Abstract
Only two Coccoloba-associated xerocomoid boletes with smooth basidiospores are currently known from the Dominican Republic, namely Boletus ruborculus and Xerocomus coccolobae. A multilocus phylogenetic analysis of four gene markers (ITS, LSU, RPB2, TEF1) reveals that B. ruborculus forms an autonomous clade in the Boletaceae corresponding to a novel genus, which is introduced here as Tropicoboletus gen. nov., whereas X. coccolobae is confirmed as a member of Xerocomus s. str. Tropicoboletus is sister to subfamily Xerocomoideae in the combined RPB2/TEF1 Boletaceae-wide analysis. Accurate morphological descriptions of the two species based on well-annotated samples are provided, accompanied by color photographs of fresh specimens in habitat and line drawings of their main anatomical features. The holotype collections of B. ruborculus and X. coccolobae were successfully sequenced and re-examined anatomically. The distribution range of Tropicoboletus ruborculus comb. nov. is extended from the original locality in Puerto Rico to the Dominican Republic and Mexico where its presence is reported for the first time. Similarly, the Dominican collections of X. coccolobae represent the first documented occurrence of this species for the Island of Hispaniola. Based on molecular and morphological evidence, we conclude that the Belizean species Xerocomus olivaceus is conspecific with X. coccolobae and is therefore reduced into synonymy. In addition, the holotypes of Xerocomus caeruleonigrescens, Xerocomus cuneipes, and Xerocomus pseudoboletinus var. pini-caribaeae were microscopically re-studied, although their exact taxonomic placement remains unresolved in the absence of any phylogenetic inference. Molecular investigation of a paratype of Boletus guadelupae resulted in a conspecificity with the recently described Singerocomus atlanticus from Brazil, extending the biogeographic coverage of Singerocomus to the Caribbean. Accordingly, the new combination Singerocomus guadelupae is proposed and S. atlanticus is synonymized. Finally, a putative novel Xerocomus s. str. species is discovered from the Dominican Republic but not formally described for the time being due to the paucity of material available.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Boletes (Boletaceae, Boletales) are one of the largest and most biodiverse groups of basidiomycetes. They play a vital role in establishing ectomycorrhizal (ECM) association with woody plants and their ecological and economic impact have become increasingly important in the last decades (Singer 1986; Watling 2008). The rapid progress of molecular phylogenetic techniques applied to the investigation of boletes has recently led to a radical re-assessment of several genera belonging to the hyperdiverse family Boletaceae, predominantly with respect to some historically established genera such as Boletus Fr., Leccinum Gray, Pulveroboletus Murrill, Tylopilus P. Karsten, Xerocomus Quél., etc., which have all been inferred to constitute artificial assemblages of unrelated species as traditionally circumscribed (Binder 1999; Binder and Hibbett 2006; Drehmel et al. 2008; Nuhn et al. 2013; Wu et al. 2014). Particularly, Boletus and Xerocomus revealed their strong polyphyly, and recently underwent major taxonomic and systematic upsets that facilitated their reciprocal separation and determined a general consensus in their current delimitation (Nuhn et al. 2013; Wu et al. 2014, 2016a, b).
Presently, based on an improved taxonomic resolution derived from advanced molecular phylogenetic inference, Boletus s. l. appears to encompass at least twenty-six autonomous monophyletic lineages at the generic rank, focused on previously defined sections or on single species or species-groups (some of which not formally proposed yet), including (1) Boletus s. str. (= Boletus sect. Boletus, corresponding to the B. edulis Bull. group, otherwise known as porcini mushrooms); (2) Butyriboletus D. Arora & J.L. Frank (= Boletus sect. Appendiculati Konrad & Maublanc emend. Lannoy & Estadès); (3) Caloboletus Vizzini (= Boletus sect. Calopodes Fr. emend. Lannoy & Estadès); (4) Retiboletus Manfr. Binder & Bresinsky (= Boletus sect. Ornatipedes Singer and sect. Grisei Singer); (5) Suillellus Murrill (= B. luridus Schaeffer complex); (6) Rubroboletus Kuan Zhao & Zhu L. Yang (= B. satanas Lenz/B. sinicus W.F. Chiu complex); (7) Imperator G. Koller et al. (= B. torosus Fr./B. rhodopurpureus Smotlacha complex); (8) Neoboletus Gelardi, Simonini & Vizzini (= B. luridiformis Rostk. complex); (9) Imleria Vizzini (= B. badius (Fr.) Fr. complex, corresponding to sect. Pseudoboleti Singer p. p.); (10) Cyanoboletus Gelardi, Vizzini & Simonini (= B. pulverulentus Opat. complex, corresponding to sect. Subpruinosi Fr. p. p.); (11) Baorangia G. Wu & Zhu L. Yang (= B. pseudocalopus Hongo/B. bicolor Peck complex, corresponding to sect. Fragrantes Lannoy & Estadès p. p. and sect. Brevitubi M. Zang p. p.); (12) Lanmaoa G. Wu, Zhu L. Yang & Halling (= B. fragrans Vittad./B. carminipes A.H. Smith & Thiers complex, corresponding to sect. Fragrantes p. p.); (13) Hemileccinum Šutara (= B. impolitus Fr. complex); (14) Parvixerocomus G. Wu & Zhu L. Yang (= B. aokii Hongo complex); (15) Crocinoboletus N.K. Zeng, Zhu L. Yang & G. Wu (= B. rufoaureus Massee complex); (16) Exsudoporus Vizzini, Simonini & Gelardi (= B. permagnificus Pöder /B. frostii J.L. Russell complex); (17) Amoenoboletus G. Wu, E. Horak & Zhu L. Yang (= B. weberi Singer/B. mcrobbii (McNabb) G. Stev. complex); (18) Corneroboletus N.K. Zeng & Zhu L. Yang (= B. indecorus Massee); (19) Cupreoboletus Simonini, Gelardi & Vizzini (= B. poikilochromus Pöder, Cetto & Zuccherelli); (20) B. morrisii Peck; (21) B. abruptibulbus Roody, Both & B. Ortiz; (22) B. lakhanpalii K. Das, D. Chakr., A. Baghela, S.K. Singh & Dentinger; (23) B. durhamensis B. Ortiz, Bessette & McConnell; (24) B. candidissimus T.H.G. Pham, A.V. Alexandrova & O.V. Morozova; (25) B. subsplendidus W.F. Chiu; and (26) Butyriboletus hainanensis N.K. Zeng, Zhi Q. Liang & S. Jiang (B. hainanensis complex) (Binder and Bresinsky 2002; Binder and Hibbett 2006; Halling et al. 2007, 2015; Šutara 2008; Zeng et al. 2012, 2014; Gelardi et al. 2013, 2015; Nuhn et al. 2013; Arora and Frank 2014; Vizzini 2014a, b, c, d, e, f; Vizzini et al. 2014; Wu et al. 2014, 2016a, b, 2021; Zhao et al. 2014a, b; Zhu et al. 2014; Das et al. 2015; Ortiz-Santana et al. 2016; Liang et al. 2016; Crous et al. 2019; Bozok et al. 2020; Biketova et al. 2022; Farid et al. 2021).
The heterogeneous Xerocomus s. l. was in turn split into twelve independent generic lineages based on molecular evidence: (1) Xerocomus s. str. (= X. subtomentosus (L.) Quél. complex, corresponding to Xerocomus sect. Subtomentosi (Fr.) Singer and sect. Pseudophyllopori Singer); (2) Xerocomellus Šutara (= X. chrysenteron (Bull.) Quél. complex, corresponding to Xerocomus sect. Chrysenteri Blum ex Bon, sect. Truncati (A.H. Smith & Thiers) H. Engel & Klofac and sect. Striatulispori Redeuilh); (3) Hortiboletus Simonini, Vizzini & Gelardi (= X. rubellus (Krombh.) Quél. complex); (4) Rheubarbariboletus Vizzini, Simonini & Gelardi (= X. armeniacus (Quél.) Quél. complex, corresponding to Xerocomus sect. Armeniaci H. Engel & Klofac); (5) Pseudoboletus Šutara (= X. parasiticus (Bull.) Quél. complex, corresponding to Xerocomus sect. Parasitici Singer); (6) Alessioporus Gelardi, Vizzini & Simonini (= X. ichnusanus Alessio, Galli & Littini complex); (7) Pulchroboletus Gelardi, Vizzini & Simonini (=X. roseoalbidus Alessio & Littini complex); (8) Hourangia Xue T. Zhu & Zhu L. Yang (= X. cheoi (W.F. Chiu) F.L. Tai complex); (9) Singerocomus T.W. Henkel & M.E. Smith (= X. inundabilis Singer complex); (10) Neotropicomus A.C. Magnago, Alves-Silva & T.W. Henkel (= X. parvogracilis T.W. Henkel & Husbands complex); (11) X. porophyllus T.H. Li, W.J. Yan & Ming Zhang; and (12) X. cyaneibrunnescens T.W. Henkel & Husbands (Binder 1999; Taylor et al. 2001, 2006; Peintner et al. 2003; Binder and Hibbett 2006; Šutara 2008; Gelardi et al. 2013, 2014; Nuhn et al. 2013; Osmundson et al. 2013; Yan et al. 2013; Wu et al. 2014, 2016b; Zhu et al. 2015; Ariyawansa et al. 2016; Crous et al. 2016, 2019; Das et al. 2016; Henkel et al. 2016; Farid et al. 2017, 2021; Frank et al. 2017, 2020; Loizides et al. 2019; Naseer et al. 2019; Xie et al. 2020; Magnago et al. 2022). Furthermore, an additional generic xerocomoid lineage might be represented by the X. brasiliensis (Rick) Singer complex (corresponding to Xerocomus sect. Brasilienses Singer) (Singer 1986; Watling 2008).
It appears clear, however, that additional monophyletic clades are expected to be uncovered as soon as supplementary molecular investigation of poorly known or critical boletoid and xerocomoid taxa will become available in the near future, especially from remote and underexplored areas of the pantropical belt.
Although it has not been as thoroughly investigated as North America and Europe, Mesoamerica has been the object of a large number of mycological contributions published in the past decades which have enhanced our understanding of the neotropical boletes diversity and biogeography with a special emphasis on boletoid or xerocomoid taxa, underpinning the health and functioning of different ecosystems in Mexico, mainland Central America, northern South America, and the Caribbean (e.g., Singer and Fiard 1977; Pegler 1983; Singer et al. 1983; Singer 1988; Halling 1989, 1992, 1997; Gonzáles-Velázquez and Valenzuela 1993; Gómez 1997; Halling and Mueller 1999, 2002, 2005; García-Jiménez 1999, 2013; Miller et al. 2000; Minter et al. 2001; Halling and Mata 2004; Flores Arzù and Simonini 2000; Franco-Molano et al. 2000; Halling et al. 2004; García-Jiménez 2013; García-Jiménez et al. 2013; Ortiz-Santana et al. 2007; Courtecuisse and Welti 2013; de la Fuente et al. 2018; Flores Arzù 2020). However, most of the neotropical bolete species described to date have been defined based solely on morphological, ecological, or biochemical taxonomic criteria and are in urgent need of phylogenetic reconsideration. Moreover, identification efforts are becoming rather more difficult because several Central American species show a wider distribution throughout the neotropics than previously assumed.
Two xerocomoid smooth-spored ECM bolete species associated with Coccoloba (Polygonaceae), namely Boletus ruborculus T.J. Baroni (Miller et al. 2000) and Xerocomus coccolobae Pegler (Pegler 1983), were originally described from the Greater and Lesser Antilles of the Caribbean, respectively. In order to consolidate the taxonomic concept of these two neotropical species, we carefully studied several collections for each species. Furthermore, the DNA of the original material of both species was sequenced for the first time and the holotype specimens re-examined anatomically. In the light of the obtained outcomes, Tropicoboletus is described as a new genus to science to accommodate B. ruborculus, whereas the generic affiliation of X. coccolobae in Xerocomus s. str. is corroborated by its phylogenetic placement. Furthermore, type specimens of additional neotropical species, including Xerocomus pseudoboletinus var. pini-caribaeae Singer, Xerocomus cuneipes Pegler, Xerocomus caeruleonigrescens Pegler, and Boletus guadelupae Singer & Fiard were anatomically re-studied, and the latter species was also phylogenetically investigated.
Materials and methods
Collection site and sampling
Specimens examined were collected in Sosúa, Puerto Plata Province, Dominican Republic and El Morro, Viejo San Juan, Puerto Rico. Dominican Republic samples are deposited at the Herbarium of Jardín Botánico Nacional of Santo Domingo, Dr. Rafael Ma. Moscoso, Dominican Republic (JBSD). The holotype collection of Xerocomus pseudoboletinus var. pini-caribaeae and a mixed type (holotype/paratype) of Boletus guadelupae examined in the present study are deposited at the Field Museum of Natural History, Chicago (F), the holotype of Boletus ruborculus is deposited at the New York Botanical Garden (NYBG), while the holotypes of Xerocomus coccolobae, X. cuneipes, and X. caeruleonigrescens and paratype specimens of B. guadelupae are all deposited at the Fungarium of the Royal Botanic Gardens Kew (K-M) (acronyms from Thiers 2022). “ANGE,” “MG,” “komille,” and “de la Fuente” refer to the personal herbarium of Claudio Angelini, Matteo Gelardi, Kurt O. Miller, and J.I. de la Fuente, respectively. Fungarium numbers, unless otherwise stated, are cited for all collections from which morphological features were examined. Author citations follow the Index Fungorum, Authors of Fungal Names (www.indexfungorum.org/authorsoffungalnames.htm). Geographic distribution of some studied species have also been checked on MyCoPortal (https://mycoportal.org).
Morphological studies
Macroscopic descriptions, macrochemical reactions (30% NH4OH, 30% KOH), and ecological information, such as habitat notations, time of fruiting, and associated plant communities, accompanied the detailed field notes of the fresh basidiomes. In the field, latitude, longitude, and elevation were determined with a global positioning system (GPS) receiver. Color terms in capital letters (e.g., White, Plate LIII) are from Ridgway (1912). Photographs of collections were taken in the natural habitat using a Nikon Coolpix 8400 camera. Microscopic anatomical features were observed and recorded from revived dried material; sections were rehydrated either in water, 5% potassium hydroxide (KOH), or in ammoniacal Congo red. All anatomical structures were measured from preparations in anionic Congo red. Colors and pigments were described after examination in water and 5% KOH. Measurements were made at 1000 × using a calibrated ocular micrometer (Nikon Eclipse E200 optical light microscope). Basidiospores were measured directly from the hymenophore of mature basidiomes, dimensions are given as (minimum) average ± standard deviation (maximum), Q = length/width ratio with the extreme values in parentheses, Qm = average quotient (length/width ratio) ± standard deviation and average spore volume was approximated as a rotation ellipsoid [V = (π.L.W2)/6 ± standard deviation]. The notation [n/m/p] indicates that measurements were made on “n” randomly selected basidiospores from “m” basidiomes of “p” collections. The width of each basidium was measured at the widest part, and the length was measured from the apex (sterigmata excluded) to the basal septum. Radial and/or vertical sections of the pileipellis were taken midway between the center and margin of the pileus. Sections of the stipitipellis were taken from the middle part along the longitudinal axis of the stipe. Metachromatic, cyanophilic, and iodine reactions were tested by staining the basidiospores in Brilliant Cresyl blue, Cotton blue, and Melzer’s reagent, respectively. Line drawings of microstructures were traced in free hand based on digital photomicrographs of rehydrated material.
The basidiospores of selected collections (Tropicoboletus ruborculus NY 577594 and JBSD133073, Xerocomus coccolobae K-M000178954 and JBSD133071, X. cuneipes K-M000178953, X. caerulonigrescens K-M000178955, Singerocomus guadelupae K-M000193859, and K-M000193867) were also observed under a Zeiss Ultra-Plus VP FEG-SEM scanning electron microscope (SEM), equipped with Oxford X-Max 80 mm2 SDD detector and operated at 2 kV, and a FEI Quanta 650 FEG operated at 5 kV.
DNA extraction, PCR amplification, and DNA sequencing
Genomic DNA for all samples, except for the K-M, NYBG and F specimens, was isolated from 25 mg of dried voucher specimens. DNA extraction and PCR amplification were performed as described by Alvarado et al. (2012). The universal primer pairs ITS1F/ITS4 (White et al. 1990; Gardes and Bruns 1993) and LR0R/LR5 (Vilgalys and Hester 1990; Cubeta et al. 1991) were used for the amplification of the internal transcribed spacer (ITS) and the nuclear large ribosomal subunit (LSU) regions of the nrDNA, respectively. The 6–7 region of the RPB2 gene (RNA polymerase II second largest subunit) was amplified using the primer pairs brpb2-6F2/brpb2-7R2 (Matheny et al. 2002, 2007). Primers EF1-983F and EF1-1567R (Rehner and Buckley 2005) were used for amplification of the translation elongation factor 1-α (TEF1) gene. The PCR products were purified with the Wizard SV Gel and PCR Clean-Up System (Promega, Madison, WI) following manufacturer’s instructions and positive reactions sequenced forward and reverse by MACROGEN Inc. (Seoul, Republic of Korea).
For the K-M, genomic DNA was extracted following an enzymatic digestion and glass-fiber filtration protocol (Dentinger et al. 2010) and for NYBG and F specimens using the NucleoSpin™ Plant II kit. Amplification of the ITS region was performed following standard conditions and using several primer combinations: ITS1F with ITS4B/ITS4/ITS2, ITS3 with ITS4B/ITS4, and ITS8F with ITS6R (White et al. 1990; Gardes and Bruns 1993; Dentinger et al. 2010). Successful amplicons were purified using ExoSAP-IT (USB) and sequenced bidrirectionally using BigDye3.1 in a ABI3730 DNA analyzer (Applied Biosystems).
All newly generated sequences were submitted to GenBank (https://www.ncbi.nlm.nih.gov/genbank/) and their accession numbers are reported in the text, Tables 1, 2, 3, and 4, and Suppl. Mat. Figure 2.
Sequence alignment, dataset assembly, and phylogenetic analysis
Newly generated sequences and sequences retrieved from public databases, GenBank (https://www.ncbi.nlm.nih.gov/genbank/) and UNITE (https://unite.ut.ee/), were combined for phylogenetic reconstructions.
Sequences obtained in this study were checked and assembled using Geneious R11 v.11.1.5 (https://www.geneious.com) and Sequencher 5.4.6 (https://www.genecodes.com/) programs and preliminary identified using the BLASTn algorithm (Altschul et al. 1990) in GenBank and UNITE. Based on the BLASTn results and recent phylogenetic studies focused on family Boletaceae (e.g., Nuhn et al. 2013; Wu et al. 2014, 2016a, b; Gelardi et al. 2015; Henkel et al. 2016; Vadthanarat et al. 2019, 2022; Badou et al. 2022; Magnago et al. 2022), different datasets were constructed with sequences retrieved from GenBank for comparative phylogenetic analysis for each DNA region (Tables 1, 2, 3 and 4). Alignments were generated for ITS, LSU, RPB2, and TEF1 datasets using MAFFT (Katoh et al. 2002) with default conditions for gap openings and gap extension penalties and manually adjusted with Geneious R11 v.11.1.5 (https://www.geneious.com). The best-fit models were estimated by the Akaike information criterion (AIC) and the Bayesian information criterion (BIC) using jModelTest v. 2.1.7 (Darriba et al. 2012) to provide a substitution model for each single alignment.
Four different datasets were assembled to elucidate the phylogenetic placement of the targeting taxa, viz. new genus Tropicoboletus and the species Xerocomus coccolobae and Boletus guadelupae. The first dataset focused on family Boletaceae and was generated from combined RPB2 and TEF1 sequences (Table 1). K80 + I + G and TPM1uf + I + G models were chosen for RPB2 and TEF1 alignments, respectively. The second and third datasets focused on the genus Xerocomus s. str. (LSU sequences, GTR + I + G model), and on X. coccolobae and allied species (ITS sequences, TrN + I + G model) (Tables 2 and 3). The fourth dataset focused on Boletus guadelupae and allied species in Singerocomus based on ITS sequences (Table 4), using TPM3uf + G as the best fit model.
For each molecular marker (LSU, RPB2, and TEF1), three extra single-locus alignments focused on Boletaceae were generated by retrieving all available sequences from GenBank (Suppl. Mat. Figures 2-4). GTR + I + G, TIM1 + I + G and TrN + I + G models were chosen for LSU, RPB2, and TEF1, respectively. The lack of sequences from different loci for the same vouchers prevented us from generating a multilocus analysis.
Phylogenetic analyses were performed using maximum likelihood (ML) with RAxML-NG v. 1.0.1 (Kozlov et al. 2019) and Bayesian Inference (BI) with MrBayes v. 3.2.7a (Ronquist et al. 2012) in the CIPRES science gateway (Miller et al. 2010). ML analyses were performed with 1000 bootstrap replicates (Felsenstein 1985), under the selected evolutionary models to obtain estimates for maximum likelihood bootstrap values (MLB). BI analyses were performed with one cold and three incrementally heated simultaneous Monte Carlo Markov chains (MCMC) run for 10 M generations, under the selected evolutionary models for each unlinked partition. Two simultaneous runs were performed independently. Trees were sampled every 1000 generations, resulting in sampling of 10,001 trees per single run with the first 2500 trees (25%) discarded as burn-in. For the remaining trees of the two independent runs, a majority rule consensus tree showing all compatible partitions was computed to obtain estimates for Bayesian posterior probabilities (BPP). Significance threshold was set ≥ 0.70 for MLB and ≥ 0.90 for BPP. For all phylogenetic trees, outgroup taxa are indicated in the legend. Pairwise percent identity values (P%I) of the ITS sequences were calculated using Geneious. Alignments are available as Suppl. Mat. Align. 1–4.
Results
Molecular analysis
A total of 33 sequences (12 ITS, 9 LSU, 7 RPB2, and 5 TEF1) from 12 specimens were newly generated during this study.
We obtained complete ITS sequences from three collections of Boletus guadelupae, K-M000193866, K-M000193867, and J.P. Fiard 563A/563B (F, holotype/paratype), the holotype of Boletus ruborculus NY 577594 (8253 T.J. Baroni) and an ITS1 sequence from the holotype of Xerocomus coccolobae K-M000178954. The holotypes of Xerocomus cuneipes K-M000178953 and X. caeruleonigrescens K-M000178955 did not yield any amplification.
Both Bayesian and ML analyses produced the same topologies. Therefore, only the ML trees with both MLB and BPP values are shown (Figs. 1, 2, 3, and 4). The combined RPB2/TEF1 Boletaceae-wide data matrix comprises 285 sequences and is 2067 bp long (Fig. 1, Suppl. Mat. Figure 1 and Table 1). The LSU data matrix of the genus Xerocomus comprises a total of 48 sequences and 865 characters (Fig. 2 and Table 2). The ITS data matrix of X. coccolobae and allied species comprises 22 sequences and 833 characters (Fig. 3 and Table 3). The ITS data matrix of B. guadelupae and allied species (Singerocomus) comprises 29 sequences and 870 characters (Fig. 4 and Table 4).
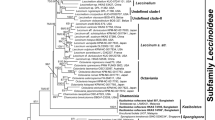
Boletaceae-wide Maximum Likelihood phylogenetic tree inferred from a two-gene dataset (RPB2 and TEF1), showing placement of the new genus Tropicoboletus. Maximum likelihood bootstrap support values (MLB ≥ 0.70) and the corresponding Bayesian posterior probabilities (BPP ≥ 0.90) are shown above the supported branches. Buchwaldoboletus lignicola and seven Chalciporus species (subfamily Chalciporoideae) were used as the outgroup taxa. All taxa belonging to subfamilies Austroboletoideae, Boletoideae, Chalciporoideae, Leccinoideae, and Zangioideae were collapsed into subfamily clades. All generic clades in the “Pulveroboletus group” that were highly supported were also collapsed. In the subfamily Xerocomoideae clade, Xerocomus s. str. was not collapsed to highlight the position of X. coccolobae and X. aff. coccolobae. Newly generated sequences are indicated in bold
Xerocomus s. str. Maximum Likelihood phylogenetic tree inferred from the LSU dataset, showing the clade of X. coccolobae and the undescribed species X. aff. coccolobae (JBSD133067). Maximum likelihood bootstrap support values (MLB ≥ 0.70) and the corresponding Bayesian posterior probabilities (BPP ≥ 0.90) are shown above the supported branches. Phylloporus pelletieri (JQ967215) and P. rubeolus (NG_042667) were used as the outgroup taxa. Newly generated sequences are indicated in bold
Selected Xerocomus s. str. species Maximum Likelihood phylogenetic tree inferred from the ITS dataset, showing the position of X. coccolobae in the genus. Maximum likelihood bootstrap support values (MLB ≥ 0.70) and the corresponding Bayesian posterior probabilities (BPP ≥ 0.90) are shown above the supported branches. Xerocomus chrysonemus (DQ066380) was used as the outgroup taxon. Newly generated sequences are indicated in bold
Singerocomus (Pulveroboletus group) Maximum Likelihood phylogenetic tree inferred from the ITS dataset, showing the phylogenetic conspecificity of the taxa Boletus guadelupae and Singerocomus atlanticus. Maximum Likelihood Bootstrap support values (MLB ≥ 0.70) and the corresponding Bayesian posterior probabilities (BPP ≥ 0.90) are shown above the supported branches. Butyriboletus species, Rubroboletus species, and Bothia castanella (DQ867114) were used as the outgroup taxa. Newly generated sequences are indicated in bold
In the combined RPB2/TEF1 Boletaceae-wide analysis (Fig. 1, Suppl. Mat. Figure 1), the subfamilies recognized in recent studies (e.g., Wu et al. 2014; Gelardi et al. 2015; Henkel et al. 2016; Vadthanarat et al. 2019, 2022; Badou et al. 2022) were also recovered. Xerocomus coccolobae and X. aff. coccolobae are nested in the genus Xerocomus s. str. (typified with X. subtomentosus). Sequences of X. olivaceus from Belize and USA (Florida) (including the holotype NR_175148) cluster with X. coccolobae in the same terminal clade (Figs. 2 and 3). The ITS P%I value of the “coccolobae clade” is = 99.7. Collections of B. ruborculus from Puerto Rico, the Dominican Republic and Mexico form a strongly supported clade which is sister to subfamily Xerocomoideae in the combined RPB2/TEF1 Boletaceae-wide analysis (with only MLB support) and in the single-locus TEF1 Boletaceae-wide analysis (MLB = 0.77) (Suppl. Mat. Figure 4), whereas the clade occupies an unresolved, uncertain position both in the single LSU and RPB2 loci of the Boletaceae-wide analyses (Suppl. Mat. Figures 2 and 3). The ITS P%I value of the B. ruborculus sequences (JBSD133072-ANGE208 GB acc. n. OQ108295, JBSD133073-ANGE209 GB acc. n. OQ108296, JBSD133074-ANGE1406 GB acc. n. OQ108297, MO439745-komille277 GB acc. n. OQ108298, and NY 577594-TJB 8253 GB acc. n. OQ108299 holotype) is = 99.9.
The ITS sequences of three B. guadelupae collections (K-M000193866, K-M000193867, and J.P. Fiard 563A/B, holotype/paratype) cluster together with two Xerocomus sp. from Guyana and four Singerocomus atlanticus from Brazil (including the holotype KY907177) forming the “guadelupae clade” (Fig. 4). Sequences in this clade share a P%I value of 99.4.
Taxonomy
Xerocomus coccolobae Pegler, Kew Bulletin Additional Series 9: 576. 1983. Figures 5a–d, 6, and 10a, b.
MycoBank MB 109285
= Xerocomus olivaceus B. Ortiz & T.J. Baroni, Fungal Diversity 27(2): 382. 2007.
Holotype: Lesser Antilles, Martinique, Morne Aca, on forest floor under Coccoloba sp., 26 Aug 1977, leg. J.P. Fiard, K-M000178954 (J.P. Fiard 902A).
Basidiomes small to medium-small. Pileus (1.5–) 1.9–7.0 (–8.2) cm broad, at first hemispherical then persistently convex and finally broadly pulvinate-flattened, sometimes slightly depressed at center, regularly to unevenly shaped by shallow depressions, moderately fleshy, firm at the beginning but progressively softer with age, flabby in old basidiomes; margin even to faintly wavy-lobed, initially slightly involute then curved downwards and finally completely plane or even uplifted, shortly appendiculate and extending beyond the tubes up to 1 mm; surface matt, dry, finely velvety or granulose to coarsely and densely granulose in all developmental stages, usually not cracked but sometimes areolate at maturity and especially in dry weather conditions and then showing a cream color in the cracks (Light Green-Yellow, Green-Yellow, Greenish Yellow, Pl. V); evenly dark brown, bay brown, chestnut brown or purplish brown to less frequently brownish olive to pale brown (Vinaceous-Rufous, Hay’s Russett, Kaiser Brown, Hazel, Liver Brown, Pl. XIV; Pansy Purple, Pl. XII; Dresden Brown, Mars Brown, Pl. XV; Dark Mineral Red, Pl. XXVII; Cacao Brown, Pl. XXVIII; Buffy Olive, Pl. XXX; Fawn Color, Wood Brown, Pl. XL; Light Cinnamon-Drab, Cinnamon-Drab, Pl. XLVI); unchangeable on handling or touching or when injured; subpellis layer cream yellowish (Light Green-Yellow, Green-Yellow, Greenish Yellow, Pl. V). Tubes wide at first in side view then increasingly broader with age and as long as or slightly longer or shorter than the thickness of the pileus context (up to 1.4 cm long), adnate but soon depressed around the stipe apex and decurrent with a tooth, very bright yellow then olive yellow to ochraceous yellow (Lemon Chrome, Light Cadmium, Pl. IV; Wax Yellow, Primuline Yellow, Pl. XVI; Light Viridine Yellow, Greenish Yellow, Green-Yellow, Bright Green Yellow, Viridine Yellow, Oil Yellow, Pl. V; Mustard Yellow, Primuline Yellow, Pl. XVI) at maturity, unchangeable to erratically but moderately to strongly bluing (Paris Blue, Patent Blue, Pl. VIII) when cut, particularly in aged specimens. Pores initially forming a flat or concave surface, later irregularly shaped to slightly convex, broad at first then gradually wider with age (up to 2 mm in diam.), simple, firstly labyrinthine to roundish becoming prominently angular at maturity, stretched and radially arranged towards the stipe, concolorous with the tubes and unchangeable or irregularly bluing (Paris Blue, Patent Blue, Pl. VIII) on bruising or when injured, sometimes with scattered rusty brown (Ferruginous, Pl. XIV; Mikado Brown, Pl. XXIX) stains at the orifice in aged specimens. Stipe (3.0–) 3.5–5.5 (–7.8) × 0.4–1.6 (–2.0) cm, as long as or slightly longer or shorter than the pileus diameter at maturity, central to slightly off-center, solid, firm, dry, straight or curved to occasionally sinuous, cylindrical, subcylindrical to gradually and faintly enlarged or attenuated from apex down to the base, usually ending with a short taproot at the very base; surface finely to coarsely granulose throughout, with granules more densely arranged in the upper half, devoid of reticulum or ribs, evelate; entirely ornamented by orange-brown, pale brown, brownish olive to chestnut brown granules (Carnelian Red, Vinaceous-Rufous, Cinnamon-Rufous, Hazel, Pl. XIV; Cacao Brown, Pl. XXVIII; Buffy Olive, Pl. XXX; Fawn Color, Wood Brown, Pl. XL; Light Cinnamon-Drab, Cinnamon-Drab, Pl. XLVI) on a whitish background (White, Pl. LIII) and unfrequently with a narrow purplish brown band (Pansy Purple, Pl. XII) in the upper fourth, unchangeable when pressed; basal mycelium whitish (White, Pl. LIII). Context firm and tough when young, later soft textured and eventually flabby in the pileus (up to 2.2 cm thick in the central zone, up to 1.4 cm thick halfway to margin and gradually becoming thinner towards the edge), a little more fibrous in the stipe, at first cream yellowish throughout (Baryta Yellow, Martius Yellow, Picric Yellow, Pl. IV), later very pale yellowish to whitish (Pale Viridine Yellow, Light Green-Yellow, Green-Yellow, Greenish Yellow, Pl. V; White, Pl. LIII) in the pileus and in the connection zone with the stipe, whitish in the stipe (White, Pl. LIII), rarely with scattered pinkish, purplish pink, or pinkish vinaceous spots (Amaranth Purple, Aster Purple, Pansy Purple, Pl. XII), often pale brownish to dirty brown (Citrine, Dark Citrine, Pl. IV; Isabella Color, Light Brownish Olive, Pl. XXX) at the very base; unchangeable to slowly and faintly turning pale blue (Beryl Blue, Pallid Methyl Blue, Pale Methyl Blue, Light Methyl Blue, Pl. VIII) in the pileus context and in the connection zone with the stipe when exposed to air, unchangeable elsewhere; brownish (Auburn, Pl. II; Umber Brown, Pl. III) where eroded by maggots, cream yellowish where eaten by slugs (Light Green-Yellow, Green-Yellow, Greenish Yellow, Pl. V); subhymenophoral layer cream yellowish to pale yellowish (Baryta Yellow, Martius Yellow, Picric Yellow, Pl. IV; Light Green-Yellow, Green-Yellow, Greenish Yellow, Pl. V); exsiccate pale ochraceous on the context, brownish elsewhere (Clay Color, Tawny-Olive, Saccardo’s Umber, Pl. XXIX). Odor indistinct. Taste mild. Spore print not obtained. Macrochemical spot-test reactions: 30% KOH: bright orange to vinaceous red on pileus surface, pale orange to reddish orange on context and hymenophore; 30% NH4OH: vinaceous red on pileus surface, none elsewhere.
Basidiospores [171/9/4] (8.3–) 10.7 ± 0.9 (–14.5) × (4.1–) 4.9 ± 0.2 (–5.8) μm, Q = (1.84–) 1.86–2.55 (–2.78), Qm = 2.19 ± 0.16, V = 136 ± 22 μm3, fairly variable in dimension and shape, inequilateral, cylindrical to ellipsoid or broadly ellipsoid, exceptionally nearly ovoid to allantoid in side view, ellipsoid to broadly ellipsoid in face view, smooth under light microscope and SEM, apex rounded, with a short apiculus, usually with a shallow suprahilar depression and with a slightly pronounced adaxial swelling, moderately thick-walled (0.3–0.5 μm), bright yellow colored in water and honey yellow in 5% KOH, having one or less frequently two or three large oil droplets when mature, rarely pluri-guttulate, inamyloid, acyanophilic and staining blue (orthochromatic reaction) in Cresyl blue. Basidia (25–) 28–49 (–57) × 10–14 μm (n = 34), cylindrical-clavate to clavate, moderately thick-walled (0.5–0.8 μm), predominantly 4-spored but rarely also 2-spored, usually bearing relatively short sterigmata (2–5 μm), hyaline to pale yellowish and sometimes containing straw-yellow oil guttules in water and 5% KOH, bright yellow (inamyloid) in Melzer’s, without basal clamps; basidioles cylindrical-clavate to clavate, similar in size to basidia. Cheilocystidia (40–) 42–68 (–70) × 6–11 (–13) μm (n = 34), very common, decidedly slender, projecting straight to sometimes flexuous, mostly fusiform but also irregularly cylindrical or subcylindrical to sublageniform, rarely showing a narrow and long neck, sometimes multiseptate, with rounded to subacute tip, smooth, moderately thick-walled (0.5–1.0 μm), hyaline to pale yellowish in water and 5% KOH, bright yellow (inamyloid) in Melzer’s, without epiparietal encrustations. Pleurocystidia (33–) 42–96 (–106) × (5–) 7–14 μm (n = 28), frequent, cylindrical, or subcylindrical to more frequently elongate fusiform or lageniform, rarely showing a narrow and long neck, sometimes multiseptate, longer and slightly broader than but similar in color and chemical reactions to cheilocystidia. Pseudocystidia not observed. Pileipellis a trichoderm consisting of moderately to strongly interwoven, frequently branched hyphae which become in the outermost layer a palisadoderm or physalo-palisadoderm of erect subparallel chains of short to moderately slender and restricted at septa, cylindrical hyphae (cylindrocytes), tending to be repent with age and thus turning into a cutis not embedded in gelatinous matter; terminal elements (13–) 15–64 (–82) × 5–24 μm, short cylindrical or irregularly subcylindrical, peanut-shaped, acorn-shaped or bullet-shaped to more frequently cystidioid or elongated lanceolate and then progressively tapering toward the tip, apex rounded-obtuse to pointed, moderately thick-walled (up to 1 μm), hyaline to pale yellowish in water and 5% KOH, mostly smooth but some cells with a scattered but pronounced zebra-like epiparietal brownish pigment in water which tends to be solved in KOH, inamyloid in Melzer’s; subterminal elements mostly short cylindrical, size and color similar to terminal elements. Stipitipellis a layer of slender, parallel to loosely intermingled and longitudinally running, smooth walled, adpressed hyphae, 3–12 μm wide, hyaline to very pale yellowish in water and 5% KOH; the stipe apex beset by interspersed tufts caulohymenial elements consisting of sterile caulobasidioles, sparse, predominantly 4- and 2-spored, fertile caulobasidia, (28–) 32–35 (–45) × 10–12 μm, sterigmata 2–4 μm long (n = 6) and abundant projecting mostly fusiform to sublageniform but also subcylindrical to mucronate caulocystidia similar in color to hymenial cystidia but distinctly shorter, (27–) 32–40 (–45) × 6–10 μm (n = 10), having a wall up to 0.5 μm thick. Lateral stipe stratum under the caulohymenium absent. Stipe trama composed of confusedly and densely arranged, subparallel to moderately interwoven, filamentous, smooth, inamyloid hyphae, 3–22 μm broad. Hymenophoral trama bilaterally divergent of the “Phylloporus-type,” with very slightly divergent to nearly parallel and tightly arranged, non-gelatinous hyphae (lateral strata hyphae in transversal section touching or almost touching each other, 0–5 μm apart, 3–16 μm broad), hyaline to very pale yellowish in water and 5% KOH, inamyloid in Melzer’s; lateral strata (20–) 25–40 (–50) μm thick, mediostratum (10–) 15–30 (–40) µm thick, axially arranged, consisting of a tightly adpressed, non-gelatinous bundle of hyphae, 3–10 µm broad; in Congo Red the mediostratum is concolorous with the lateral strata. Thromboplerous hyphae relatively frequent, thick-walled, rarely septate, melanized, with a golden yellow to brownish homogeneous content in 5% KOH. Clamp connections absent in all tissues. Ontogenetic development gymnocarpic.
Edibility unknown.
Ecology and phenology: solitary to gregarious, growing on limestone among litter in a seasonally dry and moist anthropogenic lowland mixed stand under a large array of neotropical broadleaved trees including Coccoloba diversifolia (Polygonaceae), which represent its potential ECM host plant. See Parra et al. (2018) for further details on lowland vegetation in the Dominican Republic. Apparently localized in the Dominican Republic. November to December.
Known distribution: Reported to date from both the Lesser and Greater Antilles of the Caribbean (Cuba, Dominican Republic, British Virgin Islands, Martinique), south-eastern USA (Florida), and Mexico but likely widespread in Mesoamerica. Its occurrence in Brazil appears to be unlikely (see discussion below).
Examined material: DOMINICAN REPUBLIC, Municipality of Sosúa, Puerto Plata Province, loc. cemetery, three km away from the seaside, 19°44′40′′N 70°32′21′′W, 100 m, 12 Dec 2017, a single aged specimen, C. Angelini, JBSD133068 (ANGE915, MG811); same loc. 01 Dec 2017, four young to mature specimens, C. Angelini, JBSD133069 (ANGE965, MG812); same loc., 16 Dec 2019, a single mature specimen, C. Angelini, JBSD133070 (ANGE1392, MG813); same loc., 14 Dec 2019, three mature specimens, C. Angelini (collection lost); same loc., 16 Dec 2019, five young to mature specimens, one of which growing on an abandoned termites nest, C. Angelini (collection lost); same loc., 23 Nov 2020, several mature specimens, C. Angelini, JBSD133071 (ANGE1405, MG849); MARTINIQUE, Morne Aca, on forest floor under Coccoloba sp., 26 Aug 1977, J.P. Fiard, K-M000178954 (J.P. Fiard 902A, holotype).
Additional examined material: Xerocomus aff. coccolobae: Dominican Republic, Municipality of Sosúa, Puerto Plata Province, loc. cemetery, three km away from the seaside, 19°44′40′′N 70°32′21′′W, 100 m, 26 Dec 2014, a single tiny young specimen, C. Angelini, JBSD133067 (ANGE446, MG810). Xerocomus pseudoboletinus var. pini-caribaeae: BELIZE, Augustine Forest Station, 500 m, under Pinus caribaea, 15 Jun 1976, T.H. Ivory, F0002163C (Ivory S-101, holotype). Xerocomus cuneipes: MARTINIQUE, Basse Pointe, 50 m, under Coccoloba uvifera, 17 Aug 1976, J.P. Fiard, K-M000178953 (J.P. Fiard 710B, holotype).
Notes: We have successfully obtained DNA sequences from the holotype material of X. coccolobae (three dried mature specimens), originally found by J.P. Fiard in Martinique and currently preserved at the Royal Botanical Gardens Kew, K-M000178954 (J.P. Fiard 902A) (Fig. 8a) and the anatomical revision of the original specimen produced the following results: basidiospores ellipsoid in side view, smooth under light microscope and SEM (Fig. 10a, b), with a suprahilar depression, apex rounded, golden-yellow, (8.3–) 10.1 ± 0.9 (–11.4) × (4.3–) 4.7 ± 0.2 (–5.2) μm, Q = (1.77–) 1.99–2.31 (2.56), Qm = 2.15 ± 0.16, Vm = 119 ± 16 μm3 [32/3/1]; basidia cylindrical-clavate to clavate, (19–) 22–32 (–35) × 8–13 μm (n = 10), sterigmata 2–3 μm long; cheilocystidia fusiform to lageniform, (24–) 29–49 (–55) × 6–12 μm (n = 9); pleurocystidia fusiform, (30–) 38–52 (–57) × 7–11 μm (n = 10); trichodermal pileipellis of interwoven cylindrical to broadly cylindrical, ocher-yellow to ocher-brown in mass, mainly encrusted hyphae, terminal elements cylindrical but tapering at apex or lageniform, (15–) 21–41 (–50) × (4–) 6–11 (–13) μm (n = 34).
With the only exception of the length of hymenial cystidia which appear to be decidedly longer in the Dominican material of X. coccolobae when compared with either the protologue or the type revision, the entire overlapping of the remnant morphological, ecological and biogeographic traits of the Dominican Republic collections with the original material described from Martinique by J.P. Fiard (Pegler 1983) coupled with the phylogenetic outcomes allow us to undoubtedly attribute them to the same species. Moreover, the ITS sequence generated from the type material of X. coccolobae perfectly match those obtained from the Dominican material, thus confirming their conspecificity. Apart from a nil macrochemical reaction on external surfaces with NH4OH, there is no other sound morphological or ecological difference, nor molecular evidence for considering Xerocomus olivaceus B. Ortiz & T.J. Baroni (Ortiz-Santana et al. 2007) a different species with respect to X. coccolobae. The ITS sequence of the holotype material of X. olivaceus clearly nested within the terminal clade of X. coccolobae and therefore we merge the Belizean bolete into X. coccolobae as a later heterotypic synonym.
Key determining features of X. coccolobae include small to medium-small sized basidiomes, finely to coarsely granulose, brownish to dark brown or less frequently chestnut brown to purplish brown pileus and stipe surfaces, bright yellow olive tubular hymenophore, whitish basal mycelium, pale yellowish to whitish context usually unchanging to irregularly staining light blue in the pileus-stipe connection zone when damaged, reddish reaction with NH4OH on pileus cuticle, ellipsoid to broadly ellipsoid, smooth basidiospores, slender pleurocystidia up to 106 μm long, hymenial cystidia (both cheilo- and pleurocystidia) sometimes multiseptate, a palisadoderm pileipellis of cylindrical hyphae, hymenophoral trama of the “Phylloporus-type” and the occurrence in lowland xero-mesophytic mixed broadleaved forests in apparent association with Coccoloba spp. (Polygonaceae) (including C. uvifera, C. diversifolia, C. spicata, C. swartzii, C. pubescens, etc.) (Pegler 1983; Ortiz-Santana et al. 2007 as “X. olivaceus”; this study). Based on our observations, the bluing oxidation of hymenophore and context in X. coccolobae is usually absent but sometimes present and quite variable in terms of range and intensity, depending on specimens age and weather conditions. It should therefore be considered a feature of low taxonomic significance. Similarly, pileus and stipe surfaces exhibit a rather considerable color variation at maturity, although always spanning in the range of brown, making the diagnostic value of these chromatic traits overestimated in the past.
This is the first verified report of X. coccolobae from the Dominican Republic. Xerocomus coccolobae has so far been reported from the Caribbean (Cuba, Dominican Republic, British Virgin Islands, and Martinique) and from Mexico (Veracruz, Quintana Roo, Yucatan) (Pegler 1983; García-Jiménez 1999; Minter et al. 2001; Ortiz-Santana et al. 2007; de la Fuente et al. 2018, 2020). According to the present outcomes, the distribution of X. coccolobae should also be extended to south-eastern USA (Florida) in association with Coccoloba uvifera. However, the exact area of occupancy of X. coccolobae is currently indefinite but based on the current known distribution and host association it is plausible to claim that its geographical range may correspond with that of the plant genus Coccoloba, which is an important constituent of the coastal mixed vegetation communities of neotropical lowland ecosystems and that most likely represents its ECM plant associate.
An additional collection Xerocomus aff. coccolobae JBSD133067 (ANGE446, MG810), consisting of a single very young specimen that was firstly identified as X. coccolobae, turned out to occupy a sister position to the main clade of X. coccolobae (Figs. 1 and 2). This fruiting body might represent a novel member of Xerocomus s.str., although it morphologically recalls X. hypoxanthus Singer (see below). Additional mature specimens will be required to assess its taxonomy.
Watling and de Meijer (1997) introduced Xerocomus cf. coccolobae from Brazil (State of Paraná), later published by de Meijer (2008) as X. basius de Meijer & Watling, differing from the Central American X. coccolobae by the innately fibrillose-squamulose, olive brown to yellowish brown pileus, reddish stipe, negative reaction with NH4OH on pileus surface, narrower basidiospores [7.8–11.0 (–12.0) × 3.0–4.0 μm], smaller basidia (22–28 × 6–8 μm), more dispersed, smaller hymenial cystidia (30–40 × 5–9 μm), absence of caulocystidia, a pileipellis structure consisting of interwoven, non-encrusted, narrower hyphae (4.5–13 μm broad) and the occurrence in mixed, dense ombrophilous montane forests, presumably in association with unknown angiosperms (Watling and de Meijer 1997; de Meijer 2008). This species has been quoted in a number of Brazilian fungal checklists (de Meijer 2001, 2006, both as “Xerocomus sp. A”; Neves and Capelari 2007, as “X. cf. coccolobae”; Sulzbacher et al. 2013, as “X. aff. coccolobae”; Magnago 2014, as “X. coccolobae”; Putzke and Putzke 2019, as “X. cf. coccolobae”).
The possibility of confusion with any of the numerous similar Xerocomus s. str. species cannot be ruled out. A certain morphological affinity exists between X. coccolobae and other xerocomoid taxa occurring in the same geographical macro-region, such as X. hypoxanthus, X. cuneipes, and X. pseudoboletinus var. pini-caribaeae.
The pan-American X. hypoxanthus resembles X. coccolobae in its general appearance but is easily separated based on the yellowish stipe with a granulose-furfuraceous bright yellow apex, yellow basal mycelium, blue-green reaction with NH4OH on pileus, and deep blue to brown reaction with KOH, longer basidiospores [(8.2–) 11–14 (–15.5) × (3.2–) 4.2–5.2 μm, 12.4 ± 1.1 × 4.6 ± 0.3, Qm = 2.6] and the growth under various frondose trees and conifers (Quercus, Pinus but also Coccoloba) and on very decayed woody debris and sawdust or trunks of palmetto. This species is known from south-eastern USA, continental Central America, the Caribbean, and is apparently allochthonous with introduced plants in South America (Brazil) (Singer 1946; Singer and Digilio 1960; Pegler 1983; Singer et al. 1983; Both 1993; Gómez 1997; Bessette et al. 2000, 2016, 2019; Pers. Obs.). The ITS sequence OL342399 (PLN 11-MAR-2022) named Xerocomus hypoxanthus voucher DUKE:0351605, USA: South Carolina, Mountain Rest (Stallman, J., Johnson, J., Roy, B., Lodge, D., Sheehan, B. and Russell, S. direct submission), shares 99.76% with OL342390 Boletaceae sp. voucher DUKE:0352590, 99.75% with Cyanoboletus bessettei A.R. Bessette, L.V. Kudzma & A. Farid voucher ARB1393A (MW675737) and ARB1393B (MW675738). It represents Cyanoboletus bessettei.
Xerocomus cuneipes is superficially very similar to X. coccolobae and shares with the latter species the same habitat and putative ECM association with Coccoloba. The revision of the holotype material (which consists of four mature specimens), originally found by J.P. Fiard in Martinique and currently preserved at the Royal Botanical Gardens Kew, K-M000178953 (J.P. Fiard 710B) (Fig. 8d) resulted as follows: basidiospores ellipsoid-fusiform to ellipsoid with suprahilar depression and sometimes with a shallow abaxial depression close to the distal end, rounded apex, pale golden-yellow, smooth under light microscope and SEM (Fig. 10c), measuring (10.3–) 11.4 ± 0.7 (–13.0) × (4.9–) 5.3 ± 0.2 (–5.7) μm, Q = (1.96–) 2.01–2.30 (–2.49), Qm = 2.15 ± 0.14, Vm = 168 ± 20 μm3 [35/2/1]; basidia clavate to broadly clavate, (19–) 22–33 (–38) × 10–13 (–15) × 2–3 μm (n = 13), sterigmata 2–3 μm long; cheilocystidia rare, lanceolate, lageniform with a thin neck to occasionally mucronate, (35–) 42–59 (–63) × 10–14 μm (n = 4); pleurocystidia variable in shape, fusiform to ventricoe fusiform or lageniform, occasionally narrowly ovate, some with a secondary septum, (22–) 26–53 (–66) × (6–) 8–14 (–16) μm (n = 19); pileipellis a trichoderm of interwoven filamentous to cylindrical, umber-brown in mass, mainly non-incrusted hyphae or with a fine granular incrustation, terminal elements cylindrical to cystidioid with pointed apex, (23–) 38–64 (–89) × (7–) 9–14 (–18) μm (n = 33); hymenophoral trama of the “Phylloporus-type”. These data match those provided in the protologue of X. cuneipes (Pegler 1983). Xerocomus cuneipes can be discriminated from X. coccolobae by the smaller size (pileus up to 2.8 cm broad, stipe up to 2.4 × 0.4 cm), a stipe distinctly tapered at base and with a deep vinaceous brown tint in the lower half, abundant yellow basal mycelium, slightly larger basidiospores [(11.0–) 11.7 ± 0.5 (–12.5) × (4.5–) 5.3 ± 0.4 (–6.0) μm, Qm = 2.2] and shorter basidia (22–26 × 11–12 μm) (Pegler 1983). Xerocomus cuneipes in addition to the Lesser Antilles (Martinique) where it was firstly described (Pegler 1983) was repeatedly reported from Mexico (García-Jiménez 1999; García-Jiménez and Garza-Ocañas 2001; de la Fuente et al. 2018, 2020) but not found in the Dominican Republic to date. Unfortunately, we were unable to generate DNA sequences from the type specimen of X. cuneipes due to its poor condition; however, according to morphological traits, it might be an additional representative of Xerocomus s. str. Moreover, since morphological and ecological traits of X. cuneipes mostly overlap those of X. coccolobae, a possible conspecificity of these two taxa cannot be ruled out. However, until further evidence is provided, we presently prefer to maintain them separate.
The holotype material of X. pseudoboletinus var. pini-caribaeae, originally collected in Belize by M.H. Ivory and housed at the Field Museum of Natural History, Chicago (F) (M.H. Ivory S/101, dupl. MG860), which consists of a single mature specimen (Fig. 8b), has been studied for a more accurate comparison with X. coccolobae and the anatomical re-examination produced the following results: basidiospores elongated fusiform to ellipsoid-fusiform in side view, ellipsoid-fusiform to ellipsoid in face view, with a short apiculus and a shallow suprahilar depression, apex rounded, (11.3–) 12.7 ± 0.8 (–14.4) × (4.6–) 5.2 ± 0.4 (–6.0) μm, Q = (1.98–) 2.17–2.93 (–2.95), Qm = 2.43 ± 0.21, V = 184 ± 32 μm3 [30/1/1], smooth, inamyloid; hymenial elements (basidia, pleurocystidia, and cheilocystidia) collapsed; hymenophoral trama bilateral divergent of the “Phylloporus-type”; pileipellis a trichoderm of mostly collapsed interwoven filamentous to broadly cylindrical, smooth hyphae with terminal elements 3–17 μm wide. The basidiospores measurements as resulted from the present re-examination of the type material are not in accordance with the original description, as they are much shorter when compared with the size provided by Singer et al. (1983) for X. pseudoboletinus var. pini-caribaeae: “11.5–17.5 (–20) × 4.5–5.8 (–6.8) μm, most frequently about 15–15.5 × 5–5.5 μm” (p. 80). In addition, the collecting date reported on the box containing the holotype sample (16 Nov 1976) (Fig. 8b) is also different from that reported in the protologue (15 Jun 1976) which probably refers to another collection (M.H. Ivory S/378) from Puerto Cabezas, Nicaragua (Singer et al. 1983). Unfortunately, we were unable to successfully extract and amplify DNA from the holotype of X. pseudoboletinus var. pini-caribaeae. Xerocomus pseudoboletinus var. pini-caribaeae was said to differ from the type (var. pseudoboletinus) in larger basidiospores and exclusive association with pines (although the type variety can also associate with pine trees) (Singer et al. 1983), but based on the aforementioned revision the actual existence of var. pini-caribaeae should be carefully evaluated. There is no doubt, however, that X. coccolobae and X. pseudoboletinus var. pini-caribaeae represent two different taxa, the latter differing in larger dimension of the basidiomes (pileus up to 12 cm diam., stipe up to 12 × 2.8 cm), predominantly reddish brown color of the pileus and bright yellow of the stipe, longer and slightly broader basidiospores, a trichoderm to ixotrichoderm pileipellis of interwoven, narrower hyphae (up to 13–15 μm broad), a blue-green reaction with NH4OH on pileus and an overall brown reaction with KOH, and different ECM host trees (P. caribaea and P. clausa) (Singer et al. 1983; Gómez 1997). Pinus caribaea Morelet is a not uncommonly encountered pine tree in montane forests of the Dominican Republic; however, the Caribbean pine is not at all present along the sea-shore areas where C. coccolobae occurs. The known distribution of X. pseudoboletinus extends from south-eastern USA to Central and/or South America (Singer et al. 1983; Both 1993; Gómez 1997; MyCoPortal). It is to be noted that in Both (1993) the type material of X. pseudoboletinus var. pini-caribaeae is incorrectly cited from Nicaragua.
Finally, the generic type X. subtomentosus (L.) Quél. is reminiscent of X. coccolobae but is promptly distinguished by the larger size (pileus up to 25 cm broad, stipe up to 12 × 4 cm), finely tomentose pileus, pale yellowish context with flesh pink hues in the lower third of the stipe, yellowish and usually coarsely ribbed or roughly pseudo-reticulate stipe, blue-green reaction with NH4OH on pileus surface, slightly longer basidiospores [(9.7–) 12.2 ± 0.9 (–17.2) × (3.8–) 4.8 ± 0.3 (–5.9) μm, Qm = 2.5] with bacillate ornamentation under SEM, trichodermal pileipellis consisting of narrow filamentous hyphae (terminal elements averaging 40 × 12 µm), more ventricose hymenial cystidia (up to 21 µm broad), ECM association with broadleaved trees (mainly Fagaceae and Betulaceae) and the occurrence in Europe in warm to temperate woodlands (Engel et al. 1996; Lannoy and Estadès 2001; Ladurner and Simonini 2003; Watling and Hills 2005; Taylor et al. 2006; Muñoz et al. 2008; Šutara 2008; Šutara et al. 2009; Knudsen and Taylor 2012; Galli 2013; Klofac and Krisai-Greilhuber 2020; Pers. obs.).
Tropicoboletus Angelini, Gelardi & Vizzini, gen. nov.
MycoBank MB847064.
Etymology: the epithet refers to the occurrence of this genus in the tropical belt.
Basidiomata pileate-stipitate with poroid hymenophore, epigeal, evelate, small-sized with a xerocomoid silhouette; pileus convex to applanate, subtomentose to glabrous; hymenophore adnate to depressed around the stipe, yellow to olive-brown; stipe solid, dry, longitudinally finely fibrillose, reticulum absent; basal mycelium yellow; context firm, whitish but pale cream-yellowish in the pileus; tissues unchangeable or turning light blue slowly and erratically when injured or exposed; taste mild to slightly sour; spore print olive-brown; sordid green reaction with ammonia on pileus cuticle; basidiospores smooth, ellipsoid-fusiform; pleuro-, cheilo and caulocystidia present; trichodermal pileipellis; hymenophoral trama bilateral-divergent of the “Phylloporus-type”; lateral stipe stratum present, of the “boletoid type”; clamp connections absent; ontogenetic development gymnocarpic; geographic distribution in the tropical belt. According to the phylogenetic analysis of the combined TEF1 and RPB2 sequences the genus is sister to subfamily Xerocomoideae.
Type: Boletus ruborculus T.J. Baroni.
Tropicoboletus ruborculus (T.J. Baroni) Angelini, Gelardi & Vizzini, comb. nov. Figures 5e–h, 7, and 9a, b
MycoBank MB847066.
Basionym: Boletus ruborculus T.J. Baroni, Mycologia 92 (3): 563. 2000.
Holotype: Greater Antilles, Puerto Rico, Arecibo, Barrio Dominguito, Mata de Platano Private Reserve, under Coccoloba sp., 08 Nov 1996, leg. T.J Baroni, S.A. Cantrell and F. Bird, NY 577594 (Baroni TJB 8253, PR-1926).
Basidiomes small. Pileus (2.2–) 2.8–4.5 (–4.7) cm broad, at first hemispherical then persistently convex and finally broadly pulvinate-flattened, sometimes slightly depressed at center, regularly to unevenly shaped by shallow depressions, moderately fleshy, firm at the beginning but progressively softer with age, flabby in old basidiomes; margin even to faintly wavy-lobed, initially slightly involute then curved downwards and finally completely plane or even uplifted, shortly appendiculate and extending beyond the tubes up to 1 mm; surface matt, dry, finely tomentose but later smooth and glabrous and then slightly greasy with moist weather, not cracked; somewhat variable in color, ranging from flesh-pink to purplish pink or pinkish vinaceous to vinaceous red (Hermosa Pink, La France Pink, Shrimp Pink, Pl. I; Safrano Pink, Orient Pink, Pl. II; Venetian Pink, Alizarine Pink, Acajou Red, Vandyke Red, Pl. XIII; Deep Vinaceous, Dark Vinaceous, Pl. XXVII) but progressively fading with age becoming pinkish gray, pinkish brown, brownish pink to grayish or pale grayish brown (Light Vinaceous-Fawn, Vinaceous-Fawn, Fawn Color, Army Brown, Buffy Brown, Pl. XL; Purple-Drab, Vinaceous-Drab, Pl. XLV; Light Cinnamon-Drab, Cinnamon-Drab, Light Drab, Pl. XLVI; Pale Mouse Gray, Light Mouse Gray, Olive Gray, Mouse Gray, Pl. LI; Storm Gray, Pl. LII) starting from the center, although tending to retain pinkish hues towards the peripheral zone even in senescence, always paler at margin, outer rim usually whitish (White, Pl. LIII); slowly reddening (Pomegranate Purple, Bordeaux, Pl. XII; Deep Vinaceous, Dark Vinaceous, Pl. XXVII) on handling or touching or more obviously when injured; subpellis layer reddish violet (Rose Pink, Pale Amaranth Pink, Mallow Pink, Pl. XII). Tubes wide at first in side view then increasingly broader with age and as long as or slightly longer or shorter than the thickness of the pileus context (up to 0.6 cm long), adnate but soon depressed around the stipe apex and decurrent with a short tooth, pale yellow to olive yellow and finally brownish olive (Buff Yellow, Pl. IV; Greenish Yellow, Bright Green Yellow, Oil Yellow, Javel Green, pl. V; Primuline Yellow, Pl. XVI; Raw Sienna, Pl. III) at maturity, unchangeable to erratically turning very light blue (Pale Blue-Green, Tyrolite Green, Pl. VII) when cut. Pores initially forming a concave surface, later flat then slightly convex, broad at first then gradually wider wth age (up to 2 mm in diam.), simple, firstly roundish becoming prominently angular at maturity, stretched and radially arranged towards the stipe, concolorous with the tubes and unchangeable or irregularly and very slowly and faintly bluing (Pale Blue-Green, Tyrolite Green, Pl. VII) on bruising or when injured, sometimes with scattered rusty brown (Ferruginous, Pl. XIV; Mikado Brown, Pl. XXIX) stains at the orifice in aged specimens. Stipe (2.3–) 2.6–5.8 (–9.0) × 0.5–1.0 (–1.3) cm, slightly longer than or as long as the pileus diameter at maturity, central to slightly off-center, solid, firm, dry, straight or curved to occasionally sinuous, cylindrical, subcylindrical to gradually and faintly swollen or conversely attenuated from apex down to the base, usually ending with a short taproot at the very base; surface longitudinally finely fibrillose throughout, non-reticulate, evelate; whitish to pale yellowish (White, Pl. LIII; Baryta Yellow, Pinard Yellow, Pl. IV) in the upper third, whitish (White, Pl. LIII) elsewhere but irregularly streaked or mottled bright flesh-pink, pinkish vinaceous or purplish pink to purplish red (Hermosa Pink, La France Pink, Shrimp Pink, Pl. I; Safrano Pink, Orient Pink, Pl. II; Venetian Pink, Alizarine Pink, Acajou Red, Vandyke Red, Pl. XIII), with a pale yellow to yellow (Baryta Yellow, Pinard Yellow, Picric Yellow, Pale Lemon Yellow, Pl. IV) basal tomentum, unchangeable or faintly reddening (Pomegranate Purple, Bordeaux, Pl. XII; Deep Vinaceous, Dark Vinaceous, Pl. XXVII) to rarely very slowly and faintly bluing (Pale Blue-Green, Tyrolite Green, Pl. VII) when pressed; basal mycelium yellow (Pale Lemon Yellow, Lemon Yellow, Pl. IV). Context firm and tough when young, later soft textured and eventually flabby in the pileus (up to 0.9 cm thick in the central zone, up to 0.7 cm thick halfway to margin and gradually becoming thinner towards the edge), a little more fibrous in the stipe, whitish, pale cream to very pale yellowish (White, Pl. LIII; Baryta Yellow, Pl. IV) in the pileus but cream to pale yellowish (Baryta Yellow, Pinard Yellow, Pl. IV) upon the tubes and upper fourth of the stipe, pinkish violet (Rose Pink, Pale Amaranth Pink, Mallow Pink, Pl. XII) underneath the cuticle, more or less evenly bright flesh-pink, purplish pink or pinkish vinaceous to vinaceous red (Hermosa Pink, La France Pink, Shrimp Pink, Strawberry Pink, Peach Red, Pl. I; Safrano Pink, Orient Pink, Grenadine Pink, Grenadine, Pl. II; Venetian Pink, Alizarine Pink, Old Rose, Pl. XIII) in the rest of the stipe but pale brownish to dirty brown (Medal Bronze, Dark Citrine, Pl. IV; Isabella Color, Light Brownish Olive, Pl. XXX) at the very base; very slowly and faintly turning pale blue (Pale Blue-Green, Pl. VII; Pale Blue, Light Cerulean Blue, Cerulean Blue, Pl. VIII) upon the tubes and more sporadically in the connection zone with the stipe when exposed to air, occasionally bluing all over the pileus context, unchangeable or nearly so elsewhere; yellowish (Pinard Yellow, Pl. IV) to dark vinaceous red (Pomegranate Purple, Bordeaux, Pl. XII; Deep Vinaceous, Dark Vinaceous, Pl. XXVII) where eroded by maggots, whitish to pale flesh-pink where eaten by slugs (Hermosa Pink, La France Pink, Pl. I; White, Pl. LIII); subhymenophoral layer pale yellowish (Baryta Yellow, Pinard Yellow, Pl. IV); exsiccate pale ochraceous on the context, brownish elsewhere (Clay Color, Tawny-Olive, Saccardo’s Umber, Pl. XXIX). Odor indistinct. Taste mild to slightly sour. Spore print olive-brown. Macrochemical spot-test reactions: 30% KOH: vinaceous red on pileus surface; 30% NH4OH: with vapors greenish black on pileus surface.
Basidiospores [189/12/7] (6.5–) 10.4 ± 0.9 (–13.0) × (3.5–) 5.0 ± 0.3 (–6.0) μm, Q = (1.53–) 1.60–3.00 (–3.25), Qm = 2.07 ± 0.17, V = 138 ± 23 μm3 (a single anomalous spore measured 14.0 × 6.0 μm), inequilateral, ellipsoid to ellipsoid-fusiform in side view, broadly ellipsoid to ellipsoid or nearly ovoid in face view, smooth under light microscope and SEM, apex rounded, with a short apiculus, usually with a shallow suprahilar depression and with a pronounced adaxial swelling, moderately thick-walled (0.3–0.6 μm), straw yellow colored in water and honey yellow in 5% KOH, having one or two large oil droplets when mature, rarely pluri-guttulate, inamyloid to very weakly dextrinoid, acyanophilic and staining blue (orthochromatic reaction) in Cresyl blue. Basidia 25–40 × 10–13 μm (n = 27), cylindrical-clavate to clavate, moderately thick-walled (0.3–0.6 μm), predominantly 4-spored but rarely also 2- or 3-spored, usually bearing relatively short sterigmata (2–5 μm), hyaline to pale yellowish and sometimes containing straw-yellow oil guttules in water and 5% KOH, bright yellow (inamyloid) in Melzer’s, without basal clamps; basidioles cylindrical-clavate to clavate, similar in size to basidia. Cheilocystidia (31–) 38–60 × (7–) 10–20 μm (n = 29), common, moderately slender, projecting straight to sometimes flexuous, fusiform to more frequently ventricose-fusiform or ampullaceous and usually showing a narrow and long neck but also sublageniform to lageniform, rarely subcylindrical or subclavate, with rounded to subacute tip, smooth, moderately thick-walled (0.5–1.0 μm), hyaline to pale yellowish in water and 5% KOH, bright yellow (inamyloid) in Melzer’s, without epiparietal encrustations. Pleurocystidia (34–) 36–59 (–61) × (7–) 9–18 (–20) μm (n = 18), relatively frequent, shape, size, color, and chemical reactions similar to cheilocystidia. Pseudocystidia not observed. Pileipellis a trichoderm consisting of strongly interwoven, elongated, filamentous and sinuous to less frequently slightly enlarged, frequently branched hyphae tending to be repent in the outermost layer and thus turning into a cutis not or only partially embedded in gelatinous matter; terminal elements 35–137 × (2–) 3–14 (–17) μm, particularly long and slender, cylindrical to rarely cystidioid, apex rounded-obtuse, moderately thick-walled (up to 1 μm), hyaline to more often straw yellow in water and 5% KOH, some cells with a scattered brownish vacuolar pigment in water which tends to be solved in KOH, inamyloid in Melzer’s, smooth; subterminal elements similar in shape, size and color to terminal elements. Stipitipellis a layer of slender, parallel to loosely intermingled and longitudinally running, smooth walled, adpressed hyphae, 3–17 μm wide, hyaline to yellowish in water and 5% KOH; the stipe apex covered by a well-developed caulohymenial layer consisting of sterile caulobasidioles, sparse, predominantly 2- and 1-spored, fertile caulobasidia, (25–) 28–38 (–42) × 8–12 μm, sterigmata 2–4 (–6) μm long (n = 10) and abundant projecting caulocystidia similar in size, shape, and color to hymenial cystidia, (28–) 31–47 (–54) × 10–19 (–22) μm (n = 10), having a wall up to 1 μm thick. Lateral stipe stratum under the caulohymenium present and well differentiated from the stipe trama, of the “boletoid type”, at the stipe apex a (25–) 30–80 (–90) μm thick layer consisting of divergent, inclined and running towards the external surface, loosely intermingled and rarely branched hyphae remaining separate and embedded in a gelatinous substance. Stipe trama composed of confusedly and densely arranged, subparallel to moderately interwoven, filamentous, smooth, inamyloid to barely dextrinoid hyphae, 2–8 (–10) μm broad. Hymenophoral trama bilaterally divergent of the “Phylloporus-type”, with very slightly divergent to nearly parallel and tightly arranged, non-gelatinous hyphae (lateral strata hyphae in transversal section touching or almost touching each other, 0–4 μm apart, 4–13 μm broad), hyaline to very pale yellowish in water and 5% KOH, inamyloid in Melzer’s; lateral strata (15–) 20–30 (–40) μm thick, mediostratum (10–) 15–35 (–40) µm thick, axially arranged, consisting of a tightly adpressed, non-gelatinous bundle of hyphae, 3–9 µm broad; in Congo Red the mediostratum is concolorous with or at most slightly darker than the lateral strata. Thromboplerous hyphae very abundant especially in the hymenophore, thick-walled, rarely septate, melanized, with a golden yellow to brownish homogeneous content in 5% KOH. Clamp connections absent in all tissues. Ontogenetic development gymnocarpic.
Edibility unknown.
Ecology and phenology: solitary to gregarious, growing on limestone among litter in a seasonally dry and moist anthropogenic lowland mixed stands under a large array of neotropical broadleaved trees including Coccoloba spp. (C. uvifera, C. diversifolia, C. pubescens, C. spicata, etc.) (Polygonaceae), which represent its putative ECM host trees. See Parra et al. (2018) for further details on lowland vegetation communities in the Dominican Republic. November and December.
Known distribution: It is known to date only from Mexico and the Greater Antilles of the Caribbean (Dominican Republic and Puerto Rico) but almost certainly also occurring in Belize and neighboring countries of mainland Central America. Apparently localized and infrequent.
Examined material: DOMINICAN REPUBLIC, Municipality of Sosúa, Puerto Plata Province, cemetery, three km away from the seaside, 19°44′40′′N 70°32′21′′W, 100 m, 16 Dec 2013, a single mature specimen, C. Angelini, JBSD133072 (ANGE208, MG808); same loc., 19 Dec 2013, five mature specimens, C. Angelini, JBSD133073 (ANGE209, MG809); same loc., 14 Dec 2019, three young to mature specimens, C. Angelini (collection lost); same loc., 23 Nov 2020, six mature specimens, C. Angelini, JBSD133074 (ANGE1406, MG850); same loc., 22 Nov 2020, a single young specimen, C. Angelini, JBSD133075 (ANGE1479, MG851); PUERTO RICO, Viejo San Juan, El Morro, along the Camino Escénico, 18°28′17′′N 66°07′23′′W, 22 Nov 2020, three mature specimens, K.O. Miller, MO439745 (komille277); Arecibo, Barrio Dominguito, Mata de Platano Private Reserve, under Coccoloba sp., 08 Nov 1996, T.J Baroni, S.A. Cantrell and F. Bird, NY 577594 (Baroni TJB 8253, PR-1926, holotype); MEXICO, Quintana Roo, Santa Elena, close to rio Hondo in the vicinity of the borderline with Belize, 12 Oct 2019, J.I. de la Fuente, JIF-451-ITCV (de la Fuente 451).
Notes: Tropicoboletus is a novel genus segregated from the polyphyletic Boletus s.l. Multilocus phylogenetic analysis clearly resolved Boletus ruborculus with strong statistical support in a monophyletic lineage sister to subfamily Xerocomoideae (Fig. 1). The isolated phylogenetic placement of Tropicoboletus justifies its recognition as an independent genus.
There does not appear to be one exclusive morphological trait that could serve alone to separate Tropicoboletus from similar genera in the Boletaceae; however, a combined set of features allows a prompt circumscription of this new genus. The only known species T. ruborculus can be recognized, even in the field, with reasonable certainty as it is easily distinguished by a combination of macro-morphological characters: basidiomes with a diminutive size and xerocomoid silhouette, flesh-pink, pinkish red or vinaceous red to brownish red pileus and stipe surfaces, yellowish olive tubular hymenophore, slowly and erratically bluing tissues on exposure, vivid yellow basal mycelium, a sordid green reaction on pileus surface with ammonia vapors and the occurrence in lowland mixed broadleaved tropical woodlands in probable association with Coccoloba spp. In addition, some anatomical key features integrating macroscopic identification include ellipsoid-fusiform, smooth basidiospores, predominantly and distinctly ventricose-fusiform to ampullaceous hymenial cystidia, a trichodermal pileipellis and the hymenophoral trama of the “Phylloporus-type” (Miller et al. 2000; this study). Beside the peculiar ecosystem where this bolete resides, the distinctive and conspicuous yellow basal mycelium is the most reliable diagnostic attribute for a proper recognition of the species in the field. This clear-cut feature, however, has not been previously emphasized. In the protologue of B. ruborculus (Miller et al. 2000) nothing is said about the color of the basal mycelium, either because its importance was underestimated or because it was simply overlooked. The association with Coccoloba species is most likely but not yet confirmed by direct observation of the ectomycorrhizae. Interestingly, specimens collected in Puerto Rico under C. uvifera (including the type specimen) exhibit a brighter red pileal surface when compared to basidiomes occurring with C. diversifolia from the Dominican Republic, but they are otherwise identical from both morphological and phylogenetic aspects.
Confident identification of T. ruborculus is also reinforced in the present study by the availability of additional verified samples recently yielded in Puerto Rico and Mexico which were placed in the same clade as the Dominican vouchers. However, just a handful of collections of this rare species are presently known from the neotropics, making T. ruborculus a sparingly encountered species. Prior to the present study, T. ruborculus resulted unnoticed from Mexico and the Dominican Republic as this species was known only from the type locality in Puerto Rico (Miller et al. 2000). Presumably, it is native to Central America and most likely widespread throughout the neotropics but to what extent is the actual distribution range of T. ruborculus remains to be determined. One might hypothesize that the distribution of this species roughly overlaps with that of Coccoloba, which appears to represent its alleged strict symbiotic partner.
For the sake of completeness, a careful re-examination of the holotype material of B. ruborculus (which consists of a single mature specimen divided in half) originally collected in Puerto Rico and housed at the New York Botanical Garden (NY 577594, TJB 8253, dupl. MG855) (Fig. 8c) has been carried out, resulting in the following anatomical data: basidiospores ellipsoid-fusiform, ellipsoid to broadly ellipsoid, smooth under light microscope and SEM (Fig. 9a, b), measuring (9.0–) 10.5 ± 0.6 (–12.0) × (4.8–) 5.3 ± 0.3 (–6.0) μm, Q = (1.63–) 1.76–2.18 (–2.22), Qm = 1.98 ± 0.13, V = 155 ± 22 μm3 [30/1/1], cylindrical-clavate to clavate or occasionally sphaeropedunculate basidia, 28–33 (–36) × 10–13 (n = 6), sterigmata 2–3 μm long, rare and mostly collapsed, subfusiform or fusiform to subclavate hymenial cystidia (pleurocystidia), 37–50 × 6–9 μm (n = 3) and a trichodermal pileipellis of interwoven filamentous to broadly cylindrical, smooth hyphae with terminal elements 3–15 (–19) μm wide. These data almost perfectly match those provided by T.J. Baroni (Miller et al. 2000) for B. ruborculus in the protologue. Furthermore, we successfully generated an ITS sequence from the holotype of B. ruborculus (GB acc. n. OQ108299) which shares a P%I = 99.9 with other sequences of B. ruborculus obtained in the present study (GB acc. n. OQ108295–OQ108298), therefore confirming their conspecificity.
Type materials. a Holotype collection of Xerocomus coccolobae K-M000178954 (J.P. Fiard 902A); b holotype collection of Xerocomus pseudoboletinus var. pini-caribaeae M.H. Ivory S/101 (F); c holotype collection of Boletus ruborculus NY 577594 (TJB 8253); d holotype collection of Xerocomus cuneipes K-M000178953 (J.P. Fiard 710B); e authentic collections of Boletus guadelupae K-M000193859, K-M000193860 (J.P. Fiard 563B, isoparatype), and K-M000193861; f holotype/paratype collection of Boletus guadelupae J.P. Fiard 563A (labeled as “Fiard 563”) and J.P. Fiard 563B (F); g holotype collection of Xerocomus caeruleonigrescens K-M000178955 (J.P. Fiard 905A). Photos a, d, e, g by A. Yu. Biketova; b, c, f by T. Yu. Svetasheva
Despite its resemblance with several other red-colored xerocomoid boletes, macro- and micro-morphological features of T. ruborculus are reliable and compelling enough to allow a clear-cut delimitation from lookalikes such as Boletus guadelupae and Xerocomus caeruleonigrescens, which grow in the same ecosystem in alleged association with various species of Coccoloba.
In the present study, we have generated ITS sequences from three different B. guadelupae collections: J.P. Fiard 563A/B (F) (holotype/paratype, see below), K-M000193866 (D.N. Pegler 2981) and K-M000193867 (D.N. Pegler 2745). Based on the resulting phylogenetic tree (Fig. 4), B. guadelupae is clearly nested in Singerocomus, forming a strongly supported lineage (MLB 0.94, PP 0.97) which is sister to a clade containing S. inundabilis (Singer) T.W. Henkel & Husbands and S. rubriflavus T.W. Henkel & Husbands. Sequences of the recently described S. atlanticus A.C. Magnago (including the holotype KY907177) cluster in the same clade of B. guadelupae, sharing a P%I = 99.4 and thus indicating there are contaxic. The following new combination is therefore required:
Singerocomus guadelupae (Singer & Fiard) Gelardi, Biketova, Martinez-Suz & Vizzini, comb. nov.
MycoBank MB847067.
Basionym: Boletus guadelupae Singer & Fiard, Bulletin de la Société Mycologique de France 92(4): 445. 1976 (“1977”).
≡ Xerocomus guadelupae (Singer & Fiard) Pegler, Kew Bulletin Additional Series 9: 575. 1983.
= Singerocomus atlanticus A.C. Magnago, Acta Botanica Brasilica 32 (2): 3. 2018.
Holotype: Lesser Antilles, Guadelupe, Matouba, Basse Terre, 700 m, 31 Jul 1975, leg. J.P. Fiard, J.P. Fiard 563A (labeled as “Fiard 563”), F.
Examined material: GUADELUPE, Trace de Sofaia, 250 m, on soil, twigs, and rotting roots in forest, 25 Jul 1975, J.P. Fiard, K-M000193860 (isoparatype); same locality (F, J.P. Fiard 563B paratype, or J.P. Fiard 563A/563B mixed holotype/paratype); same locality, on forest soil and decayed trunk in lower mountain rainforest, 23 Aug 1975, J.P. Fiard, K-M000193859 (original collection number unknown!); Basse Terre, Courbayre, Morne Cadet, 300 m, 09 Oct 1977, D.N. Pegler, K-M000193866 (D.N. Pegler 2981). MARTINIQUE, Plateau Perdrix, on the ground and among fallen leaves in forest at start of rains, 05 Jul 1976, J.P. Fiard, K-M000193861 (J.P. Fiard 563C); Absalon, Clark Ravine, 15 Sep 1977, D.N. Pegler, K-M000193867 (D.N. Pegler 2745).
Additional examined material: Xerocomus caeruleonigrescens: MARTINIQUE, Morne Aca, solitary on the forest floor, 250 m, 28 Aug 1977, J.P. Fiard, K-M000178955 (J.P. Fiard 905A, holotype).
Singerocomus guadelupae is delimited from T. ruborculus by the presence of an olivaceous yellow pruina covering pileus surface in the early developmental stage, white to whitish yellow basal mycelium, unchanging tissues on bruising or exposure, a blue reaction with NH4OH and a yellow reaction with KOH on the pileus, considerably smaller, broadly ellipsoid to ovoid basidiospores [(6.0–) 7.3 ± 0.3 (–9.0) × (3.3–) 4.5 ± 0.2 (–6.0) μm, Qm = 1.6] which are smooth, fusoid to lageniform or ventricose-rostrate, narrower hymenial cystidia (34–62 × 4–11 μm) which are sometimes thick-walled (up to 2.7 μm thick) and narrower caulocystidia (31–43 × 6–9.8 μm) (Singer and Fiard 1977; Pegler 1983; Singer et al. 1983; Magnago et al. 2018, as “Singerocomus atlanticus”). Singerocomus guadelupae may establish ECM association with Polygonaceae, Nyctaginaceae, and Fabaceae and to date it has been reported from both the Greater and Lesser Antilles (Cuba, Martinique, Guadeloupe, Dominica) and from all along the Atlantic Forest in Brazil (Singer and Fiard 1977; Pegler 1983; Singer et al. 1983; Minter et al. 2001; Magnago et al. 2018, as “Singerocomus atlanticus”; Putzke and Putzke 2019) but has not as yet been found in the Dominican Republic. Having been confirmed as a member of Singerocomus, S. guadelupae broadens the biogeographic range of the genus to the Caribbean. For additional considerations on type collections of B. guadelupae, see below. The anatomical study of the authentic specimen K-M000193859 of B. guadelupae collected in Guadelupe by J.P. Fiard and lodged at the Royal Botanic Gardens Kew (K-M) resulted as follows: basidiospores broadly ellipsoid to ovoid in side view, smooth under light microscope and SEM (Fig. 9d), with an indistinct suprahilar depression and rounded apex, pale golden-yellow, (6.4–) 7.2 ± 0.5 (–8.4) × (4.1–) 4.5 ± 0.3 (–5.4) μm, Q = (1.32–) 1.49–1.69 (–1.80), Qm = 1.59 ± 0.10, Vm = 80 ± 15 μm3 [33/3/1]; basidia cylindrical-clavate to clavate, 28–35 (–41) × 8–11 μm (n = 12), sterigmata 2–3 μm long; cheilo- and pleurocystidia fusiform to lageniform, cheilocystidia (40–) 43–55 (–58) × (7–) 9–12 μm (n = 10); pleurocystidia (22–) 31–53 (–60) × 6–10 μm (n = 10); trichodermal pileipellis of interwoven filamentous to cylindrical, reddish in mass, smooth to rarely encrusted hyphae, terminal elements cylindrical but tapering at apex, (14–) 19–40 (–66) × 4–9 (–12) μm (n = 33). These microscopic data are consistent with those reported for B. guadelupae in the protologue (Singer and Fiard 1977).
A re-examination of the anatomical features of the holotype material of X. caeruleonigrescens K-M000178955 (J.P. Fiard 905A), originally found in Martinique and consisting of a single mature specimen (Fig. 8g), produced the following results: basidiospores ellipsoid to broadly ellipsoid without suprahilar depression, golden-yellow, smooth under light microscope and with very finely bacillate (rodlet) encrustation on most of the surface under SEM, although some smooth patches may occur [Fig. 10d–f; similar to those of X. ferrugineus (Schaeff.) Alessio (see Šutara 2008, Fig. 2, for comparison)], measuring (10.3–) 11.2 ± 0.6 (–13.0) × (5.0–) 6.0 ± 0.3 (–6.6) μm, Q = (1.65–) 1.74–2.01 (–2.19), Qm = 1.87 ± 0.13, Vm = 211 ± 29 μm3 [33/1/1]; basidia cylindrical-clavate to clavate, 25–30 (–32) × 9–12 μm (n = 12), sterigmata 2–3 μm long; cheilocystidia fusiform to clavate, (29–) 31–40 (–43) × 6–10 μm (n = 6); pleurocystidia fusiform to lageniform with elongated neck, with pale yellow content, (29–) 35–52 (–54) × 6–12 μm (n = 12); pileipellis a palisadoderm of interwoven cylindrical to broadly cylindrical, umber-brown in mass hyphae, some with ocher-brownish content, terminal elements cylindrical with rounded apex, (13–) 23–36 (–43) × (4–) 6–8 μm (n = 33); hymenophoral trama of the “Phylloporus-type”. These data are consistent with those provided in the protologue of X. caeruleonigrescens (Pegler 1983). Xerocomus caeruleonigrescens is separated from T. ruborculus by the smaller size of the fruiting bodies (pileus 2.5 cm diam., stipe 3.3 × 0.4 cm), tissues bruising deep bluish black throughout, slightly larger basidiospores [(10.0–) 11.3 ± 0.8 (–13.0) × (5.2–) 6.0 ± 0.4 (–6.5) μm, Qm = 1.9] which are finely bacillate under SEM, smaller basidia (25–28 × 9–11 μm), narrower hymenial cystidia (40–50 × 9–11 μm) and a palisadoderm pileipellis with shorter and narrower terminal cells (21–65 × 5–7 μm) (Pegler 1983). This species has also been reported from Mexico (García-Jiménez 1999; García-Jiménez and Garza-Ocañas 2001; de la Fuente et al. 2018, 2020). Unfortunately, various attempts to sequence and amplify the ITS region of the holotype material of X. caeruleonigrescens resulted unsuccessful and therefore we could not determine the exact phylogenetic placement of this taxon.
The pine-associated Boletus caribaeus (Singer) Singer, originally introduced as a subspecies of Boletus rubellus Krombh. (= Hortiboletus rubellus (Krombh.) Simonini, Vizzini & Gelardi), differs from T. ruborculus, aside from the different ECM host tree (Pinus caribaea), by larger-sized basidiomes (pileus up to 15 cm broad, stipe up to 3.5 cm wide), yellow context discoloring dark blue throughout on exposure, whitish basal mycelium, an indistinct reaction on pileus surface with NH4OH, slightly longer basidiospores [(9.0–) 9.5–14.2 (–14.5) × (4.0–) 4.2–6.5 μm], smaller cheilocystidia (20–23 × 6.5–7 μm), narrower pleurocystidia (23–70 × 7.5–11 μm) and caulocystidia (29–45 × 8–11 μm), narrower pileipellis hyphae (up to 7 μm wide), hymenophoral trama of the “Boletus-type” and sometimes by the the tendency to develop sterile gasteroid basidiomes (carpophoroidism) (Singer 1945, 1947; Singer et al. 1983). This species occurs from south-eastern USA (Florida) south to continental Central America (Belize) (Singer 1945, 1947; Singer et al. 1983). Presently unknown from the Dominican Republic.
Molecular separation of T. ruborculus from the European H. rubellus is morphologically substantiated by the presence in this latter taxon of larger-sized basidiomes (pileus up to 12 cm broad, stipe up to 12 × 3 cm), a reddish stipe surface turning blue on handling, a more constant and intense bluing of pileal context when exposed, a dirty ochraceous yellow stipe context which usually exhibits carrot orange to flame red tiny punctuations at the very base, a negative to pale yellow reaction with NH4OH on the pileus, slightly longer basidiospores [(9.2–) 12.0 ± 0.9 (–15.1) × (3.8–) 5.2 ± 0.4 (–6.7) μm, Qm = 2.3], narrower hymenial cystidia (40–60 × 8–13 μm), a physalo-palisadoderm pileipellis of subparallel, broader hyphae, and the occurrence preferably on woodland edges, grassy clearings, tracksides, cultivated lands or similar rural environments, mainly in association with Fagaceae (Quercus, Castanea, Fagus) (Engel et al. 1996; Lannoy and Estadès 2001; Ladurner and Simonini 2003; Peintner et al. 2003; Watling and Hills 2005; Muñoz et al. 2008; Šutara et al. 2009; Knudsen and Taylor 2012; Galli 2013; Klofac and Krisai-Greilhuber 2020; Pers. obs.). In addition, H. rubellus is phylogenetically very distantly related to T. ruborculus (Wu et al. 2016a, b).
More collections of T. ruborculus are surely needed for a better taxonomic understanding of the generic limits of Tropicoboletus and a more accurate knowledge of its ecology and geographic distribution patterns in Mesoamerica. Furthermore, future extensive research in undersampled ecological niches might uncover additional members of Tropicoboletus, especially from the paleotropics.
Clarification on type collections of Boletus guadelupae
There appears to be much confusion with dates and collection numbers of the type specimens of B. guadelupae. Based on data provided in the protologue (Singer and Fiard 1977), the holotype was collected in Basse Terre, Matouba, Guadelupe, 31 Jul 1975 (collection number not specified), whereas J.P. Fiard 563B (collected in Trace de Sofaia, Guadelupe, 25 Jul 1975) is designated as paratype, both collections having been deposited at the Field Museum of Natural History, Chicago (F).
In Singer et al. (1983), the collection number of the holotype from Matouba is specified as J.P. Fiard 563C. This is inconsistent with the information reported in Pegler (1983), where the holotype collection from Matouba is reported under the number J.P. Fiard 563A (with a wrong date “July 1075”) and strangely enough the type is said to be housed at K-M instead of F as previously reported in Singer and Fiard (1977). This is another contradictory information which can be explained by the fact that the type was later duplicated at K-M. However, according to ICN rules, there cannot be two holotypes of the same species deposited at two different official herbaria. Therefore, we must assume that part of J.P. Fiard 563A was housed at F (labeled as “Fiard 563” and representing the holotype as reported in the protologue and based on the collection date provided by Pegler) and part at K-M (representing an isotype, but see below). The same thing probably happened to the paratype J.P. Fiard 563B, which was split between F and K-M. We have also been able to retrieve J.P. Fiard 563C (K-M000193861) at K-M (Fig. 8e), but unlike what written by Singer et al. (1983) this sample originates from Martinique and is dated 05 Jul 1976. Consequently, J.P. Fiard 563C cannot be considered the holotype and the type data reported in Singer et al. (1983) are evidently wrong. Likewise, the specimen K-M000193860 (which is referred to as J.P. Fiard 563B on 25 Jul 1975, heavily infected by Hypomyces sp.) housed at the Royal Botanic Gardens Kew as the holotype of B. guadelupae is not the holotype nor an isotype but a simple isoparatype (Fig. 8e). We are not able to address why the letter “B” after Fiard’s collection number was handwritten modified as “A” in the accompanying label (Fig. 8e), but no doubt the correct number is the original J.P. Fiard 563B. Another authentic specimen K-M000193859, which was collected by Fiard on 23 Jul 1975 and not metioned in the protologue, has a wrong collection number (J.P. Fiard 563B, again with the label handwritten modified by replacing the letter “A” with the letter “B” after Fiard’s collection number) (Fig. 8e), but it can be neither J.P. Fiard 563B nor J.P. Fiard 563A, because these vouchers were collected on 25 Jul 1975 and 31 Jul 1975, respectively. Accordingly, the original collection number of K-M000193859 presently remains unknown. K-M000193861 (J.P. Fiard 563C) (Fig. 8e) from Martinique is an additional authentic collection.
With regard to the type material preserved at F, which consists of three fragmented dried specimens (Fig. 8f), it is clearly visible that the upper right label (coll. Fiard 563B) refers to the paratype (although the date 25 Aug 1957 is obviously incorrect) while the bottom left label (Fiard 563, dated 31 Jul 1975) refers to the holotype. Unfortunately, it is impossible to know which of the two collections the dried specimens refer to. We are inclined to believe that the specimens refer to the holotype collection, but we have no proof to demonstrate it. One might hypothesize that J.P. Fiard 563A and J.P. Fiard 563B could have mixed into a single bag, so as to become a sole collection (this would explain the presence of two labels in the same packet). On the other hand, considering all material of B. guadelupae presently housed in the Fungarium of the Kew Gardens, we must conclude that an isotype of this species has never been deposited at K-M, since none of the available collections is dated 31 Jul 1975, unless we accept that the type at F is a mixture of the holotype and paratype specimens, in this case K-M000193860 would actually represent the isotype of B. guadelupae.
Notes on basidiospore ornamentation of Xerocomus s. str. under SEM
According to Šutara (2008), species of the genera Xerocomus and Phylloporus Quél. have bacillate spore ornamentation under SEM. Heinemann et al. (1988) were the first to observe such ornamentation in X. subtomentosus (bacillate) and X. ferrugineus (bacillate or roughened to smooth). The bacillate ornamentation of the basidiospores of X. subtomentosus, X. chrysonemus A.E. Hills & A.F.S. Taylor, X. silwoodensis A.E. Hills, U. Eberh. & A.F.S. Taylor, and X. doodhcha K. Das, D. Chakr., Baghela, S.K. Singh & Dentinger is detectable under × 6 K – × 15 K magnification, while that of X. ferrugineus can be observed with × 30 K or higher magnification (Oolbekkink 1991; Šutara 2008; Das et al. 2016). Based on the current study, X. coccolobae has smooth basidiospores under SEM, as well as X. cuneipes (a single specimen K-M000178953), which has not yet been proven genetically to be a member of Xerocomus s.str. (Fig. 10c). A single specimen of Xerocomus caeruleonigrescens (K-M000178955), which has not been successfully sequenced, showed a finely bacillate (rodlet) encrustation which was observed on most of the basidiospore surface, altough some patches appeard to be smooth (Fig. 10d–f).
This kind of incrustation can be explained by hydrophobin (surface active protein) coating of basidiospores and has similar rodlet pattern to assembled Class I hydrophobins on aerial hyphae of Schizophyllum commune Fr. and pileus surface of Agaricus bisporus (J.E. Lange) Imbach (Wösten et al. 1993; Lugones et al. 1996). Hydrophobins of mushroom-forming fungi (Agaricomycetes) are far from being well studied (Li et al. 2021). So far, Pisolithus tinctorius (Mont.) E. Fisch. (putative P. albus (Cooke & Massee) Priest), Suillus luteus (L.) Roussel, and Paxillus involutus (Batsch) Fr. are the only species of the order Boletales where hydrophobins, which are predominantly produced during ectomycorrhiza formation, were formally detected and characterized (Tagu et al. 1996; Duplessis et al. 2001; Rineau et al. 2017). However, there is no information on basidiospore coat hydrophobins in Boletales in the available literature. They likely play an important role in spore protection and germination, as it has been shown in anamorphic fungi (Cai et al. 2021).
Additional remarks on some incertae sedis genera
In the combined RPB2/TEF1 phylogeny focused on the whole family Boletaceae, we recovered a well-supported clade (MLB = 0.93, PP = 0.98) containing Solioccasus Trappe, Osmundson, Manfr. Binder, Castellano & Halling, Bothia Halling, T.J. Baroni & Manfr. Binder and Phylloporopsis Angelini, A. Farid, Gelardi, M.E. Smith, Costanzo & Vizzini (provisionally named “Bothia clade” in Farid et al. 2018), and another clade with no statistical support comprising Pseudoboletus Šutara, Gymnogaster J.W. Cribb and Baorangia G. Wu & Zhu L. Yang (Fig. 1). All these genera are currently settled in the Boletaceae but with an uncertain phylogenetic placement (incertae sedis). The “Bothia clade” might represent an additional subfamily within the Boletaceae, although the only known synapomorphy of this grouping appears to be the cyanophily of the basidiospore wall as previously highlighted by Farid et al. (2018). On the other hand, Pseudoboletus, Gymnogaster and Baorangia do not appear to be strictly related to one another from morphological, ecological, or trophic standpoints and their phylogenetic close proximity could be only artifactual. Obviously, further investigation on morphological, ecological, and chemical features and additional molecular loci will be required to better clarify the taxonomic boundaries of these genera and their reciprocal phylogenetic relationships. Accordingly, we believe it is premature to introduce formal ranks for these two groups of genera and we therefore refrain from proposing new subfamilies for the time being.
Data availability
Sequence data are deposited in GenBank. The datasets generated and analyzed during the current study are in the tables or available from the corresponding author upon request.
References
Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ (1990) Basic local alignment search tool. J Mol Biol 215:403–410
Alvarado P, Moreno G, Manjón JL (2012) Comparison between Tuber gennadii and T. oligospermum lineages reveals the existence of the new species T. cistophilum (Tuberaceae, Pezizales). Mycologia 104(4):894–910
Ariyawansa HA, Hyde KD, Jayasiri SC, Buyck B, Chethana KWT et al (2016) Fungal divers notes 111–252—taxonomic and phylogenetic contributions to fungal taxa. Fungal Divers 75(1):27–274. https://doi.org/10.1007/s13225-015-0346-5
Arora D, Frank JL (2014) Clarifying the butter boletes: a new genus, Butyriboletus, is established to accommodate Boletus sect. Appendiculati, and six new species are described. Mycologia 106(3):464–480. https://doi.org/10.3852/13-052
Badou SA, Furneaux B, De Kesel A, Khan FK, Houdanon RD et al (2022) Paxilloboletus gen. nov., a new lamellate bolete genus from tropical Africa. Mycol Prog 21:243–256
Bessette AE, Roody WC, Bessette AR (2000) North American boletes. Syracuse University Press, Syracuse, A color guide to the fleshy pored mushrooms
Bessette AE, Roody WC, Bessette AR (2016) Boletes of eastern North America. Syracuse University Press, Syracuse
Bessette AE, Bessette AR, Lewis DP (2019) Mushrooms of the Gulf Coast States: a field guide to Texas, Louisiana, Mississipi, Alabama, and Florida. University of Texas Press, Austin
Biketova AYu, Gelardi M, Smith ME, Healy RA, Simonini G et al (2022) Reappraisal of the genus Exsudoporus (Boletaceae) worldwide based on multi-gene phylogeny, morphology and biogeography, and insights on Amoenoboletus. J Fungi 8(2):101. https://doi.org/10.3390/jof8020101
Binder A (1999) Zur molekularen Systematische der Boletales: Boletineae und Sclerodermatineae subordo nov. PhD Dissertation, Universität Regensburg
Binder M, Bresinsky A (2002) Retiboletus, a new genus for species-complex in the Boletaceae producing retipolites. Feddes Repertorium 113(1–2):30–40. https://doi.org/10.1002/1522-239X(200205)113:1/2%3c30::AID-FEDR30%3e3.0.CO;2-D
Binder M, Hibbett DS (2006) Molecular systematics and biological diversification of Boletales. Mycologia 98(6):971–981. https://doi.org/10.3852/mycologia.98.6.971
Both EE (1993) The boletes of North America. A compendium. Buffalo Museum of Science, Buffalo
Bozok F, Assyov B, Taşkin H, Doğan HH, Büyükalaca S (2020) Molecular phylogenetic studies of Turkish boletes with emphasis on some recently described species. Nova Hedwigia 110(1–2):99–129. https://doi.org/10.1127/nova_hedwigia/2019/0563
Cai F, Zhao Z, Gao R, Chen P, Ding M et al (2021) The pleiotropic functions of intracellular hydrophobins in aerial hyphae and fungal spores. PLOS Genet 17(11):e1009924. https://doi.org/10.1371/journal.pgen.1009924
Courtecuisse R, Welti S (2013) Liste préliminaire des Fungi recensés dans les îles françaises des Petites Antilles: Martinique, Guadeloupe et dépendances. II – Basidiomycètes non lamellés (espèces gastéroïdes, rouilles et charbons exclus). Doc Mycol 35:47–173
Crous PW, Wingfield MJ, Richardson DM, Le Roux JJ, Strasberg D et al (2016) Fungal planet description sheets: 400–468. Persoonia 36:316–458. https://doi.org/10.3767/003158516X692185
Crous PW, Wingfield MJ, Lombard L, Roets F, Swart WJ et al (2019) Fungal planet description sheets: 951–1041. Persoonia 43:223–425. https://doi.org/10.3767/persoonia.2019.43.06
Cubeta MA, Echandi E, Abernethy T, Vilgalys R (1991) Characterization of anastomosis groups of binucleate Rhizoctonia species using restriction analysis of an amplified ribosomal RNA gene. Phytopathology 81:1395–1400
Darriba D, Taboada GL, Doallo R, Posada D (2012) jModelTest 2: more models, new heuristics and parallel computing. Nat Methods 9:772
Das K, Chakraborty D, Baghela A, Singh SK, Dentinger BTM (2015) Boletus lakhanpalii, a new species in Boletaceae from Sikkim (India) with uncertain phylogenetic placement. Sydowia 67:11–19. https://doi.org/10.12905/0380.sydowia67-2015-0011
Das K, Chakraborty D, Baghela A, Singh SK, Dentinger BTM (2016) New species of xerocomoid boletes (Boletaceae) from Himalayan India based on morphological and molecular evidence. Mycologia 108(4):753–764. https://doi.org/10.3852/15-206
de la Fuente JI, Ayala-Vásquez O, Garza-Ocañas F, López CY, García-Jiménez J (2018) Some interesting Boletales (Basidiomycota) from Quintana Roo, Mexico. Scientia Fungorum 48:77–86
de la Fuente JI, García-Jiménez J, López CY, Oros-Ortega I, Vela-Hernández RY et al (2020) An annotated checklist of the macrofungi (Ascomycota, Basidiomycota, and Glomeromycota) from Quintana Roo. Mexico Check List 16(3):627–648. https://doi.org/10.15560/16.3.627
de Meijer AAR (2001) Mycological work in the Brazilian state of Parana. Nova Hedwigia 72:105–159
de Meijer AAR (2006) Preliminary list of the macromycetes from the Brazilian State of Paraná. Bol Mus Bot Munic Curitiba 68:1–55
de Meijer AAR (2008) Macrofungos notáveis das Florestas do Pinheiro-do-Paraná. Brazilian Agricultural Research Corporation, Colombo
Dentinger BTM, Margaritescu S, Moncalvo JM (2010) Rapid and reliable highthroughput methods of DNA extraction for use in barcoding and molecular systematics of mushrooms. Mol Ecol Res 10(4):628–633
Drehmel D, James T, Vilgalys R (2008) Molecular phylogeny and biodiversity of the boletes. Fungi 1(4):17–23
Duplessis S, Sorin C, Voiblet C, Palin B, Martin F, Tagu D (2001) Cloning and expression analysis of a new hydrophobin cDNA from the ectomycorrhizal basidiomycete Pisolithus. Curr Genet 39(5–6):335–339. https://doi.org/10.1007/s002940100224
Engel H, Dermek A, Klofac W, Ludwig E, Brückner T (1996) Schmier- und Filzröhrlinge s.l. in Europa. Die Gattungen Boletellus, Boletinus, Phylloporus, Suillus, Xerocomus. Verlag Heinz Engel, Weidhausen-Coburg
Farid A, Franck AR, Garey JR (2017) Boletus rubricitrinus belongs in Pulchroboletus (Boletaceae). Czech Mycol 69(2):143–162
Farid A, Gelardi M, Angelini C, Franck AR, Costanzo F et al (2018) Phylloporus and Phylloboletellus are no longer alone: Phylloporopsis gen. nov. (Boletaceae), a new smooth-spored lamellate genus to accommodate the American species Phylloporus boletinoides. FUSE 2:341–359. https://doi.org/10.3114/fuse.2018.02.10
Farid A, Bessette AE, Bessette AR, Bolin JA, Kudzma LV et al (2021) Investigations in the boletes (Boletaceae) of southeastern USA: four novel species, and three novel combinations. Mycosphere 12(1):1038–1076. https://doi.org/10.5943/mycosphere/12/1/12
Felsenstein J (1985) Confidence limits on phylogenies: an approach using the bootstrap. Evolution 39:783–791
Flores Arzù R, Simonini G (2000) Contributo alla conoscenza delle Boletales del Guatemala. Riv Micol 43(2):121–145
Flores Arzù R (2020) Diversity and importance of edible ectomycorrhizal fungi in Guatemala. In: Pérez-Moreno J, Guerin-Laguette A, Flores Arzù R, Yu FQ (Eds): Mushrooms, humans and nature in a changing world - Perspectives from ecological, agricultural and social sciences. Chapter 4. Springer Nature Switzerland, Cham
Franco-Molano AE, Aldana-Gómez R, Halling RE (2000) Setas de Colombia. Agaricales, Boletales y otros hongos: guía de campo. Colciencias, Universidad de Antioquia Medellín, Colombia
Frank JL, Bessette AR, Bessette AE (2017) Alessioporus rubriflavus (Boletaceae), a new species from the eastern United States. North American Fungi 12(2):1–8. https://doi.org/10.2509/naf2017.012.002
Frank JL, Siegel N, Schwartz CF, Araki B, Vellinga EC (2020) Xerocomellus (Boletaceae) in western North America. FUSE 6:265–288. https://doi.org/10.3114/fuse.2020.06.13
Galli R (2013) I Boleti: atlante pratico–monografico per la determinazione dei boleti. 4th ed. Micologica, Vergiate
García-Jiménez J (1999) Estudio sobre la taxonomía, ecología y distributión de algunos hongos de la familia Boletaceae (Agaricales, Basidiomycetes) de México. Universidad Autónoma de Nuevo León, Tesis de Maestría
García-Jiménez J, Garza-Ocañas F (2001) Conocimiento de los hongos de la familia Boletaceae de México. Ciencia UANL 4(3):336–343
García-Jiménez J (2013) Diversidad de macromicetos en el estado de Tamaulipas. PhD en Ciencias Naturales, Universidad Autónoma de Nuevo León, México
García-Jiménez J, Singer R, Estrada E, Garza-Ocañas F, Valenzuela R (2013) Dos especies nuevas del género Boletus (Boletales: Agaricomycetes) en México. Rev Mex Biodivers 84:152–162. https://doi.org/10.7550/rmb.31988
Gardes M, Bruns TD (1993) Community structure of ectomycorrhizal fungi in a Pinus muricata forest: above- and below-ground views. Can J Bot 74:1572–1583
Gelardi M, Vizzini A, Ercole E, Voyron S, Sun JZ et al (2013) Boletus sinopulverulentus, a new species from Shaanxi Province (central China) and notes on Boletus and Xerocomus. Sydowia 65(1):45–57
Gelardi M, Simonini G, Ercole E, Vizzini A (2014) Alessioporus and Pulchroboletus (Boletaceae, Boletineae), two novel genera for Xerocomus ichnusanus and X. roseoalbidus from the European Mediterranean basin: molecular and morphological evidence. Mycologia 106(6):1168–1187. https://doi.org/10.3852/14-042
Gelardi M, Simonini G, Ercole E, Davoli P, Vizzini A (2015) Cupreoboletus (Boletaceae, Boletineae), a new monotypic genus segregated from Boletus sect Luridi to reassign the Mediterranean species B poikilochromus. Mycologia 107(6):1254–1269. https://doi.org/10.3852/15-070
Gómez LD (1996) (“1997”) Basidiomicetes de Costa Rica: Xerocomus, Chalciporus, Pulveroboletus, Boletellus, Xanthoconium (Agaricales: Boletaceae). In: Carranza J, Mueller GM (Eds) Fungi of Costa Rica: selected studies on ecology and biodiversity. Rev Biol Trop 44(4):59–89
Gonzáles-Velázquez A, Valenzuela R (1993) Los boletáceos y gonfidiáceos del Estado de México I: Discusiones sobre su distribución en diferentes tipos de vegetación, asociaciones ectomicorrizógenas, fenología y comestibilidad. Rev Mex Micol 9:35–46
Halling RE (1989) A synopsis of Colombian Boletes. Mycotaxon 34:93–113
Halling RE (1992) A new species of Boletus section Luridi from Colombia. Brittonia 44(3):322–325. https://doi.org/10.2307/2806931
Halling RE (1996) (“1997”) Boletaceae (Agaricales): latitudinal biodiversity and biological interactions in Costa Rica and Colombia. In: Carranza J, Mueller GM (Eds) Fungi of Costa Rica: selected studies on ecology and biodiversity. Rev Biol Trop 44(Suppl 4):111–114
Halling RE, Mueller GM (1999) New boletes from Costa Rica. Mycologia 91(5):893–899
Halling RE, Mueller GM (2002) Agarics and Boletes of neotropical oakwoods. In: Watling R, Frankland JC, Ainsworth AM, Isaac S, Robinson CH (Eds) Tropical Mycology. Vol 1. Macromycetes, CAB International, Wallingford
Halling RE, Mata M (2004) Boletus flavoruber, un nouveau bolet du Costa Rica. Bull Soc Mycol Fr 120(1–4):257–262
Halling RE, Mata M, Mueller GM (2004) Three new boletes for Costa Rica. Mem NY Bot Gard 89:141–147
Halling RE, Mueller GM (2005) Common mushrooms of the Talamanca Mountains, Costa Rica. Memoirs of the New York Botanical Garden Vol 90. New York Botanical Garden Press, Bronx
Halling RE, Baroni TJ, Binder M (2007) A new genus of Boletaceae from eastern North America. Mycologia 99(2):310–316. https://doi.org/10.3852/mycologia.99.2.310
Halling RE, Fechner N, Nuhn M, Osmundson TW, Soytong K, Arora D, Binder M, Hibbett DS (2015) Evolutionary relationships of Heimioporus and Boletellus (Boletales), with an emphasis on Australian taxa including new species and new combinations in Aureoboletus, Hemileccinum and Xerocomus. Aust Syst Bot 28:1–22. https://doi.org/10.1071/SB14049
Heinemann P, Rammeloo J, Rullier E (1988) L’ornementation sporale des Xerocomaceae à spores dites lisses. Bull Jard Bot Nat Belg 58:513–534
Henkel TW, Obase K, Husbands D, Uehling JK, Bonito G et al (2016) New Boletaceae taxa from Guyana: Binderoboletus segoi gen. and sp. nov., Guyanaporus albipodus gen. and sp. nov., Singerocomus rubriflavus gen. and sp. nov., and a new combination for Xerocomus inundabilis. Mycologia 108(1):157–173. https://doi.org/10.3852/15-075
Katoh K, Misawa K, Kuma K, Miyata T (2002) MAFFT: a novel method for rapid multiple sequence alignment based on fast Fourier transform. Nucleic Acids Res 30(14):3059–3066
Klofac W, Krisai-Greilhuber I (2020) Revised key for the determination of fresh collections of European species of Boletales with tubulate hymenophore. Österr Z Pilzk 27:81–303
Knudsen H, Taylor AFS (2012) Boletales E.-J. Gilbert. In: H Knudsen, J Vesterholt (eds). Funga Nordica – agaricoid, boletoid, clavarioid, cyphelloid and gastroid genera. Nordsvamp, Copenhagen
Kozlov AM, Darriba D, Flouri T, Morel B, Stamatakis A (2019) RAxML-NG: a fast, scalable and user-friendly tool for maximum likelihood phylogenetic inference. Bioinformatics 35(21):4453–4455
Ladurner H, Simonini G (2003) Xerocomus s.l. Fungi Europaei 8. Edizioni Candusso, Alassio
Lannoy G, Estadès A (2001) Flore mycologique d’Europe 6 – les bolets. Documents Mycologiques, Mémoire hors série 6. CRDP de l’académie d’Amiens, Amiens
Li X, Wang F, Liu M, Dong C (2021) Hydrophobin CmHYD1 is involved in conidiation, infection and primordium formation, and regulated by GATA transcription factor CmAreA in edible fungus. Cordyceps Militaris J Fungi (basel) 7(8):674. https://doi.org/10.3390/jof7080674
Liang ZQ, An DY, Jiang S, Su MS, Zeng NK (2016) Butyriboletus hainanensis (Boletaceae, Boletales), a new species from tropical China. Phytotaxa 267(4):256–262. https://doi.org/10.11646/phytotaxa.267.4.2
Loizides M, Bellanger JM, Assyov B, Moreau PA, Richard F (2019) Present status and future of boletoid fungi (Boletaceae) on the island of Cyprus: cryptic and threatened diversity unravelled by ten-year study. Fungal Ecol 41:65–81. https://doi.org/10.1016/j.funeco.2019.03.008
Lugones L, Bosscher J, Scholtmeijer K, Vries O, Wessels J (1996) An abundant hydrophobin (ABH1) forms hydrophobic rodlet layers in Agaricus bisporus mushrooms. Microbiology 142(5):1321–1329. https://doi.org/10.1099/13500872-142-5-1321
Magnago AC (2014) Taxonomia e sistemática de Boletaceae (Boletales) para o Brasil. Algas e Plantas, Universidade Federal de Santa Catarina, Florianópolis, Dissertação Pós-Graduação em Biologia de Fungos
Magnago AC, Henkel T, Neves MA, Borges da Silveira RM (2018) Singerocomus atlanticus sp. nov., and a first record of Singerocomus rubriflavus (Boletaceae, Boletales) for Brazil. Acta Bot Brasilica 32(2):222–231. https://doi.org/10.1590/0102-33062017abb0320
Magnago AC, Alves-Silva G, Henkel TW, Borges da Silveira RM (2022) New genera, species, and combinations of Boletaceae from Brazil and Guyana. Mycologia 114(3):607–625. https://doi.org/10.1080/00275514.2022.2037307
Matheny PB, Liu YJ, Ammirati JF, Hall BD (2002) Using RPB1 sequences to improve phylogenetic inference among mushrooms (Inocybe, Agaricales). Am J Bot 89(4):688–698
Matheny PB, Wang Z, Binder M, Curtis JM, Lim YW et al (2007) Contributions of rpb2 and tef1 to the phylogeny of mushrooms and allies (Basidiomycota, Fungi). Mol Phyl Evol 43:430–451
Miller OK Jr, Lodge DJ, Baroni TJ (2000) New and interesting ectomycorrhizal fungi from Puerto Rico, Mona, and Guana Islands. Mycologia 92(3):558–570. https://doi.org/10.2307/3761516
Miller MA, Pfeiffer W, Schwartz T (2010) Creating the CIPRES science gateway for inference of large phylogenetic trees. In: Proceedings of the gateway computing environments workshop (GCE), 14 November 2010. New Orleans, LA, pp 1–8
Minter DW, Rodríguez-Hernández M, Mena-Portales J (2001) Fungi of the Caribbean. PDMS Publishing, Isleworth, An annotated checklist
Muñoz JA, Cadiñanos Aguirre JA, Fidalgo E (2008) Contribución al catálogo corológico del género Xerocomus en la Peninsula Iberica. Bol Soc Micol Madr 32:249–277
Naseer A, Sarwar S, Khalid AN, Healy R, Smith ME (2019) Hortiboletus kohistanensis (Boletaceae), a new bolete species from temperate and subalpine oak forests of Pakistan. Phytotaxa 388(3):239–246. https://doi.org/10.11646/phytotaxa.388.3.3
Neves MA, Capelari M (2007) A preliminary checklist of Boletales from Brazil and notes on Boletales specimens at the Instituto de Botânica (SP) Herbarium, São Paulo, SP. Brazil Sitientibus Série Ciências Biologicas 7(2):163–169
Nuhn ME, Binder M, Taylor AFS, Halling RE, Hibbett DS (2013) Phylogenetic overview of the Boletineae. Fungal Biol 117(7–8):479–511. https://doi.org/10.1016/j.funbio.2013.04.008
Oolbekkink G (1991) The taxonomic value of the ornamentation of spores in the Xerocomus group of Boletus. Persoonia 14(3):245–273
Ortiz-Santana B, Lodge DJ, Baroni TJ, Both EE (2007) Boletes from Belize and the Dominican Republic. Fungal Divers 27(2):247–416
Ortiz-Santana B, Bessette AE, McConnell OL (2016) Boletus durhamensis sp. Nov. from North Carolina. Mycotaxon 131(3):703–715. https://doi.org/10.5248/131.703
Osmundson TW, Robert VA, Schoch CL, Baker LJ, Smith A et al (2013) Filling gaps in biodiversity knowledge for macrofungi: contributions and assessment of an herbarium collection dna barcode sequencing project. PLoS ONE 8(4):e62419. https://doi.org/10.1371/journal.pone.0062419
Parra AL, Angelini C, Ortiz-Santana B, Mata G, Billette C et al (2018) The genus Agaricus in the Caribbean. Nine new taxa mostly based on collections from the Dominican Republic. Phytotaxa 345(3):219–271. https://doi.org/10.11646/phytotaxa.345.3.2
Pegler DN (1983) Agaric flora of the Lesser Antilles. Kew Bulletin Additional Series 9. HMSO, London
Peintner U, Ladurner H, Simonini G (2003) Xerocomus cisalpinus sp. nov., and the delimitation of species in the X. chrysenteron complex based on morphology and rDNA–LSU sequences. Mycol Res 107(6):659–679. https://doi.org/10.1017/S0953756203007901
Putzke J, Putzke MTL (2019) Cogumelos (fungos Agaricales) no Brasil - Ordens Boletales (Boletaceae e Paxillaceae), Polyporales (Polyporaceae/Lentinaceae), Russulales (Russulaceae) e Agaricales (Cortinariaceae, Inocybaceae, Pluteaceae e Strophariaceae). Vol 2. E-book, São Gabriel
Rehner SA, Buckley E (2005) A Beauveria phylogeny inferred from nuclear ITS and EF1-a sequences: evidence for cryptic diversification and links to Cordyceps teleomorphs. Mycologia 97(1):84–98
Ridgway R (1912) Color standards and color nomenclature. Self-published, Washington DC
Rineau F, Lmalem H, Ahren D, Shah F, Johansson T et al (2017) Comparative genomics and expression levels of hydrophobins from eight mycorrhizal genomes. Mycorrhiza 27:383–396. https://doi.org/10.1007/s00572-016-0758-4
Ronquist F, Teslenko M, van der Mark P, Ayres DL, Darling A et al (2012) MrBayes 3.2: efficient Bayesian phylogenetic inference and model choice across a large model space. Syst Biol 61:539–542
Šutara J (2008) Xerocomus s.l. in the light of the present state of knowledge. Czech Mycol 60(1):29–62
Šutara J, Mikšík M, Janda V (2009) Hřibovité houby. Academia, Praha
Singer R (1946) The Boletineae of Florida with notes on extralimital species. II. The Boletaceae (Gyroporoideae). Farlowia 2(2):223–303
Singer R (1945) New Boletaceae from Florida (a preliminary communication). Mycologia 37(6):797–799
Singer R (1947) The Boletoideae of Florida. The Boletineae of Florida with notes on extralimital species III. Am Midl Nat 37(1):1–135. https://doi.org/10.2307/2421647
Singer R, Digilio APL (1960) Las Boletaceas De Sudamerica Tropical Lilloa 30:141–164
Singer R, Fiard JP (1976) (“1977”) Agaricales nouvelles des Antilles françaises. Bull Soc Mycol Fr 92(4):445–447
Singer R, Araujo I, Ivory MH (1983) The ectotrophically mycorrizal fungi of the neotropical lowlands, especially Central Amazonia (litter decomposition and ectomycorrhiza in Amazonian forests 2.). Nova Hedwigia Beih 77:1–352
Singer R (1986) The Agaricales in modern taxonomy, 4th edn. Koeltz Scientific Books, Koenigstein
Singer R (1988) La fitogeografia de las Boletineas (Basidiomycetes, Agaricales) en relacion a las especies Mexacanas. Rev Mex Micol 4:267–274
Sulzbacher MA, Grebenc T, Jacques RJS, Antoniolli ZI (2013) Ectomycorrhizal fungi from southern Brazil—a literature-based review, their origin and potential hosts. Mycosphere 4(1):61–95. https://doi.org/10.5943/mycosphere/4/1/5
Tagu D, Nasse B, Martin F (1996) Cloning and characterization of hydrophobins-encoding cDNAs from the ectomycorrhizal Basidiomycete Pisolithus tinctorius. Gene 168(1):93–97. https://doi.org/10.1016/0378-1119(95)00725-3
Taylor AFS, Jonsson L, Jonsson M, Rosling A, Hills AE et al (2001) Species delineation within European species of Xerocomus using Internal Transcriber Spacer sequence data. Micol Veget Medit 16(2):171–192
Taylor AFS, Hills AE, Simonini G, Both EE, Eberhardt U (2006) Detection of species within the Xerocomus subtomentosus complex in Europe using rDNA-ITS sequences. Mycol Res 110(3):276–287. https://doi.org/10.1016/j.mycres.2005.11.013
Thiers B (2022) (continuously updated) Index Herbariorum: a global directory of public herbaria and associated staff. New York botanical garden’s virtual herbarium. http://sweetgum.nybg.org/ih/. Accessed 20 Sep 2022
Vadthanarat S, Lumyong S, Raspé O (2019) Cacaoporus, a new Boletaceae genus, with two new species from Thailand. MycoKeys 54:1–29
Vadthanarat S, Raspé O, Lumyong S (2022) Rubinosporus auriporus gen. et sp. nov. (Boletaceae: Xerocomoideae) from tropical forests of Thailand, producing unusual dark ruby spore deposits. J Fungi 8:278. https://doi.org/10.3390/jof8030278
Vilgalys R, Hester M (1990) Rapid genetic identification and mapping of enzymatically amplified ribosomal DNA from several Cryptococcus species. J Bacteriology 172:4238–4246
Vizzini A (2014a) Nomenclatural Novelties Index Fungorum 146:1–2
Vizzini A (2014b) Nomenclatural Novelties Index Fungorum 147:1
Vizzini A (2014c) Nomenclatural Novelties Index Fungorum 176:1
Vizzini A (2014d) Nomenclatural Novelties Index Fungorum 183:1
Vizzini A (2014e) Nomenclatural Novelties Index Fungorum 188:1
Vizzini A (2014f) Nomenclatural Novelties Index Fungorum 192:1
Vizzini A, Simonini G, Ercole E, Voyron S (2014) Boletus mendax, a new species of Boletus sect. Luridi from Italy, and insights on the B. luridus complex. Mycol Prog 13(1):95–109. https://doi.org/10.1007/s11557-013-0896-4
Vizzini A (2015) Nomenclatural Novelties. Index Fungorum 244:1
Watling R, de Meijer AAR (1997) Macromycetes from the State of Paraná, Brazil 5. Poroid and lamellate boletes. Edinburgh J Bot 54(2):231–251. https://doi.org/10.1017/S0960428600004042
Watling R, Hills AE (2005) Boletes and their allies – Boletaceae, Strobilomycetaceae, Gyroporaceae, Paxillaceae, Coniophoraceae, Gomphidiaceae. British Fungus Flora, Agarics and Boleti. Vol 1. HMSO, Edinburgh
Watling R (2008) A manual and source book on the boletes and their allies. Synopsis Fungorum 24. Fungiflora, Oslo
White TJ, Bruns T, Lee S, Taylor J (1990) Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis MA, Gelfand DH, Sninsky J et al (eds): PCR protocols: a guide to methods and applications. Academic Press, San Diego
Wösten H, De Vries O, Wessels J (1993) Interfacial self-assembly of a fungal hydrophobin into a hydrophobic rodlet layer. Plant Cell 5(11):1567–1574. https://doi.org/10.1105/tpc.5.11.1567
Wu G, Feng B, Xu JP, Zhu XT, Li YC et al (2014) Molecular phylogenetic analysis redefines seven major clades and reveal 22 new generic clades in the fungal family Boletaceae. Fungal Divers 69(1):93–115. https://doi.org/10.1007/s13225-014-0283-8
Wu G, Zhao K, Li YC, Zeng NK, Feng B et al (2016a) Four new genera of the fungal family Boletaceae. Fungal Divers 81(1):1–24. https://doi.org/10.1007/s13225-015-0322-0
Wu G, Li YC, Zhu XT, Zhao K, Han LH et al (2016b) One hundred noteworthy boletes from China. Fungal Divers 81(1):25–188. https://doi.org/10.1007/s13225-016-0375-8
Wu G, Li MX, Horak E, Yang ZL (2021) Phylogenetic analysis reveals the new genus Amoenoboletus from Asia and New Zealand. Mycologia 114(1):144–156. https://doi.org/10.1080/00275514.2021.1971450
Xie HJ, Lin WF, Jiang S, Xue R, Wu LL et al (2020) Two new species of Hortiboletus (Boletaceae, Boletales) from China. Mycol Prog 19(11):1377–1386. https://doi.org/10.1007/s11557-020-01634-z
Yan WJ, Li TH, Zhang M, Li T (2013) Xerocomus porophyllus sp. nov., morphologically intermediate between Phylloporus and Xerocomus. Mycotaxon 124:255–262
Zeng NK, Cai Q, Yang ZL (2012) Corneroboletus, a new genus to accommodate the southeast Asian Boletus indecorus. Mycologia 104(6):1420–1432. https://doi.org/10.3852/11-326
Zeng NK, Wu G, Li YC, Liang ZQ, Yang ZL (2014) Crocinoboletus, a new genus of Boletaceae (Boletales) with unusual boletocrocin polyene pigments. Phytotaxa 175(3):133–140. https://doi.org/10.11646/phytotaxa.175.3.2
Zhao K, Wu G, Feng B, Yang ZL (2014a) Molecular phylogeny of Caloboletus (Boletaceae) and a new species in East Asia. Mycol Prog 13(4):1127–1136. https://doi.org/10.1007/s11557-014-1001-3
Zhao K, Wu G, Yang ZL (2014b) A new genus, Rubroboletus, to accommodate Boletus sinicus and its allies. Phytotaxa 188(2):61–77. https://doi.org/10.11646/phytotaxa.188.2.1
Zhu XT, Li YC, Wu G, Feng B, Zhao K et al (2014) The genus Imleria (Boletaceae) in East Asia. Phytotaxa 191(1):81–98. https://doi.org/10.11646/phytotaxa.191.1.5
Zhu XT, Wu G, Zhao K, Halling RE, Yang ZL (2015) Hourangia, a new genus of Boletaceae to accommodate Xerocomus cheoi and its allied species. Mycol Prog 14(6):37. https://doi.org/10.1007/s11557-015-1060-0
Acknowledgements
Claudio Angelini wishes to thank P. Suarez, F. Jiménez, T. Clase, E. Septimo, M.C. Nova (Jardín Botánico Nacional Dr. Rafael M. Moscoso, Santo Domingo, Dominican Republic) for their interest and encouragement in studying fungi of the Dominican Republic and for their active cooperation in providing herbarium material preserved at their institution. We are indebted to L. Davies (Royal Botanic Gardens, Kew, UK), G.M. Mueller, and T. Widhelm (Field Museum of Natural History, Chicago, USA), as well as R.E. Halling and L. Briscoe (New York Botanical Garden, New York, USA) for their assistance in providing the collections (predominantly types) of X. coccolobae, X. caerulonigrescens, X. cuneipes, X. pseudoboletinus var. pini-caribaeae, S. guadelupae, and T. ruborculus for sampling. We would like to acknowledge Á. Kovács (Biological Research Center, Szeged, Hungary), R. Woods, E. Arrigoni, and A. Byrne (Royal Botanic Gardens, Kew, UK) for their help with the molecular work on type specimens and B.T.M. Dentinger (University of Utah, Salt Lake City, USA) for initial funding to sequence some of the specimens. We also express our gratitude to I.S. Druzhinina (Royal Botanic Gardens, Kew, UK), I. Clatworthy (National History Museum, UK), and B. Dobrić (Serbia) for assistance in preparing specimens, SEM photography, and interpretation of results. A.C. Magnago (Universidade Federal do Espírito Santo, Vitória, Brazil) provided valuable literature. Thanks are also given to the anonymous reviewers for their constructive comments and valuable suggestions.
Funding
Open access funding provided by Università degli Studi di Torino within the CRUI-CARE Agreement. Tatiana Yu. Svetasheva was supported by the project RFBR 20–04-00349.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study, conception, and design. Claudio Angelini, Kurt O. Miller, Javier Isaac de la Fuente, and Jesús García Jiménez collected specimens and contributed to the morphological work and descriptions. Matteo Gelardi and Alona Yu. Biketova performed microscopical descriptions. Matteo Gelardi made the line drawings. Enrico Ercole and Alfredo Vizzini did the molecular lab work and performed the phylogenetic analyses. Alona Yu. Biketova, Laura M. Suz, Tatiana Yu. Svetasheva, Kurt O. Miller, Javier Isaac de la Fuente, and Jesús García Jiménez provided some DNA sequences. Matteo Gelardi and Alfredo Vizzini wrote the manuscript, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Informed consent was obtained from all individual participants included in the study.
Competing interests
The authors declare no competing interests.
Additional information
Responsible Editor: Zhu-Liang Yang.
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
11557_2023_1876_MOESM1_ESM.pdf
Supplementary file1 Suppl. Fig. 1 Boletaceae-wide Maximum Likelihood phylogenetic tree inferred from a two-gene dataset (RPB2 and TEF1), showing placement of the new genus Tropicoboletus. Maximum Likelihood Bootstrap support values (MLB ≥ 0.70) are shown above the supported branches. Buchwaldoboletus lignicola and seven Chalciporus species (subfamily Chalciporoideae) were used as the outgroup taxa. Tree identical to that of Fig. 1 but the subfamilies Austroboletoideae, Boletoideae, Chalciporoideae, Leccinoideae, and Zangioideae are not collapsed. Newly generated sequences are indicated in bold (PDF 101 KB)
11557_2023_1876_MOESM2_ESM.pdf
Supplementary file2 Suppl. Fig. 2 Boletaceae-wide Maximum Likelihood phylogenetic tree inferred from the LSU dataset, showing placement of the new genus Tropicoboletus. Maximum Likelihood Bootstrap support values (MLB ≥ 0.70) are shown above the supported branches. Chalciporus species (subfamily Chalciporoideae) were used as the outgroup taxa. Newly generated sequences are indicated in bold (PDF 78 KB)
11557_2023_1876_MOESM3_ESM.pdf
Supplementary file3 Suppl. Fig. 3 Boletaceae-wide Maximum Likelihood phylogenetic tree inferred from the RPB2 dataset, showing placement of the new genus Tropicoboletus. Maximum Likelihood Bootstrap support values (MLB ≥ 0.70) are shown above the supported branches. Buchwaldoboletus lignicola, Chalciporus (including Rubinoboletus) species (subfamily Chalciporoideae) were used as the outgroup taxa. Newly generated sequences are indicated in bold (PDF 98 KB)
11557_2023_1876_MOESM4_ESM.pdf
Supplementary file4 Suppl. Fig. 4 Boletaceae-wide Maximum Likelihood phylogenetic tree inferred from the TEF1 dataset, showing placement of the new genus Tropicoboletus. Maximum Likelihood Bootstrap support values (MLB ≥ 0.70) are shown above the supported branches. Buchwaldoboletus lignicola, Chalciporus (including Rubinoboletus) species (subfamily Chalciporoideae) were used as the outgroup taxa. Newly generated sequences are indicated in bold (PDF 100 KB)
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Gelardi, M., Angelini, C., Biketova, A.Y. et al. Coccoloba-associated xerocomoid boletes (Boletaceae) from the Caribbean and Mexico: Tropicoboletus ruborculus gen. et comb. nov., revision of Xerocomus coccolobae, phylogenetic assessment of Singerocomus guadelupae comb. nov., and type studies of Xerocomus caeruleonigrescens, X. cuneipes, and X. pseudoboletinus var. pini-caribaeae. Mycol Progress 22, 29 (2023). https://doi.org/10.1007/s11557-023-01876-7
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11557-023-01876-7