22–2.92 (n = 20 from spore print, F2021050/ PP175835) ellipsoid, smooth, inamyloid. Basidia 21–24 × 7–9 μm, clavate, 4–spored. Basidioles 19.5–22 × 6–8 μm, fusoid to narrowly clavate. Pleurocystidia absent. Cheilocystidia abundant, Siccus-type broom cells, main body 7.5–17 × 6.5–10 μm, clavate to broadly clavate, sub-globose, with terminal setules 2–5 × 1–1.5 μm, blunt to sub-acute, occasionally branched and thick-walled. Pileipellis a hymeniderm of Siccus-type broom cells, main body 8–17 × 6–10 μm, clavate to broadly clavate, sub-globose to irregular, at times branched, apical setules 2–5 × 1–1.5 μm, blunt, occasionally bifid and thick-walled, occurring densely or sparsely and at times in distinct groups. Pileal trama inamyloid to faintly dextrinoid, hyphae 3–4 μm diam. Lamellar trama occasionally contains inflated hyphae (to 24 μm diam.) with normal hyphae 4–7 μm diam. Stipe hyphae parallel, cylindrical, smooth, cortical hyphae dextrinoid, 5–5.5 μm diam., medullary hyphae inamyloid, 5–6 μm diam. Caulocystidia absent. Clamp connections present in all tissues.
Habit, habitat, and distribution: Marasmius tropicus consists of tough, fine, black, branching rhizomorphs, 0.1 mm diam., attached to understorey sapling trunks, vines and fallen leafy branches in wet tropical rainforest, especially in riparian zones. The rhizomorphs form aerial tangles capturing fallen leaf litter, attaching to the litter by byssi (small discs of adhering mycelium), and in the wet season producing a few to many sporophores directly from the rhizomorphs or occasionally from twigs. Tangles are often sprawling between healthy plants, without apparent soil attachment. Specimens collected in this study in north Queensland are from Murray Falls in Girramay National Park, near Cardwell to Malanda and Speewah Conservation Parks in the Cairns Hinterland. In addition, a specimen collected by Richard Davis of Northern Australian Quarantine Strategy (NAQSG/DAFF) from Malaita, Solomon Islands, while surveying for horsehair blight in neglected cocoa plantations, was sequenced and is shown to belong to this taxon.
Notes: Marasmius tropicus is difficult to distinguish from the other cryptic species, M. crinis-equi and M. kabakada morphologically. However, of the three, it is more likely to develop yellowish-brown to sienna coloration in the pileus. This character is noted in the con-specific Thai collection (identified as M. crinis-equi) where the pileus is said to be ‘reddish brown to orangish brown’ (Wannathes et al. 2009). It forms a monophyletic clade, including the Thai (NW348) and Solomon Island sequences, with strong support, that is distinct from M. crinis-equi and M. kabakada. Its sister clade, with low support, is M. arachnotropus Oliveira (BS 52, PP 0.64) and more distantly a group of undescribed species from Cameroon, Marasmius sp.7 RAK and M. sp.1 RAK, which appears to be the same as sequences labelled M. crinis-equi from Ghana – GH76 & GH36 (Gp 1A, Fig. 3).
Additional specimens examined: QLD, Barron Gorge NP, Speewah Con. Park sect., in wet tropical rainforest understorey and lawyer vine, 12 Feb 2021, F.E. Guard F2021057, O. Albert-Mitchell & T. Lebel (BRI AQ1043691 GenBank ITS PP175837, LSU PP175761) and F2021050 (BRI AQ1043690 GenBank ITS PP175835, LSU PP175760); Cairns Hinterland, Malanda Con. Park, on dead leaves in wet tropical rainforest, 17 Feb 2021, F.E. Guard F2021074 & R. Philpot (BRI AQ1043692 GenBank ITS PP175837, LSU PP175761); Girramay N.P., Murray Falls Section, in riparian rainforest on saplings, 9 Feb 2021, F.E. Guard F2021035 & T. Lebel (BRI AQ1043686 GenBank ITS PP175834, LSU PP175764), F2021036 (BRI AQ1043687 GenBank ITS PP175840, LSU PP175766), F2021037 (BRI AQ1043688 GenBank ITS PP175841, LSU PP175762) and 3 Feb 2023, F.E. Guard F2023015 & R. Philpot (BRI AQ1045311 GenBank ITS PP175839, LSU PP175765); Topaz, Galaji Nature Refuge, in abandoned bird nest wall, 8 Feb 2023, F.E. Guard F2023040, M. Clarkson & J. Clarkson (BRI AQ 1045946 GenBank ITS PP175836, LSU PP175795); Solomon Islands, Malaita on Theobroma cacao trees in cocoa plantation, 26 Oct 2018, R.I. Davis, RID7970 (BRIP69145 GenBank ITS PP175832, LSU PP175794).
Marasmius perumbilicatus F.E. Guard, T. Lebel, Dearnaley sp. nov. Figs. 10 and 11.
MycoBank: MB 853128.
Etymology. ‘umbilicus’ is Latin for umbilicus (navel), to refer to the central pileal depression, which is prominent in this tiny Marasmius.
Holotype: Australia, QLD, Balmoral Ridge, Dilkusha Nature Refuge, road verge, S 26° 44′ 19.9″, E 152° 53′ 39.9″, 350 m asl., in leaf litter of regenerating subtropical rainforest under Neolitsea dealbata, 3 Feb 2018, F.E. Guard F2018010 (BRI AQ799985; GenBank ITS OP562719, LSU OP562713).
Description: Basidiomata small, marasmioid. Pileus 1.5–5 mm diam., parabolic to slightly campanulate, umbilicate, with or without a tiny orange-brown papilla, off-white (2B), pinkish buff (52) to pale apricot, dry, deeply sulcate; flesh very thin, white. Lamellae distant, 8–10, with rare lamellulae, adnate to narrow collar, white, margins usually non-coloured, occasionally concolorous with pileus. Stipe central, filiform, 30–55 × 0.1–0.2 mm, glossy, black base, dark brown throughout the length and buff upper end, insititious, hollow; some sterile stipes present, and juvenile stipes paler with off-white upper half. Rhizomorphs black, 0.1–0.2 mm diam., binding leaf litter. Spore print white. Basidiospores (15)16–19.5 × 4–5 μm, mean 17.5 × 4.5 μm, Q = 3.3–4.7, Qm = 4, narrowly clavate, hyaline, inamyloid (n = 20). Basidia 4-spored, 23–34 × 8–11 μm, sterigmata 3.5–5 μm. Basidioles clavate or fusoid. Pleurocystidia absent. Cheilocystidia common Siccus-type broom cells, narrowly to broadly clavate, globose, short cylindric to long with tapered base, occasionally bifid (9)15.5–25.5 × 7.5–11 μm, with sparse to crowded obtuse short setulae, 0.5–3.5 × 0.5–2 μm, sometimes thick-walled and refractive; rare smooth cells with irregular outline. Pileipellis is a hymeniderm composed of Siccus-type broom cells, cylindric, clavate to broadly clavate, mostly thin-walled, rarely branched or deeply bifid, 9.5–16 × 6–8.5 μm, with crowded short setulae, some thick-walled, refractile, 2–4 × 0.5–1.5 μm. In KOH, thick-walled digits are golden yellow. Pileal trama inamyloid to mildly dextrinoid, 3.5–6(8) μm diam. Lamellar trama inamyloid to mildly dextrinoid, 3–5(7) μm diam. Stipe hyphae parallel, cortical hyphae dextrinoid, thick-walled, medullary hyphae inamyloid, 2.5–5 μm diam. Caulocystidia absent. Clamp connections present.
Habit, habitat, and distribution: Gregarious, fruiting in troops on the rainforest floor, on fallen leaves and petioles of numerous rainforest species including Sloanea woollsii, Neolitsea dealbata and Ficus spp., often in disturbed areas and road verges. Sporophores are found in summer and early autumn after significant rain. To date, this species has been collected on privately conserved land in Dilkusha Nature Refuge, Maleny and Palmwoods, Sunshine Coast, SEQ, as well as in Speewah Conservation Park in FNQ and northern NSW as far south as Iluka. It has been observed and photographed in other parts of SEQ and also Lake Barrine in FNQ. It is expected that the distribution is much more widespread. (Fig. 11). Notes: Marasmius perumbilicatus is characterised by its tiny, umbilicate, buff sporophores on tall filiform stipes, lacking aerial rhizomorphs, gregarious habit, and substrate of rainforest leaves and petioles. It has long, narrowly clavate spores. The characteristics of lamellae adnate to a collar, insititious stipe, Siccus type broom cells and the absence of pleurocystidia place this species in subgen. Marasmius, sect. Crinis-eques, although the longish spores (to 20 μm) are outside the usual range for this section. Other members of the section include the widespread Australian species of the M. crinis-equi complex. Marasmius perumbilicatus has been mislabelled M. crinis-equi in Australian field guides (McCann 2003; Young 2005). However, species of the M. crinis-equi complex differ by having an aerial habit with sparse fruitbodies, pale through brown to brownish-orange caps, often fewer lamellae, very short stipes (< 12 mm) usually arising directly from plentiful black rhizomorphs, and much smaller spores (9–13 × 3.5–5 μm vs. 16–19.5 × 4–5 μm). Marasmius madagascariensis differs in having a slightly larger (2–6 mm) orange-brown cap, slightly more lamellae (9–11), shorter stipe (10–23 mm) and much shorter spores (8.8–12.8 × 4–5.6 μm) (Shay et al. 2017). Marasmius guyanensis Mont. first described from French Guyana, and also found in Africa, Thailand, Indonesia, Malaysia, and Brazil has a yellow to yellowish-orange cap, more lamellae (8–12) in Malaysian collections (Tan et al. 2009), though only 7–10 in Javan collections (Desjardin et al. 2000), shorter stipe (12–23 mm), a wide range of spore sizes (9–16 × 3–5 μm.) (Tan et al. 2009). Molecularly, Marasmius perumbilicatus is a well-supported species in a poorly supported group (Gp 1B, Fig. 3) with M. kabakada and Marasmius sp.4 RAK.
Additional specimens examined: Australia, NSW, Iluka Nature Reserve, littoral rainforest, leaf litter, 25 Feb 2022, F.E. Guard F2022030, T. Lebel & A.G. Boxshall (BRI AQ1041077, GenBank ITS PP175845, LSU PP175793); Rocky Creek Dam Road, Big Scrub Loop walking track, on roadside cutting, in leaf litter, 20 Feb 2022, F.E. Guard F2022009 (BRI AQ1041076, GenBank ITS PP175844, LSU PP175792); QLD, Balmoral Ridge, Dilkusha Nature Refuge, regenerating subtropical rainforest on road verge, leaf litter, 9 Feb 2019, F. Guard F2019001 (MEL2458227, GenBank ITS OP562722, LSU OP562715), 29 Jan 2020, F. Guard F2020020 (BRI AQ1017488 GenBank LSU PP175788 and in leaf litter under Ficus sp. and exotic fruit trees, 24 Apr 2019, F.E. Guard F2019026 (BRI AQ1077371, MEL2469585; GenBank ITS OP562723, LSU OP562716); Barron Gorge National Park, Speewah Campground, in leaf litter, 31 Dec 2020, L. M. Reinhold LMR FNQ8 (BRI AQ1021683, GenBank ITS PP175846, LSU PP175791); Palmwoods, 531 Landershute Rd, in leaf litter, 9 Feb 2020, W.G. Boatwright WGB756 (envt., GenBank ITS PP175843, LSU PP175790).
Marasmius argillaceus F.E. Guard, Laidlaw, T. Lebel, Dearnaley sp. nov. Figs. 12 and 13.
MycoBank: MB 853129.
Holotype: Australia, VIC, Tanjil South, 690 Moe-Willowgrove Rd, S 38° 05′ 38.2″, E 146 \(^\circ\) 13′ 20.8″, under Acacia melanoxylon, Leptospermum and Eucalyptus species, 16 July 2020, E. Laidlaw EL006 (MEL 2485436; GenBank ITS PP175865, LSU PP175807).
Etymology: ‘argillaceus’ is the Latin term for clay-coloured and is used in reference to its clay pink pilei.
Description: Basidiomata small, marasmioid. Pileus 2–11 mm diam., broadly parabolic to broadly convex with or without slightly flared margin, mature caps clay pink (30) with dark brown central depression, immature caps purplish date (22), centrally umbonate becoming umbilicate with maturity, the umbo eventually forming a central conical papilla which may disappear with maturity, deeply sulcate, smooth dry surface; flesh very thin, white. Lamellae distant, 9–13, adnate to a narrow collar, broadening at cap margin to produce scalloped effect, off-white with gill margin sometimes concolorous with cap, lamellulae occasional with or without some shallow cross-venations. Stipe central, filiform, 20–30 × 0.2 mm, blackish at base, dark brown throughout length and pale, off-white at apex, smooth, hollow, insititious though slightly enlarged at base. Sterile stipes common, but rhizomorphs absent. Spore print white.
Basidiospores 15.5–19 × 4–5 μm, mean 17.5 × 4.5 μm, Q = 3.24–4.60, Qm = 3.91, narrowly clavate, smooth, inamyloid, (n = 20). Basidia 4-spored, 19–23.5 × 7–7.5 μm, with sterigmata 3.5–5 μm in length (measured from EMTas002). Basidioles fusiform, narrowly clavate, 20–21 × 5.5 μm. Pleurocystidia absent. Cheilocystidia Siccus-type broom cells, body cylindric to clavate, 8–18 × 4–7 μm, setules apical, 2–5 × 1–2 μm. Pileipellis a hymeniderm composed of Siccus-type broom cells, body clavate, cylindric, ovoid, irregular, at times bifid 9.5–17 × 6–12 μm, setules apical, divergent, often bifid, obtuse, 1–5 × 1–1.5 μm, thick-walled and golden in KOH. Pileal trama slightly dextrinoid. Stipe hyphae parallel, cortical hyphae 3.5–7.5 μm diam., thick-walled. Caulocystidia absent. Clamp connections present and common. Rhizomorphs absent.
Habit, habitat, and distribution: Gregarious, fruiting in troops on fallen phyllodes and twigs of Acacia melanoxylon, also on mossy well-rotted logs in temperate wet sclerophyll forest. Sporophores found in autumn and winter. This species has been found in Tasmania, Victoria, and South Australia. To date, it has not been recorded in similar habitat in New South Wales or Queensland.
Notes: Marasmius argillaceus is characterised by its distinctive small clay pink, deeply sulcate caps on relatively short filiform stipes, marginate lamellae, gregarious habit and substrate of fallen Acacia phyllodes and twigs. It has long narrowly clavate spores, and its characters of collariate lamellae, insititious stipe, and Siccus-type broom cells traditionally place it in sect. Marasmius, subsect Sicciformes. Several morphological features separate it from M. crinis-equi. These include the substrate (leaf litter, not aerial rhizomorphs), the abundance and size of fruitbodies, which are larger than M. crinis-equi, number of lamellae and the clavate spores which are significantly longer than those of M. crinis-equi, (15.5–19 × 4–5 vs 11–12.5 × 4.5–5 μm). Marasmius argillaceus has been mislabelled as M. crinis-equi in one Australian field guide (Gates and Ratkowsky 2016), but it is morphologically distinct and molecularly distant. It appears to be a temperate zone taxon. It is morphologically and molecularly (Fig. 3) similar to the tropical species, M. brevicollus Corner, as described by Wannathes et al. (2009) and Tan et al. (2009), but with larger pilei, more lamellae, and shorter basidiospores. It is also similar to M. purpureobrunneolus Henn., described from Java in 1900, but has slightly larger pilei (2–11 vs 2–5 mm diam.) and larger basidiospores (15.5–19 × 4–5 μm vs (12)13–16 × 3–4 μm). Molecularly they are sister species with moderate support (BS 73, PP 0.85). Marasmius chrysocephalus Singer has a golden pileus, shorter basidiospores (11.7–15.2 × 3.2–4.2 μm) and produces black rhizomorphs (Singer 1976), which according to Koch et al. (2020) are used in bird nest construction in Guyana. Marasmius sanguirotalis Singer, as described by Oliveira et al. (2020), has a darker purplish-brown, slightly smaller (2–5 mm vs 2–11 mm diam.) pileus, a shorter, broader stipe (11–30 × 0.3–0.6 mm vs 20–30 × 0.2 mm) and overlapping, but on average shorter, narrower basidiospores (12–19 × 2.5–4 μm (mean 15 × 3 μm) vs 15.5–19 × 4–5 μm (mean 17.5 × 4.5 μm). Marasmius rubrobrunneus Shay and Desjardin from Madagascar is sister to M. sanguirotalis, and morphologically very similar (Oliveira et al. 2020). In the new classification of Marasmius by Oliveira et al. (2024b), M. argillaceus would be part of subgenus Marasmius, sect. Sanguirotalis, which includes M. sanguirotalis, M. rubrobrunneus, M. chrysocephalus, M. purpureobrunneolus, and M. brevicollus sensu Wannathes. However, they are not a well-supported clade in our analysis.
Additional materials examined: Australia, SA, Southern Lofty Region, Mt Crawford Forest, on twigs and fallen logs in Pinus radiata plantation, 19 July 2003, P.S. & D.E. Catcheside, (ADC51207 GenBank ITS PP175866) and 24 July 2006, P.S. Catcheside, (ADC54694 GenBank ITS PP175868, LSU PP175808); TAS, Nicholls Rivulet, Dogwood Forest, near Fannys Creek, on twigs of dogwood (Pomaderris apetala), 1 June 2020, Elaine McDonald EMTas007, (GenBank ITS PP175864, LSU PP175805) and 1 May 2021, EMTas002 (GenBank LSU PP175806) and EMTas003 (GenBank ITS PP175867, LSU PP175809); Blackwood, south of Simmons Reef Rd, 20 m. west of Jack Cann Reserve, by footpath, 13 July 2006, N.H. Sinnott (MEL2305227 GenBank ITS OP562720, LSU OP562717); Junee Caves, 24 May 2003, David Ratkowsky (MEL2257866, GenBank ITS OP562721, LSU OP562718).
Discussion
Re-collecting and analysing specimens from the holotype/lectotype areas in Australia and re-examination of the historical collections in K have been important in clarifying the concept and boundaries of M. crinis-equi. Finding three distinct species within eastern Australia, on molecular analysis of two gene regions (ITS and LSU), despite their morphologically similar nature, raises the issue of cryptic species. Species concepts including cryptic species have been examined widely (Lücking et al. 2020). A review by Cao et al. (2021) suggested that there are three categories of crypticity—(1) pseudocryptic, where morphological differences do exist, but the species are so similar that they are hard to distinguish; (2) semi-cryptic, where phenotype-related characters such as ecology and distribution are the only distinguishing features; (3) strictly cryptic, which are indistinguishable on morphological characters. On those criteria, Marasmius kabakada is pseudocryptic, with its usually lowland, tropical distribution within Queensland and small morphological differences helping to delimit it as a distinct taxon. Marasmius crinis-equi and M. tropicus have overlapping distributions in FNQ, though only the former has been found in SEQ and NSW to date, and the latter has a Pacific and south-east Asian as well as its Australian distribution. Morphologically, M. tropicus more commonly has a yellowish-brown to orange pileus when fresh, though occasionally off-white to buff. On the other hand, M. crinis-equi usually has off-white to buff pileus, only becoming fulvous on drying or ageing. This suggests that they should also be considered pseudocryptic. However, the possibility that M. tropicus can be pathogenic has not been excluded and would separate the two into semi-cryptic species. The overlapping distributions of all three make for more difficult separation of the species in the Cardwell (Rockingham Bay) region of north Queensland.
Recent studies from Africa and the Neotropics confirm there are many taxa of rhizomorph-producing, litter trap-forming Marasmius across the globe. The authentic horsehair fungus, Marasmius crinis-equi and sister taxa M. kabakada and M. tropicus from Australia are just three of these. This is in sharp contrast to the idea of a single cosmopolitan species accepted 100 years ago. Oliveira et al. (2024a) recently coined another term, ‘spider fungi’, to describe the global guild of fungi with an aerial rhizomorph, leaf litter trapping habit. These aerial networks, like spiderwebs, are considered to be important components of the fungal decomposer communities in wet tropical and subtropical forests (Lodge and Cantrell 1995; Snaddon et al. 2012). Australia’s horsehair fungi can be considered a part of this guild, given the similar ecological niche they are found in. They are also a valuable source of material for many bird species in nest building (Koch et al. 2020). All three rhizomorph-forming taxa in this study have been found to be used in bird nests, either in the walls or as a lining pad.
Not surprisingly as sequences are added from new geographic areas, new relationships become apparent. Perhaps a little surprising is that for many of the Australian taxa described here, the sister taxa are African (Cameroon, Ghana, Madagascar) and central American (Guyana) species rather than SE Asian (Thailand, Malaysia) or South American. This may simply reflect a lack of molecular data from other parts of SE Asia, tropical Australia (e.g. Northern Territory, northern Western Australia), or the Pacific islands (i.e. Papua New Guinea, New Caledonia, Cook Islands, Vanuatu).
In Australia, while aerial rhizomorph tangles have been found in unpruned tea bushes in FNQ, they are rare. Well-managed tea plantations in Australia have not had any problem with horsehair blight. In natural habitats, observations of aerial leaf traps suggest they are persistent, long-term structures (more than 2 years) that trap falling litter and do not affect the health of their hosts. Thus far, there is no evidence to suggest that the authentic Marasmius crinis-equi causes leaf necrosis or other damage to the host plants. The study of Su et al. (2011) needs to be repeated with molecular data of the fungus involved. It is likely that it is other fungi, either non-Marasmius or undescribed Marasmius species are the cause of horsehair blight and thread blight disease in SE Asian and African coffee, cocoa, and tea plantations, as suggested by Petch (1915) and demonstrated in Ghana (Amoako-Attah et al. 2020). In South America, M. infestans Huamán, Ramos C & Diaz Val has recently been described as a cause of thread blight disease in cocoa plantations in Peru (Huamán-Pilco et al. 2023). This species is closely related to M. neosessiliformis (Madagascar) and the M. tenuissimus complex causing thread blight in Ghana (Fig. 3). The newly described Marasmius tropicus was implicated in horsehair blight in a neglected cocoa plantation in the Solomons, with minor impact; however, this is based upon a single collection. Further investigation of aerial rhizomorph tangles in Australian and Pacific Islands plantations and native vegetation is necessary to determine causal agent identity and pathogenicity potential. Clarifying the concept and circumscription of M. crinis-equi will have consequences internationally as the variable pathogenicity of the horsehair fungus can now be proposed as likely due to non-Australian species in other countries.
Conclusion
Re-collection, morphological examination, molecular analysis, and epitypification of the species have been important for stabilising the taxon boundaries. We choose to continue the use of the name Marasmius crinis-equi, pending a binding decision on the validity of Marasmius equicrinis F. Muell., and have chosen one of the three Australian taxa to represent the species concept. As such, the name should no longer be applied to collections from other regions with aerial leaf trapping rhizomorphs and similar morphology. Molecular data across two or more gene regions will assist in clarifying the position of other as yet undescribed species of ‘horsehair/spider’ fungi across the globe and their relationship to M. crinis-equi. The discovery that horsehair fungi in Australia form a complex of three species, including M. crinis-equi, is unsurprising, but produces challenges in identification, which may not be resolved in the field. However, we have concluded that species lacking aerial rhizomorph networks are not part of this complex.
Clarification of mislabelled species in Australia is of value to field mycology. While the complete geographic distribution of M. perumbilicatus and M. argillaceus is not yet resolved, it is clear that M. perumbilicatus is a widespread tropical, subtropical, and temperate species in eastern Australia and M. argillaceus appears to be a temperate species.
Data Availability
All sequences in this study will be publicly available from GenBank.
References
Amoako-Attah I et al (2020) Identification and characterization of fungi causing thread blight diseases on cacao in Ghana. Plant Dis 104(11):3033–42. https://doi.org/10.1094/PDIS-03-20-0565-RE
Antonin V (1991) Studies in marasmioid fungi –VI. A new subsection Sicciformes within Marasmius, section Marasmius and a key to the European species of Marasmius section Marasmius. Acta Mus Moraviae Sci Nat 76:145–147
Antonin V (2007) Fungus flora of tropical Africa (J. Degreef Ed. Vol. 1). Belgium: National Botanic Garden
Ariyawansa HA et al (2014) Epitypification and neotypification: guidelines with appropriate and inappropriate examples. Fungal Diversity 69(1):57–91. https://doi.org/10.1007/s13225-014-0315-4
Berkeley M (1881) Australian fungi. II Received principally from Baron F. von Mueller. Bot J Linn Soc 18(111):383–389
Cao B et al (2021) Delimiting species in Basidiomycota: a review. Fungal Divers 109(1):181–237. https://doi.org/10.1007/s13225-021-00479-5
Dassanayake NC, Wanigasundara WADP, Balasuriya A, Amaratunge MKSLD (2009) A field assessment of the factors affecting horse hair blight (Marasmius equicrinis) in tea in the Ratnapura District. J Agric Sci-Sri Lanka 4(2):59–66
Dennis RWG (1951) Some Agaricaceae of Trinidad and Venezuela. Leucosporae. Part 1 Trans. Brit Mycol Soc 34:411–482
Desjardin DE, Horak E (1997) Marasmius and Gloiocephala in the south Pacific region: Papua New Guinea, New Caledonia and New Zealand, vol 2. Cramer, J, Berlin
Desjardin D, Retnowati A, Horak E (2000) Agaricales of Indonesia. 2. A preliminary monograph of Marasmius from Java and Bali. Sydow 52(2):92–193
Dowe JL, Maroske S (2020) John Dallachy (1804–71): from gardener to botanical collector. Hist Rec Aust Sci 31:87–100
Gardes M, Bruns T (1993) ITS primers with enhanced specificity for basidiomycetes - application to the identification of mycorrhizae and rusts. Mol Ecol 2(2):113–118. https://doi.org/10.1111/j.1365-294X.1993.tb00005.x
Gates G, Ratkowsky D (2016) A field guide to Tasmanian fungi (2nd ed.) Hobart, Tasmania: Tasmanian Field Naturalists Club
George A (2009) Australian botanist’s companion, 1st edn. Four Gables Press, Kardinya, Western Australia
Grace CL, Desjardin DE, Perry BA, Shay JE (2019) The genus Marasmius (Basidiomycota, Agaricales, Marasmiaceae) from Republic of Sao Tome and Principe. West Africa Phytotaxa 414(2):55–104. https://doi.org/10.11646/phytotaxa.414.2.1
Grgurinovic CA (1997) Larger fungi of South Australia. Adelaide: The Botanic Gardens of Adelaide and State Herbarium and the Flora and Fauna of South Australia Handbooks Committee: Adelaide
Huamán-Pilco AF et al (2023) Morphological, phylogenetic and genomic evidence reveals the causal agent of thread blight disease of cacao in Peru is a new species of Marasmius in the section Neosessiles Marasmius infestans sp. nov. F1000Research 12:1327. https://doi.org/10.12688/f1000research.140405.2
Huelsenbeck JP, Ronquist F (2001) MRBAYES: Bayesian inference of phylogenetic trees. Bioinforma 17(8):754–755. https://doi.org/10.1093/bioinformatics/17.8.754
Kalchbrenner K (1880) Fungi of Australia. I. Basidiomycetes. Grevillea 8:151–154
Katoh K, Standley DM (2013) MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Mol Biol Evol 30(4):772–780. https://doi.org/10.1093/molbev/mst010
Killermann S (1928) Unterklasse Eubasidii. Reihe Hymenomyceteae. In: Engler A (ed.) Die Natürlichen Pflanzenfamilien, 2nd edn. vol. 6. Verlag von Wilhelm Engelmann: Leipzig, pp 99–283
Koch RA, Liu J, Brann M, Jumbam B, Siegel N, Aime MC (2020) Marasmioid rhizomorphs in bird nests: species diversity, functional specificity, and new species from the tropics. Mycologia 112(6):1086–1103
Lodge DJ, Cantrell S (1995) Fungal communities in wet tropical forests: variation in time and space. Can J Bot 73(S1):1391–1398
Lücking R et al (2020) Unambiguous identification of fungi: where do we stand and how accurate and precise is fungal DNA barcoding? IMA Fungus 11(1):14. https://doi.org/10.1186/s43008-020-00033-z
Maroske S, Vaughan A (2014) Ferdinand Mueller’s female plant collectors: a biographical register. Muelleria 32:92–172
May T, Wood A (1997) Fungi of Australia Vol. 2A, Catalogue and bibliography of Australian macrofungi 1. Basidiomycota p.p. (A. E. Orchard Ed. Vol. 2A). Australia: CSIRO
McCann I (2003) Australian fungi illustrated. Vermont, 3138: Macdown Productions
Mueller F von (1875) Ferdinand von Mueller to Miles Berkeley, 1875–04–15. Correspondence of Ferdinand von Mueller
Mueller F von (1879) Ferdinand von Mueller to Miles Berkeley, 1879–09–18. Correspondence of Ferdinand von Mueller.
Oliveira JJ, Moncalvo J-M, Margaritescu S, Capelari M (2020) Phylogenetic and morphological analyses of species of Marasmius sect. Marasmius from the Atlantic Rainforest, Brazil. Plant Syst Evol 306(2):1–4. https://doi.org/10.1007/s00606-020-01659-7
Oliveira JJS, Vargas-Isla R, Cabral TS, Rodrigues DP, Ishikawa NK (2024a) Spider fungi: new species of Marasmius and Pusillomyces in the aerial rhizomorph web-making guild in Amazonia. Fungal Syst Evol 14:35–55. https://doi.org/10.3114/fuse.2024.14.03
Oliveira JJS, Desjardin DE, Jenkinson TS et al (2024b) Taxonomic revision of Marasmius Fr. and Marasmiaceae Roze ex Kuhner based on multigene phylogenetics and morphological evidence. Fungal Divers 30:1–54. https://doi.org/10.1007/s13225-024-00534-x
Pegler DN (1965) Studies on Australasian Agaricales. Aust J Bot 13:323–356
Pegler D (1986) Agaric flora of Sri Lanka. Kew Bull Addit Ser 12:1–519
Petch T (1915) Horse hair blights. Ann Royal Botanic Gardens Peradeniya 6:43–68
Petch T (1923) The diseases of the tea bush. McMillan & Co., Ltd, London
Petch T (1948) A revision of Ceylon Marasmii. Trans Brit Mycol Soc 31:19–44
Royal Botanic Gardens Edinburgh (1969) Flora of British fungi colour identification chart. Her Majesty’s stationery office, Edinburgh
Shay JE, Desjardin DE, Perry BA, Grace CL, Newman DS (2017) Biodiversity and phylogeny of Marasmius (Agaricales, Basidiomycota) from Madagascar. Phytotaxa 292:101–149. https://doi.org/10.11646/phytotaxa.292.2.1
Singer R (1976) Marasmieae (Basidiomycetes - Tricholomataceae). Flora Neotropica 17:1–347
Singer R (1986) The Agaricales in modern taxonomy, 4th edn. Koeltz Scientific Books, Koenigstein
Snaddon J, Turner E, Fayle T, Khen C, Eggleton P, Foster WA (2012) Biodiversity hanging by a thread: the importance of fungal litter-trapping systems in tropical rainforests. Biol Let 8:397–400. https://doi.org/10.1098/rsbl.2011.1115
Stamatakis A (2014) RAxML version 8: a tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics 30(9):1312–1313. https://doi.org/10.1093/bioinformatics/btu033
Su HJ, Thseng FM, Chen JS, Ko W-H (2011) Production of volatile substances by rhizomorphs of Marasmius crinisequi and its significance in nature. Fungal Divers 49:199–202. https://doi.org/10.1007/s13225-010-0084-7
Tan Y-S, Desjardin D, Perry B, Vikineswary S, Noorlidah A (2009) Marasmius sensu stricto in peninsular Malaysia. Fungal Divers 37:9–100
Turland NJ, Wiersema JH, Barrie FR, Greuter W, Hawksworth DL, Herendeen PS, Knapp S, Kusber W-H, Li D-Z, Marhold K, May TW, McNeill J, Monro AM, Prado J, Price MJ, Smith GF (eds) (2018) ‘International Code of Nomenclature for algae, fungi, and plants (Shenzhen Code) adopted by the Nineteenth International Botanical Congress Shenzhen, China, July 2017’. Regnum Vegetabile 159. Glashütten, Koeltz Botanical Books
Van Overeem C (1927) Fragmente aus: Die Nutzpilze Niederlaendisch-Indiens. Bulletin Du Jardin Botanique De Buitenzorg, Ser 3(9):8–22
Vilgalys R, Hester M (1990) Rapid genetic identification and mapping of enzymatially amplified ribosomal DNA from several Cryptococcus species. J Bacteriol 172(8):4238–4246. https://doi.org/10.1128/jb.172.8.4238-4246.1990
von Mueller F (1880) Marasmius equi-crinis F.v.M. Fragmenta Phytographiae Australiae 11:90. https://doi.org/10.5962/bhl.title.287
Wannathes N, Desjardin D, Hyde K, Perry B, Lumyong S (2009) A monograph of Marasmius (Basidiomycota) from northern Thailand based on morphological and molecular (ITS sequences) data. Fungal Divers 37:209–306
White T, Bruns T, Lee S, Taylor J (1990) Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis M, Gelfand D, Sninsky J, White T (eds) PCR protocols: a guide to methods and applications. Academic Press, San Diego, USA, pp 315–322
Young A (2005) A field guide to the fungi of Australia: University of New South Wales Press Ltd.
Acknowledgements
The authors wish to sincerely thank the staff of Kew Fungarium for their extensive assistance in finding original Australian collections of Marasmius crinisequi in K, and for the work of Isabella Miles-Bunch (Fungarium Curator) and Lee Davies (Fungarium Collections Manager) for photographing the specimens, protologues, and other miscellaneous information on packets and sharing that with us. We thank Vladimir Antonin for his comments on the state of the K collections and their lack of identifiable sporophores.
Other institutions in Australia, (National Herbarium of Victoria MEL, State Herbarium of South Australia AD, Queensland Herbarium BRI, and Queensland Plant Pathology Herbarium BRIP) have assisted with loans of their material for which we are grateful. We thank the staff of the Department of Agriculture, Fisheries & Forestry (DAFF), N. Qld for information on surveys for horsehair blight in Solomon Islands, PNG and FNQ, esp. Richard Davis.
We thank Eileen Laidlaw and members of the Queensland Mycological Society who have contributed collections and images.
A special thank you goes to the Jabalbina Yalanji Aboriginal Corporation, with whom we discussed the naming of Marasmius kabakada and for their interest in the project and enthusiasm for the use of their language name ‘kabakada’ meaning ‘a rainy place’, describing the Daintree rainforest, where the species was first found.
We thank Sara Maroske (Mueller Correspondence Project, Royal Botanic Gardens Victoria) for sharing her knowledge of Mueller’s correspondence and his network of collectors.
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection, and analysis were performed by Frances Guard, Teresa Lebel, and John Dearnaley. The first draft of the manuscript was written by Frances Guard, and all authors commented on versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Declarations
We are grateful for financial assistance through a Hansjörg Eichler Australian Systematic Botany Society (ASBS) grant for field work for F.E. Guard.
The authors have no other relevant financial or non-financial interests to disclose.
Additional information
Section Editor: Rui-Lin Zhao
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Guard, F.E., Dearnaley, J., May, T.W. et al. Untangling horsehair fungi in Australia: Marasmius crinis-equi (Marasmiaceae) and related taxa. Mycol Progress 23, 60 (2024). https://doi.org/10.1007/s11557-024-01995-9
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11557-024-01995-9