Abstract
Protoilludene-type sesquiterpene aryl esters are a unique and very diverse compound class that were exclusively isolated from members of the genus Armillaria (Agaricomycetes, Physalacriaceae) up to this point. Herein, we describe the isolation and structural characterization of 5′-O-methyl-14-hydroxyarmillane (1), a new armillane-type derivative, that was obtained from submerged cultures of Guyanagaster necrorhiza (CBS 138623) together with the known congeners melleolide G (2), melleolide B (3), and 10-dehydroxy-melleolide B (4). ROESY data and coupling constants assigned the relative configurations of 1, while common absolute configurations were confirmed from comparison of its ECD spectrum to the one of 10-hydroxy-5′-methoxy-6′-chloroarmillane (5). Additionally, the configuration of melleolide G (2) was revised based on observed ROESY correlations. It is the first time that protoilludene-type sesquiterpene aryl esters were isolated from another genus, namely, Guyanagaster, that is closely related to Armillaria. 1–4 were evaluated for their biological activities in a serial dilution assay against several yeast, fungi, and bacteria, as well as in a cytotoxicity assay against different cell lines. Compound 4 was moderately active against Bacillus subtilis, Staphylococcus aureus, and Mucor hiemalis. Furthermore, 1, 3, and 4 showed weak cytotoxic effects against the mouse fibroblast cell line L929 and the cervix carcinoma cell line KB3.1.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
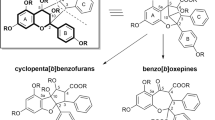
The protoilludene-type sesquiterpene aryl esters are a chemically very diverse class of compounds among the natural products that can be obtained from fungal sources. Up to this point, more than 70 congeners of this compound class were discovered and described in literature (Dörfer et al. 2019). Often casually summarized as melleolides, strictly speaking protoilludene-type aryl esters have to be divided into three groups according to the localization of the double bond within the protoilludene backbone. Melleolides are Δ2-3-unsaturated and armillyl orsellinates are Δ2-4-unsaturated, while armillanes are characterized by the absence of the double bound within the 6-membered ring (Engels et al. 2021). Alongside their chemical diversity, several members of the protoilludene-type aryl esters show a wide range of bioactivities, including antibacterial, antifungal, and cytotoxic effects (Dörfer et al. 2019). Interestingly, this class of natural products was exclusively isolated from cultures of the genus Armillaria (Agaricomycetes, Physalacriaceae) until now. Armillaria spp. are well-known and globally distributed parasitic fungi that affect a broad range of different hosts in timber and agronomic systems, where they play a critical role as root pathogens and decomposers (Baumgartner et al. 2011; Coetzee et al. 2018). In 2010, a gasteroid fungus that is closely related to Armillaria was described as Guyanagaster necrorhiza (Henkel et al. 2010) and together with Desarmillaria, these genera were later assigned to the Armillarioid clade (Koch et al. 2017; Kedves et al. 2021). Phylogenetic reconstruction showed that Guyanagaster species are extant taxa that diverged from early members of the Armillarioid clade when this lineage evolved in Eurasia about 51 million years ago (Koch et al. 2017). During this process, the mushroom ancestor of Guyanagaster underwent a process referred to as gasteromycation leading to the gasteroid species that were only found in the Guiana Shield region (Guyana) up to this point (Henkel et al. 2010; Koch et al. 2017). The close taxonomic relationship of the genera Armillaria and Guyanagaster led to the endeavor to investigate the secondary metabolism of Guyanagaster species for the production of protoilludene-type sesquiterpene aryl esters. Herein, we describe the isolation and structural characterization of 5′-O-methyl-14-hydroxyarmillane (1), a new armillane-type derivative, that was obtained from submerged cultures of G. necrorhiza (CBS 138623) together with the known congeners melleolide G (2), whose configuration we revise, melleolide B (3), and 10-dehydroxy-melleolide B (4).
Results and discussion
Guyanagaster necrorhiza was cultivated in liquid YM6.3 medium. Extraction of the harvested mycelium and culture broth led to extracts that were purified by preparative HPLC. This resulted in the isolation and characterization of the new meroterpenoid 5′-O-methyl-14-hydroxyarmillane (1) that showed a molecular ion cluster at m/z 451.2333 [M+H]+ (calcd. for C24H35O8, 451.2326) in the HRESIMS spectrum revealing the molecular formula C24H34O8. Its 1H and 13C NMR (Table 1) data were highly similar to those of armillane (Donnelly and Hutchinson 1990). The key difference is the methylation of the hydroxy group at C-5′ and the presence of an additional hydroxy group at C-14 for 1. The relative configuration was confirmed by coupling constants and ROESY data: the large coupling constants JH2,H3 = JH3,H13 = 11.0 Hz, observed in the triplet of H-3, indicate that all of these three protons are in axial position on alternating sides of the ring structure. ROESY correlations between H3-15/H-9 and H3-15/H-13, as well as between H2-14 and H-3, H-10α, H-12α confirm an unchanged configuration compared to 5. The absolute configuration was assigned as 2R,3S,4R,5R,7R,9S,11R,13R, which is characteristic for melleoid sesquiterpenoids, since the ECD spectra of 5′-O-methyl-14-hydroxyarmillane (1) and the one of 10-hydroxy-5′-methoxy-6′-chloroarmillane (5) (Mándi and Kurtán 2019) are very similar. Due to the absence of the double bond in the 6-membered ring of the protoilludene backbone, 1 can be assigned to the armillane-type sesquiterpene aryl esters.
Furthermore, with melleolide G (2), melleolide B (3), and 10-dehydroxy-melleolide B (4) three known congeners, previously described from Armillaria spp. in the literature, were isolated from the same batch of submerged G. necrorhiza cultures. While 2 and 3 were reported from cultures of A. mellea (Arnone et al. 1986; Arnone et al. 1988), 4 was obtained from an undefined species Armillaria sp. (Yin et al. 2012). As their names already imply, 2–4 belong to the subclass of melleolides, due to their characteristic double bond localization (Δ2-3-unsaturated). Although our 13C data of 2 is virtually identical to those of Arnone et al. (1988), the assignment of C-2 and C-3 has to be exchanged based on HMBC data (Table S1, Figure S10). More importantly, the published confirmation of 2 is not consistent with the ROESY correlations we observed: while methyl H3-15 shows ROESY correlations to both methines H-9 and H-13, oxymethylene H2-14 has correlations with H-10α, H-12α, and H3-8 (Table S1, Figure S11). Therefore, the stereoconfigurational assignment of C-11 has to be revised, with the oxymethylene (CH2-14) pointing below the main molecular plane.
Protoilludene-type sesquiterpene aryl esters are well known for their broad spectrum of biological activities and several derivatives were reported as antibacterial, antifungal, or cytotoxic (Dörfer et al. 2019). Therefore, the potential bioactivities of compounds 1–4 were evaluated in a serial dilution assay against several yeast, fungi, and bacteria, as well as in a cytotoxicity assay against different cell lines. In the serial dilution assay, only 4 showed moderate activities against B. subtilis, S. aureus, and M. hiemalis (Table 2). The relationship between structures and biological activities of this compound class has not been systematically investigated yet; thus, no conclusions to explain the activities of 4 can be made. Solely the weak or missing cytotoxic effects of 1–4 (Table 3) can be associated with the hydroxylation of C-1, since especially derivatives with an aldehyde function at this position are reported to be the ones with the highest potential for cytotoxicity (Dörfer et al. 2019).
For the first time, protoilludene-type sesquiterpene aryl esters are reported from another genus than Armillaria. Our data confirm previous results describing the genus Guyanagaster as closely related to Armillaria (Henkel et al. 2010; Koch et al. 2017; Kedves et al. 2021).
Material and methods
General experimental procedures
A PerkinElmer 241 polarimeter was used to measure the optical rotation. To record the UV spectra, a Shimadzu UV-Vis spectrophotometer UV-2450 (Duisburg, Germany) was used. NMR spectra were measured with the Bruker Avance III 500-MHz spectrometer (Bremen, Germany) equipped with a BBFO (plus) SmartProbe (1H 500 MHz, 13C 125 MHz) and the Bruker Avance III 700-MHz spectrometer (Bremen, Germany) equipped with a 5-mm TCI cryoprobe (1H 700 MHz, 13C 175 MH). Selected chemical shifts of acetone-d6 (1H: 2.05 ppm, 13C: 29.92 ppm) were used to reference the NMR data. The Agilent 1200 series HPLC-UV system (Santa Clara, CA, USA) in combination with an ESI-TOF-MS (Maxis, Bruker, Bremen, Germany) was used to record HRESIMS data. These measurements were performed with the 2.1 × 50 mm, 1.7 μm, C18 Acquity UPLC BEH (Waters, Milford, MA, USA) column, using MilliQ water + 0.1% formic acid as solvent A and acetonitrile + 0.1% formic acid as solvent B (gradient: 5% B for 0.5 min increasing to 100% B in 19.5 min and maintaining 100% B for 5 min, flow rate: 0.6 mL/min, UV/Vis detection: 200–600 nm).
Fungal material
The culture of G. necrorhiza we studied (original code MCA 3950) was kindly provided by M. Cathie Aime, who also deposited the strain at the Westerdijk Fungal Biodiversity Institute (Utrecht, Netherlands) under the accession number CBS 138623. The strain was previously used in the study by Koch and Aime (2018).
Fermentation of G. necrorhiza and extraction
Forty 500-mL Erlenmeyer shape culture flasks, each of them containing 200 mL of YM6.3 medium (10 g/L malt extract, 4 g/L D-glucose, 4 g/L yeast extract, pH 6.3), were inoculated with three 50-mm2 sized pieces of well-grown mycelium from YM6.3 agar plates per flask. The cultures were incubated on a rotary shaker at 23 °C and 140 min−1. To monitor the consumption of the glucose, test strips (Medi-Test Glucose, Macherey-Nagel, Düren, Germany) were used. After 24 days, the culture broth was tested negative for glucose and 2 days later the cultures were harvested (26 days in total). Mycelium and supernatant were separated by centrifugation at 5100 rpm for 30 min. The mycelium was extracted with acetone in an ultrasonic bath for 30 min, twice. Subsequently, the mycelium was separated from the liquid phase by filtration and the organic solvent was evaporated at 40 °C on a rotary evaporator. Dilution of the remaining aqueous phase with H2O was followed by an extraction against EtOAc (1:1, twice). The obtained organic phase was evaporated to dryness on a rotary evaporator at 40 °C leading to 126 mg mycelial extract. Extraction of the supernatant was carried out against EtOAc (1:1) in a separatory funnel, twice. After phase separation, the organic phase was kept and evaporated to dryness (40 °C) leading to 360 mg of supernatant extract.
Analytical HPLC
The extracts that were obtained from the mycelium and supernatant of G. necrorhiza were dissolved with acetone to yield a concentration of 10 mg/mL. This was followed by a filtration of 100 μL of the solution with Whatman Mini-UniPrep™ syringeless filter devices (cytiva, Marlborough, MA, USA). All samples were analyzed using an analytical HPLC device (Dionex UltiMate 3000 series) coupled to an ion trap mass spectrometer (amazon speed by Bruker). After injection of 2 μL of the samples, the separation was carried out with an ACQUITY-UPLC BEH C18 column (50 × 2.1 mm; particle size: 1.7 μm) by Waters. As mobile phase HPLC grade H2O and MeCN with 0.1% formic acid were used at a flow rate of 600 μL/min. The gradient started at 5% of MeCN, continued with an increase to 100% MeCN in 20 min, and remained at 100% MeCN for 5 min. All obtained chromatograms were analyzed with the software Data Analysis 4.4 (Bruker).
Isolation of compounds 1–4 via reversed phase liquid chromatography
Purification of the obtained extracts was carried out using a Gilson PLC 2250 purification system coupled to a Gemini LC column 250 × 50 mm, 110 Å, 10 μm (Phenomenex, Torrance, CA, USA). HPLC grade H2O + 0.1% formic acid (solvent A) and MeCN + 0.1% formic acid (solvent B) were used as mobile phase at a flow rate of 50 mL/min. The gradient for the purification of the supernatant extract started with isocratic conditions at 15% B for 5 min, followed by an increase to 80% B in 60 min, another increase to 100% B in 5 min and remained at 100% B for 10 min. The fraction at 37.8–38.5 min led to 2.6 mg of 1, the fraction at 45.5–46.5 min led to 1.5 mg of 2, the fraction at 52.0–53.0 min led to 1.1 mg of 3, and the fraction at 60.3–61.0 min led to 0.2 mg of 4. The gradient for the purification of the mycelial extract started with isocratic conditions at 25% B for 5 min, followed by an increase to 100% B in 75 min and remained at 100% B for 10 min. The fraction at 35.0–36.5 min led to 0.5 mg of 2, the fraction at 39.5–41.0 min led to 0.5 mg of 3, and the fraction at 71.0–72.5 min led to 0.5 mg of 4 (Fig. 1).
Spectral data
5′-O-methyl-14-hydroxyarmillane (1): colorless oil; [α]20D = −13 (c 0.1, MeOH); UV/Vis (0.01 mg/mL, MeOH) λmax(logε): 301 (4.30), 265 (4.01), 212 (3.59) nm; ECD (0.25 mg/mL, MeOH) λ(Δε) 302 (1.1), 263 (−1.1), 238 (0.0), 219 (−0.7), 198 (1.8) nm, Fig. 2; 1H and 13C NMR data (acetone-d6), Table 1; ESIMS m/z 451.25 [M+H]+, 449.30 [M-H]−; HRESIMS m/z 451.2333 [M+H]+ (calcd. for C24H35O8, 451.2326); tR = 9.2 min (analytical HPLC).
Evaluation of antimicrobial activity
To determine minimum inhibitory concentrations (MICs) against several yeast, fungi, and bacteria, serial dilution assays in 96-well microtiter plates were performed as described by Harms et al. (2021). Compounds 1–4 were tested against Bacillus subtilis, Staphylococcus aureus, Mycobacterium smegmatis, Escherichia coli, Pseudomonas aeruginosa, Chromobacterium violaceum, Schizosaccharomyces pombe, Candida albicans, Pichia anomala, Mucor hiemalis, and Rhodotorula glutinis.
Evaluation of cytotoxicity
The cytotoxicities of compounds 1–4 were assessed by in vitro assays in 96-well plates as described by Harms et al. (2021). They were tested against the mouse fibroblast cell line L929 and the cervix carcinoma cell line KB3.1.
Conclusions
With 5′-O-methyl-14-hydroxyarmillane (1), a new armillane-type derivative, and the known congeners melleolide G (2), melleolide B (3), and 10-dehydroxy-melleolide B (4), four protoilludene-type sesquiterpene aryl esters were obtained from submerged cultures of G. necrorhiza (CBS 138623). It is the first time that members of this compound class were isolated from cultures of another genus than Armillaria. These results confirmed the close relationship between Armillaria and Guyanagaster leading to the assumption that more congeners, including new derivatives, can be found during further investigations on the secondary metabolism of Guyanagaster species. Therefore, G. necrorhiza (CBS 138623) can be cultivated under different conditions or other specimen can be investigated for their secondary metabolite production.
References
Arnone A, Cardillo R, Modugno VD, Nasini G (1988) Secondary mould metabolites. XXII. Isolation and structure elucidation of melledonals D and E and melleolides E-H, novel sesquiterpenoid aryl esters from Clitocybe elegans and Armillaria mellea. Gazz Chim Ital 118:517–521
Arnone A, Cardillo R, Nasini G (1986) Structures of melleolides B-D, three antibacterial sesquiterpenoids from Armillaria mellea. Phytochemistry 25(2):471–474
Baumgartner K, Coetzee MPA, Hoffmeister D (2011) Secrets of the subterranean pathosystem of Armillaria. Mol Plant Pathol 12:515–534. https://doi.org/10.1111/j.1364-3703.2010.00693.x
Coetzee MPA, Wingfield BD, Wingfield MJ (2018) Armillaria root-rot pathogens: species boundaries and global distribution. Pathogens 7:83. https://doi.org/10.3390/pathogens7040083
Donnelly DMX, Hutchinson RM (1990) Armillane, a saturated sesquiterpene ester from Armillaria mellea. Phytochemistry 29:179–182. https://doi.org/10.1016/0031-9422(90)89033-6
Dörfer M, Gressler M, Hoffmeister D (2019) Diversity and bioactivity of Armillaria sesquiterpene aryl ester natural products. Mycol Prog 18:1027–1037. https://doi.org/10.1007/s11557-019-01508-z
Engels B, Heinig U, McElroy C et al (2021) Isolation of a gene cluster from Armillaria gallica for the synthesis of armillyl orsellinate–type sesquiterpenoids. Appl Microbiol Biotechnol 105:211–224. https://doi.org/10.1007/s00253-020-11006-y
Harms K, Surup F, Stadler M et al (2021) Morinagadepsin, a depsipeptide from the fungus Morinagamyces vermicularis gen. et comb. nov. Microorganisms 9:1191
Henkel TW, Smith ME, Aime MC (2010) Guyanagaster, a new wood-decaying sequestrate fungal genus related to Armillaria (Physalacriaceae, Agaricales, Basidiomycota). Am J Bot 97:1474–1484. https://doi.org/10.3732/ajb.1000097
Kedves O, Shahab D, Champramary S et al (2021) Epidemiology, biotic interactions and biological control of armillarioids in the Northern Hemisphere. Pathogens 10:76. https://doi.org/10.3390/pathogens10010076
Koch RA, Aime MC (2018) Population structure of Guyanagaster necrorhizus supports termite dispersal for this enigmatic fungus. Mol Ecol 27:2667–2679. https://doi.org/10.1111/mec.14710
Koch RA, Wilson AW, Séné O et al (2017) Resolved phylogeny and biogeography of the root pathogen Armillaria and its gasteroid relative. Guyanagaster. BMC Evol Biol 17:33. https://doi.org/10.1186/s12862-017-0877-3
Mándi A, Kurtán T (2019) Applications of OR/ECD/VCD to the structure elucidation of natural products. Nat Prod Rep 36:889–918. https://doi.org/10.1039/C9NP00002J
Yin X, Feng T, Liu J-K (2012) Structures and cytotoxicities of three new sesquiterpenes from cultures of Armillaria sp. Nat Prod Bioprospect 2:245–248. https://doi.org/10.1007/s13659-012-0077-1
Acknowledgements
The authors are grateful for the help of C. Kakoschke and E. Surges by measuring the NMR spectra, as well as for Wera Collisi by performing the bioassays. Furthermore, we wish to thank Aileen Gollasch for recording the HRESIMS spectra. S. Pfütze is grateful for the expert advisory assistance and support of E. Charria, J-P. Wennrich, A. Skiba, and S. Reinecke.
Funding
Open Access funding enabled and organized by Projekt DEAL. S. Pfütze is thankful for a grant from the Life Science-Stiftung zur Förderung von Wissenschaft und Forschung (LSS).
Author information
Authors and Affiliations
Contributions
The study including cultivation, extraction, and compound isolation was conducted by D. Nedder with support from S. Pfütze. Analysis and structure elucidation was conducted by S. Pfütze. The first draft of the manuscript was written by S. Pfütze and revised by F. Surup and M. Stadler. All authors approved the final version.
Corresponding author
Ethics declarations
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Conflict of interest
The authors declare no competing interests.
Additional information
Section Editor: Ji-Kai Liu
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
ESM 1
(PDF 1.15 mb)
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Pfütze, S., Nedder, D.L., Surup, F. et al. 5′-O-methyl-14-hydroxyarmillane, a new armillane-type sesquiterpene from cultures of Guyanagaster necrorhiza. Mycol Progress 22, 70 (2023). https://doi.org/10.1007/s11557-023-01920-6
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11557-023-01920-6