Abstract
Preparative high-performance liquid chromatography (HPLC) purification of the ethyl acetate extract derived from dried basidiomes of the European mushroom Hericium coralloides led to the identification of two previously undescribed isoindolinone derivatives named corallocins D and E (1-2). The structures of the compounds were elucidated based on HR-ESI-MS (high-resolution electron spray ionization mass spectrometry), interpretation of 1D and 2D NMR spectra, electronic circular dichroism (ECD) experiments, and comparisons with published and theoretical data. The metabolites were tested for their cytotoxic and antimicrobial effects in vitro where weak to moderate biological effects were observed against HeLa cells (KB 3.1), Mucor hiemalis and Bacillus subtilis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Basidiomycota fungi, are well-known for their profuse production of bioactive molecules (Sandargo et al. 2019; Sum Chemutai et al. 2023b). In particular, fungal meroterpenoids have displayed various bioactivities and structural diversities (Geris and Simpson 2009; Jiang et al. 2021). This class of terpenoids, majorly produced via the mixed terpene biosynthetic pathways, is found ubiquitously in animals, plants, Actinobacteria, and fungi (Jiang et al. 2021). Among the potent meroterpenoid drugs are mycophenolic acid and its derivative (mycophenolate mofetil), used as an immunosuppressive drug in organ transplant and cancer chemotherapy (Benjanuwattra et al. 2020).
Mushrooms belonging to the genus Hericium, especially H. erinaceus (Lion’s Mane), are majorly cultivated as foods, and remain a valuable reservoir of meroterpenoids (Thongbai et al. 2015). According to a recent review, Hericium mushrooms global export and import market values were USD 963.4 million and USD 978.83 million in 2021, respectively (Niego et al. 2023). Meroterpenoids have been widely reported from various species of the fungal genus for their neuroprotective effects (Wang et al. 2015; Wittstein et al. 2016; Ryu et al. 2021; Sum Chemutai et al. 2023a). Nonetheless, Hericium meroterpenes have also been reported for other health-promoting capabilities such as their antidiabetic, antimicrobial, anticancer, immunomodulatory, and hypolipidemic effects (Thongbai et al. 2015). So far, few reports of bioactive natural products from H. coralloides exist (Kim et al. 2018; Wittstein et al. 2016). Notably, new isoindolinone and benzofuranone derivatives named corallocins A-C were shown to possess neurotrophic properties, thus inducing the expression of neurotrophins NGF (nerve growth factor) and BNDF (brain-derived neurotrophic factor) in human astrocytes, in addition to the antiproliferative effects of corallocin B against human breast and cervical cancer cell lines (Wittstein et al. 2016). Another study similarly isolated new antioxidants from the culture broth of the fungus, with IC50 values in the range of 29–66 µM in the radical scavenging assay (Kim et al. 2018).
Thus, as a part of our ongoing research on novel antimicrobials from fungi belonging to the phylum Basidiomycota, we discovered two hitherto unprecedented isoindolinone-type meroterpenoids from H. coralloides. Their chemical analyses and bioassays are herein described.
Materials and methods
General experimental procedures
HPLC-DAD/MS analyses were performed on the amaZon speed ETD ion trap mass spectrometer (Bruker Daltonics, Bremen, Germany) in the positive and negative ionization modes simultaneously. The HPLC system [C18 Acquity UPLC BEH (Waters) column (stationary phase) and solvent A (H2O + 0.1% formic acid) + solvent B (MeCN + 0.1% formic acid) (solvent system)] were used. The elution gradient applied was an initial 5% B for 0.5 min, followed by an increase to 100% B in 20 min. Thereafter, an isocratic condition was maintained at 100% B for 10 min. The flow rate was 0.6 mL/min, and the UV/Vis detections were recorded at 190–600 nm and 210 nm.
HR-ESI-MS (high-resolution electrospray ionization mass spectrometry) analyses were measured on the MaXis ESI-TOF (time of flight) mass spectrometer (Bruker Daltonics) coupled to an Agilent 1260 series HPLC-UV system (Agilent Technologies, Santa Clara, CA, USA). The HPLC system (C18 Acquity UPLC BEH (ultraperformance liquid chromatography) (ethylene bridged hybrid) (Waters) column (stationary phase) and solvent A (H2O + 0.1% formic acid) + solvent B (MeCN + 0.1% formic acid) (solvent system)) were used. The separation gradient employed was an initial 5% B for 0.5 min, followed by an increase to 100% B in 19.5 min. Subsequently, the gradient was held at isocratic conditions at 100% B for 5 min. The flow rate was maintained at 0.6 mL/min (40 °C) and the UV/Vis detection at 200–600 nm. The Compass DataAnalysis 4.4 SR1 software was used to determine the molecular formulas of the compounds using the Smart Formula algorithm (Bruker Daltonics).
The NMR spectra were recorded on Avance III 500 (Bruker, Bremen, Germany) (1H: 500 MHz, 13C: 125 MHz) spectrometer locked to the respective deuterium signal of the solvent. Deuterated DMSO-d6 was used as a solvent in the NMR measurements, where chemical shifts were determined in parts per million (ppm) and coupling constants in Hertz (Hz). Optical rotation was measured in dimethylsulfoxide (Uvasol, Merck, Darmstadt, Germany) solvent using Anton Paar MCP-150 Polarimeter (Graz, Austria) with sodium D line at 589 nm and 100 mm path length. The UV spectra were recorded on the Shimadzu UV/Vis 2450 spectrophotometer (Kyoto, Japan), and ECD (electronic circular dichroism) spectra were measured on the J-815 spectropolarimeter (Jasco, Pfungstadt, Germany) in dimethylsulfoxide solvent (Uvasol).
Chemicals and solvents (analytical and HPLC grade) used were obtained from AppliChem GmbH (Darmstadt, Germany), Avantor Performance Materials (Deventer, Netherlands), Merck KGaA (Darmstadt, Germany), and Carl Roth GmbH & Co. KG (Karlsruhe, Germany).
Fungal material
The dried fruiting bodies of H. coralloides used in this study were obtained from KÄÄPÄ Biotech, Finland, where they had been grown artificially.
Extraction of metabolites
The dried basidiomes were ground in a mortar to yield a powdered sample (9 g). The powder was soaked in acetone (200 mL), and placed on a shaker (120 rpm), at 25 °C for 48 h. The sample was thereafter filtered, and the residue was extracted twice with acetone (200 mL). The filtrate obtained was then dried under reduced pressure using a rotary evaporator and extracted with ethyl acetate as previously described (Wittstein et al. 2016). This yielded 272 mg of ethyl acetate crude extract.
Isolation of compounds 1 and 2
The total extract obtained above (272 mg) was purified using a preparative reverse-phase liquid chromatography system (PLC 2020; Gilson, Middleton, WI, USA) equipped with C18 VP-Nucleodur column 100−5 (250 mm × 40 mm, 7 μm, Machery-Nagel, Düren, Germany), while using deionized water + 0.1% formic acid (solvent A) and acetonitrile (MeCN) + 0.1% formic acid (solvent B), as a solvent system. The applied gradient to elute the metabolites was as follows: initial holding at 5% B for 10 min, 5–45% solvent B (50 min), 45–90% B (5 min), 90–100% B (5 min), and isocratic conditions at 100% solvent B (20 min), to yield 1 (1.0 mg; tR = 74 min) and 2 (0.7 mg; tR = 76 min). The flow rate was maintained at 40 mL/min, and the UV detections were made at 190, 210, and 280 nm.
Corallocin D (1): Off-white amorphous solid; \({\left[\alpha \right]}_{\text{D}}^{20}\) + 24 (c 0.1, DMSO); UV/Vis (DMSO): λmax (log ε) 257.5 (0.9); NMR data (1H: 500 MHz, 13C: 125 MHz, DMSO-d6) see Table 1; HR-ESI-MS: m/z 388.2123 [M + H]+ (calcd. 388.2118 for C22H30NO5+), m/z 410.1946 [M + Na]+ (calcd. 410.1938 for C22H29NaNO5+), m/z 775.4183 [2M + H]+ (calcd. 775.4164 for C44H59N2O10+), m/z 797.4001 [2M + Na]+ (calcd. 797.3984 for C44H58NaN2O10+).
Corallocin E (2): Off-white amorphous solid; \({\left[\alpha \right]}_{\text{D}}^{20}\) + 26 (c 0.1, DMSO); UV/Vis (DMSO): λmax (log ε) 220 (0.5); NMR data (1H: 500 MHz, 13C: 125 MHz, DMSO-d6) see Table 1; HR-ESI-MS: m/z 464.2438 [M + H]+ (calcd. 464.2431 for C28H34NO5+), m/z 486.2258 [M + Na]+ (calcd. 486.2251 for C28H33NaNO5+), m/z 927.4806 [2M + H]+ (calcd. 927.4790 for C56H67N2O10+), m/z 949.4625 [2M + Na]+ (calcd. 949.4610 for C56H66NaN2O10+).
Antimicrobial assay
Minimum inhibitory concentrations (MICs) were determined via serial dilutions on 96-well microtiter plates against various test microorganisms as previously established (Sum Chemutai et al. 2022). YMG (yeast-malt-glucose) medium was used in the growth of filamentous fungi and yeasts, whereas EBS medium (0.5% casein peptone, 0.1% meat extract, 0.1% yeast extract, 0.5% glucose, 50 mM HEPES [11.9 g/L], and pH = 7.0) was used for the growth of bacteria. The test strains included bacteria: Staphylococcus aureus (DSM 346), Bacillus subtilis (DSM 10), Acinetobacter baumannii (DSM 30008), Escherichia coli (DSM 1116), Chromobacterium violaceum (DSM 30191), Pseudomonas aeruginosa (PA14), and Mycolicibacterium smegmatis (ATCC 700084), and fungi: Candida albicans (DSM 1665), Mucor hiemalis (DSM 2656), Schizosaccharomyces pombe (DSM 70572), Rhodotorula glutinis (DSM 10134), and Pichia anomala (DSM 6766). Ciprofloxacin, kanamycin, gentamycin, and oxytetracycline were used as positive controls against bacteria, whereas nystatin was used against fungi. The lowest concentration, at which no growth of the test organism was observed, was determined as the MIC.
Cytotoxicity assay
Mammalian cell lines obtained from the DSMZ collection (Braunschweig, Germany), including mouse fibroblasts (L929), human endocervical adenocarcinoma (KB3.1), epidermoid carcinoma (A431), ovarian cancer (SKOV-3), prostate cancer (PC3), breast cancer (MCF-7), and human lung adenocarcinoma (A549) cell lines, were used in the determination of in vitro cytotoxicity (IC50) of compounds 1–2. MC-7, SKOV-3, and A431 were cultured in RPMI (Gibco), A549, KB 3.1 and L929 in DMEM (Gibco), and PC-3 in F12-K (Gibco). The cells (supplemented with 10% Gibco bovine serum) were initially grown at 37 °C for 5 days under 10% carbon dioxide (CO2). Thereafter, cytotoxicity of the compounds was determined using the MTT (3-(4,5-dimethylthiazol-2- yl)-2, 5 diphenyltetrazolium bromide) in 96-well microplates in accordance with our standard protocols (Sum Chemutai et al. 2022). Epothilone B was used as the positive control.
Computational functional theory calculations
A conformational analysis was performed to reveal all possible conformations of compound 1 within 10 kcal/mol energy window with the aid of the Omega2 software version 2.5.1.4 (2017). The generated conformations were then geometrically optimized at the B3LYP/6-31G* level of theory. On the optimized structures, frequency computations were performed, and Gibbs free energies were subsequently evaluated. Utilizing the polarizable continuum model (PCM), the initial fifty excitation states were obtained by employing time-dependent density functional theory (TDDFT)-ECD computations in the presence of DMSO. The ECD spectra were extracted and subjected to Boltzmann averaging using the SpecDis 1.71 software (Bruhn et al 2013; Bruhn 2017). The Boltzmann-weighted curve was then compared to the experimental spectra. The Gaussian09 software was utilized to perform all the executed quantum computations (Frisch et al. 2009).
Results and discussion
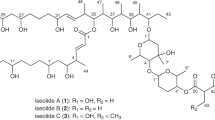
HPLC-DAD/MS investigation of the EtOAc extract derived from the fruiting bodies of H. coralloides revealed two previously undescribed isoindolinone derivatives (1 and 2 in Fig. 1), albeit in very low amounts. The compounds were isolated and subjected to antimicrobial and cytotoxicity assays. In this study, we report the identification and structure elucidation of 1 and 2, as well as their bioassay results.
Compound 1 was isolated as an off-white amorphous solid. Its molecular formula was determined to be C22H29NO5 based on HR-ESI-MS that revealed two peaks for a protonated molecule and a sodium adduct at m/z 388.2123 [M + H]+ and at m/z 410.1946 [M + Na]+ calculated for 388.2118 and 410.1938, respectively. The determined molecular formula indicated nine degrees of unsaturation. The 13C NMR spectrum of 1 (Table 1; Fig. S4) revealed the presence of twenty-two carbon resonances distinguished into nine quaternary carbons including two carbonyl carbons at δC 174.1 and δC 167.7 together with seven aromatic carbon atoms (δC 158.2, δC 150.5, δC 133.8, δC 131.6, δC 130.6, δC 121.4, δC 119.6). In addition, the 13C NMR data of 1 also revealed four tertiary carbon atoms differentiated into three olefinic (δC 124.2, δC 122.6, δC 95.9) and one aliphatic (δC 50.5), alongside with four secondary carbon atoms (δC 44.9, δC 39.4, δC 26.2, δC 22.4) and five primary carbon atoms including one interpreted as a methoxy group (δC 55.8) and four other methyl groups (δC 25.5, δC 17.6, δC 16.7, δC 16.0).
The 1H NMR, 1H-1H COSY, and HSQC spectra of 1 (Table 1; Fig. 2; Figs. S3, S5, S7), revealed a geranyl side chain presumably biosynthesized through head-to-tail addition of active dimethylallyl and isopentenyl moieties supported by the presence of two olefinic protons at δH 5.11 (tq, J = 7.2, 1.3 Hz, 1H) and at δH 5.02 (m, 1H), which exhibited long-range correlation in 1H-1H COSY spectrum to one olefinic methyl groups at δH 1.72 (d, J = 1.3 Hz, 3H) and two olefinic methyl groups at δH 1.59 (d, J = 1.4 Hz, 3H) and δH 1.52 (d, J = 1.6 Hz, 3H), respectively, together with three methylene groups at δH 3.30 (d, J = 7.2 Hz, 2H), δH 1.98 (q, J = 7.4 Hz, 2H), and δH 1.88 (t, J = 7.0 Hz, 2H). In addition, the 1H NMR spectrum of 1 showed an aromatic singlet proton at δH 6.70 and two diastereotopic protons at δH 4.25 and δH 4.54 with a common geminal coupling constant (J-value) of 17.3 Hz. A literature search of 1 indicated that it is probably a previously undescribed isoindolinone derivative related to hericerin, corallocins B and C that were previously described from the fruiting bodies of Hericium erinaceus (Kimura et al. 1991, as “erinaceum”) and H. coralloides (Wittstein et al. 2016), respectively. A detailed comparison of 1H and 13C NMR data of 1 to those reported in literature (Kimura et al. 1991; Wittstein et al. 2016), obviously revealed that 1 comprises a doublet methyl group at δH 1.38 (d, J = 7.4 Hz, 3H; δC 16.7) that was correlated by 1H-1H COSY spectrum and HMBC spectrum (Fig. 2) to an oxygenated aliphatic methine proton at δH 4.63 (q, J = 7.4 Hz, 1H; δC 50.5) and a carbonyl carbon at δC 174.1, respectively, indicating the presence of 3-hydroxybutyryl moiety. Further confirmation to the depicted structure of 1 (Fig. 1) as an isoindolinone derivative was provided by HMBC spectrum (Fig. 2) that disclosed key correlations from a methoxy group at δH 3.78 (OCH3-8), a methylene group at δH 3.30 (d, J = 7.2 Hz, H2-1') and an aromatic proton at δH 6.70 (H-7) to an oxygenated aromatic carbon at δC 158.2 (C-6) indicating the positions of geranyl side chain, methoxy group and aromatic proton to be on three adjacent carbons (C5–C7), respectively.
The location of 3-hydroxybutyryl moiety was confirmed by ROESY spectrum (Fig. 2) that revealed a key ROE correlation from a methyl doublet at δH 1.38 (d, J = 7.4 Hz, H3-3″) to a diastereotopic methylene group at δH 4.25/δH 4.54 (H2-3) indicating the 3-hydroxybutyryl moiety to be located at nitrogen atom of the isoindolinone structure (N-2). The absolute configuration of C-2″ was unambiguously determined by comparing the measured and the calculated ECD spectra (Fig. 3). This comparison unveiled a close resemblance between the measured ECD spectrum of 1 and that calculated for (2″R) which revealed an opposite pattern to the calculated ECD spectrum of its enantiomer. Hence, the absolute configuration of 1 was unambiguously confirmed to be (2″R). Based on the obtained results, compound 1 was identified as a previously undescribed isoindolinone derivative that was given a trivial name, corallocin D.
Compound 2 was obtained, similar to 1, as an off-white amorphous solid. The HR-ESI-MS of 2 established its molecular formula to be C28H33NO5 by revealing two peaks for a protonated molecule at m/z 464.2438 [M+H]+ and a sodium adduct at m/z 486.2258 [M + Na]+ calculated for 464.2431 and 486.2251, respectively, indicating thirteen degrees of unsaturation exceeding 1 by 4°. The 1H NMR spectral data of 1 and 2 (Table 1; Figures S3, S11) interpreted the additional four degrees of unsaturation in 2 by revealing five aromatic protons at δH 7.23 (m, 4H) and δH 7.14 (m, 1H) suggesting the presence of monosubstituted aromatic ring. Apart from this additional aromatic ring, the 1H and 13C NMR spectral data of 2 came in accordance with those recorded for 1 (Table 1). The 1H NMR and 1H-1H COSY spectra of 2 disclosed the presence of a geranyl side chain characterized by two olefinic protons at δH 5.09 (tq, J = 7.2, 1.4 Hz, H-2′) and at δH 5.00 (m, H-6′), which exhibited key correlation in 1H-1H COSY spectrum to olefinic methyl groups at δH 1.71 (d, J = 1.4 Hz, H3-9′), δH 1.56 (d, J = 1.5 Hz, H3-8′), and δH 1.50 (d, J = 1.4 Hz, H3-10′), respectively, together with three methylene groups at δH 3.29 (d, J = 7.2 Hz, H2-1′), δH 1.98 (q, J = 7.0 Hz, H2-5′), and δH 1.88 (t, J = 7.0 Hz, H2-4′). In addition, the 1H-1H COSY spectrum of 2 unraveled two other spin systems, one connects an aromatic proton at δH 7.14 to four other aromatic protons at δH 7.23 together with a second spin system from a methine proton at δH 3.12 (br s) and two diastereotopic methylene protons at δH 3.09 (d, J = 14.9 Hz, 1H) and at δH 3.40 (dd, J = 14.9, 4.7 Hz, 1H). Further confirmation for the depicted structure of 2 as an isoindolinone derivative was provided by HMBC and HSQC spectra (Fig. 2; Fig. S15). The HMBC spectrum of 2 revealed key correlations from the two diastereotopic methylene protons at δH 3.09/δH 3.40 to electromagnetically equivalent aromatic carbons at δC 128.5 (C-5″, C-9″) that were directly correlated via HSQC spectrum to two aromatic protons at δH 7.23 indicating the presence of additional aromatic ring in 2 as 3-hydroxy-4-phenylbutyryl moiety. The HMBC spectrum of 2 revealed similar key correlations as in 1, which revealed the position of the geranyl side chain, methoxy group, and a singlet aromatic proton (δH 6.68, H-7) to be located on three adjacent aromatic carbons (C5–C7) in the isoindolinone nucleus. To locate the 3-hydroxy-4-phenylbutyryl moiety, the ROESY spectrum of 2 was measured and it revealed key ROE correlations from aromatic protons at δH 7.23 (C-5″, C-9″) to a diastereotopic methylene group at δH 4.22/δH 4.33 (d, J = 17.0 Hz, H2-3) indicating its binding at nitrogen atom (N-2) of the isoindolinone moiety. Based on the related structures of 1 and 2 suggesting a common biosynthetic origin, sharing a similar sole chiral center and close positive \({\left[\alpha \right]}_{\text{D}}^{20}\) values (+24 and +26, respectively), the absolute configuration of 2 was concluded to be (2''R). Consequently, compound 2 was identified as a previously undescribed isoindolinone derivative featuring 3-hydroxy-4-phenylbutyryl moiety in its structure and it was named as corallocin E.
Biological activities of compounds 1–2
The isolated isoindolinone derivatives 1 and 2 were evaluated for their antimicrobial effects, based on the serial dilution assay method against bacteria and fungi (Table S1). Compound 1 was weakly active against Mucor hiemalis at 66.6 µg/mL, whereas compound 2 was similarly active against Bacillus subtilis at 66.6 µg/mL. Nystatin and oxytetracycline were used as positive controls for fungi and bacteria, respectively. The compounds were mostly inactive in the other test microorganisms. Furthermore, cytotoxicity tests revealed that the compounds were non-cytotoxic (Table S1).
Conclusions
Two new isoindolinone derivatives denoted as corallocins D (1) and E (2), were isolated from the fruiting bodies of the basidiomycete H. coralloides. Isoindolinone metabolites have been already isolated several times as the major metabolites of basidiomata (Wittstein et al. 2016; Ryu et al. 2021) and cultures (Wang et al. 2015; Sum Chemutai et al. 2023a) of the genus Hericium. It is noteworthy that the isoindolinone derivatives seem to be more abundant in the solid cultures and basidiomes (Wittstein et al. 2016; Ryu et al. 2021; Sum Chemutai et al. 2023a), while the submerged cultures produce benzofuranones (Sum Chemutai et al. 2023a).
Data availability
Not applicable.
References
Benjanuwattra J, Chaiyawat P, Pruksakorn D, Koonrungsesomboon N (2020) Therapeutic potential and molecular mechanisms of mycophenolic acid as an anticancer agent. Eur J Pharmacol 887:173580. https://doi.org/10.1016/J.EJPHAR.2020.173580
Bruhn T, Schaumlöffel A, Hemberger Y, Bringmann G (2013) SpecDis 1.53, Würzburg, Germany: University of Würzburg. Chirality 25:243–249. https://doi.org/10.1002/chir.22138
Bruhn T (2017) SpecDis Version 1.71; Berlin
Frisch GWTJ, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Mennucci B, Petersson GA, Nakatsuji H, Caricato M, Li X, Hratchian HP, Izmaylov AF, Bloino J, Zheng G, Sonnenberg JL, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Vreven T, Montgomery J, Peralta JE, Ogliaro F, Bearpark M, Heyd JJ, Brothers E, Kudin KN, Staroverov VN, Kobayashi R, Normand J, Raghavachari K, Rendell A, Burant JC, Iyengar SS, Tomasi J, Cossi M, Rega N, Millam JM, Klene M, Knox JE, Cross JB, Bakken V, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Martin RL, Morokuma K, Zakrzewski VG, Voth GA, Salvador P, Dannenberg JJ, Dapprich S, Daniels AD, Farkas Ö, Foresman JB, Ortiz JV, Cioslowski J, Fox DJ (2009) Gaussian, Inc.; Wallingford, CT
Geris R, Simpson TJ (2009) Meroterpenoids produced by fungi. Nat Prod Rep 26:1063–1094. https://doi.org/10.1039/B820413F
Jiang M, Wu Z, Liu L, Chen S (2021) The chemistry and biology of fungal meroterpenoids (2009–2019). Org Biomol Chem 19:1644–1704. https://doi.org/10.1039/D0OB02162H
Kim JY, Woo EE, Lee IK, Yun BS (2018) New antioxidants from the culture broth of Hericium coralloides. J Antibiot 71(9):822–825. https://doi.org/10.1038/s41429-018-0067-6
Niego AGT, Lambert C, Mortimer P, Thongklang N, Rapior S, Grosse M, Schrey H, Charria-Girón E, Walker A, Hyde KD, Stadler M (2023) The contribution of fungi to the global economy. Fungal Divers 121:95–137. https://doi.org/10.1007/s13225-023-00520-9
OMEGA, 2.5.1.4. OpenEye Scientific Software; ,Santa Fe, NM, USA., 2017
Ryu SH, Hong S, Khan Z, Lee S, Vishwanath M, Turk A, Yeon S, Jo Y, Lee D, Lee J, Huang B (2021) Neurotrophic isoindolinones from the fruiting bodies of Hericium erinaceus. Bioorg Med Chem Lett 31:127714. https://doi.org/10.1016/j.bmcl.2020.127714
Sandargo B, Chepkirui C, Cheng T, Chaverra-Muñoz L, Thongbai B, Stadler M, Hüttel S (2019) Biological and chemical diversity go hand in hand: Basidiomycota as source of new pharmaceuticals and agrochemicals. Biotechnol Adv 37:107344. https://doi.org/10.1016/J.BIOTECHADV.2019.01.011
Sum Chemutai W, Ebada SS, Kirchenwitz M, Kellner H, Ibrahim MAA, Stradal TEB, Matasyoh JC, Stadler M (2023a) Hericioic acids A-G and hericiofuranoic acid; neurotrophic agents from cultures of the European mushroom Hericium flagellum. J Agric Food Chem 71(29):11094–11103. https://doi.org/10.1021/acs.jafc.3c02897
Sum Chemutai W, Ebada SS, Matasyoh C, Stadler M (2023b) Recent progress in the evaluation of secondary metabolites from Basidiomycota. Curr Res Biotechnology 6:100155. https://doi.org/10.1016/j.crbiot.2023.100155
Sum Chemutai W, Mitschke N, Schrey H, Wittstein K, Kellner H, Stadler M, Matasyoh JC (2022) Antimicrobial and cytotoxic cyathane-xylosides from cultures of the basidiomycete Dentipellis fragilis. Antibiotics 11:1072. https://doi.org/10.3390/ANTIBIOTICS11081072/S1
Thongbai B, Rapior S, Hyde KD, Wittstein K, Stadler M (2015) Hericium erinaceus, an amazing medicinal mushroom. Mycol Prog 14:91. https://doi.org/10.1007/S11557-015-1105-4
Wang K, Bao L, Qi Q, Zhao F, Ma K, Pei Y, Liu H (2015) Erinacerins C-L, isoindolin-1-ones with α-glucosidase inhibitory activity from cultures of the medicinal mushroom Hericium erinaceus. J Nat Prod 78:146–154. https://doi.org/10.1021/np5004388
Wittstein K, Rascher M, Rupcic Z, Löwen E, Winter B, Köster RW, Stadler M (2016) Corallocins A-C, nerve growth and brain-derived neurotrophic factor inducing metabolites from the mushroom Hericium coralloides. J Nat Prod 79:2264–2269. https://doi.org/10.1021/acs.jnatprod.6b00371
Acknowledgements
We deeply thank Wera Collisi for assistance with the bioassays, Christel Kakoschke for conducting the NMR spectroscopic measurements, and Aileen Gollasch and Esther Surges for running the LC-MS samples. We also thank Joette Crosier and her colleagues of KÄÄPÄ Biotech, Finland, for providing the mushroom material.
Funding
Open Access funding enabled and organized by Projekt DEAL. W. C. S. was supported by a doctoral scholarship funding from the German Academic Exchange Service (DAAD) program number 57507871. The Alexander von Humboldt (AvH) foundation funded S. S. E. via the Georg-Forster Fellowship for Experienced Researchers (Ref 3.4-1222288-EGY-GF-E).
Author information
Authors and Affiliations
Contributions
W. C. S.: conceptualization, screening, isolation of compounds, structure elucidation, antimicrobial assays, and preparing the original draft; D. G.: isolation of compounds; M. A. A. I.: ECD calculation; S. S. E.: structure elucidation, correcting, editing and polishing the final draft; M. S.: supervision, funding acquisition, correcting, editing, and polishing the final draft. All authors have read and agreed to the published version of the manuscript.
Corresponding authors
Ethics declarations
Institutional Review Board statement
Not applicable.
Informed consent
Not applicable.
Conflict of interest
The authors declare no competing interests.
Additional information
Section Editor: Ji-Kai Liu
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Sum, W.C., Gonkhom, D., Ibrahim, M.A.A. et al. New isoindolinone derivatives isolated from the fruiting bodies of the basidiomycete Hericium coralloides. Mycol Progress 23, 4 (2024). https://doi.org/10.1007/s11557-023-01941-1
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11557-023-01941-1