Abstract
Purpose
Cochlea implant surgery with proper positioning of the cochlear electrode can be challenging. Intraoperative real-time hybrid laser-fluoroscopic-guided navigation based on a multiplanar cone beam computed tomography (CBCT) dataset opens up the opportunity to immediate radiological control of primary electrode misalignments and offering new insights into the cochlea electrode insertion routes and favorable cochlear implant-insertion angle.
Methods
In this retrospective study, 50 cases (29 males, 18 females) of conventional electrode implantation (without intraoperative image control; group A) and nine cases (7 males, 2 females) of CBCT-laser-fluoroscopic-guided surgery (group B) were included in the present study. CBCT-laser-guided surgery under real-time fluoroscopic control was conducted using an intraoperative C-arm CBCT. All patients received preoperative cross-sectional imaging (CT and MRI), in which cochlear malformation could be excluded. Postoperatively, we looked for electrode misplacements.
Results
In group A, electrode misalignment was detected postoperatively in 14 of 50 cases (28.0%). In group B, primary electrode misalignment was detected intraoperatively in two patients (22.2%). In both patients, the misalignments were corrected in the same session. The comparison of cochlear insertion angles showed significant differences. Group A: 47.5 ± 2.6° (actual conventional surgery) vs 17.6 ± 2.8° (theoretical CBCT-laser-fluoroscopic-guided surgery) P < 0.001. Group A vs group B: 47.5 ± 2.6° (actual conventional surgery; Group A) vs 17.9 ± 2.5° (actual CBCT-laser-fluoroscopic-guided surgery; Group B) P < 0.001.
Conclusion
We consider that an intraoperative hybrid CBCT-laser-fluoroscopic-controlled approach in cochlear implant surgery using a C-arm CT can be beneficial, because electrode misalignments can be reduced and if it does occur, remedied in the same surgical session.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cochlear implant surgery is constantly evolving. Not only preoperative diagnostics and planning are crucial for a good result, but also more often intraoperative image-guided electrode navigation. For optimal hearing results in cochlear implant surgery, the implanted electrode should be located completely within the scala tympani.
The theoretically achievable insertion depth is not often achievable in practice for many patients. In addition, accidental scale shift into the scala vestibuli is to be avoided [1]. Equally, electrode tip fold-over and other dislocations of the electrode array affect the correct functioning of the implant negatively, leading to complete dysfunction in the worst case [2,3,4].
The importance of accurate navigation in cochlear implantation has been widely recognized. Previously, alternative methods like the three-dimensional CT-based method for preoperative imaging have been proposed [5]; however, they do not lend real-time guidance to the surgeon during surgery. In past years, electrophysiological measurements [6], fluoroscopy [7], and transorbital or head X-ray [8, 9] have been available during surgery in a routine setting, and they have shown clear benefits with regard to correct electrode placement. With timely use of intraoperative radiological diagnostics, the need for revisions can be largely obviated [10].
We have developed a hybrid laser-fluoroscopic-guided CBCT approach to resolve the above challenging issues in the cochlear implantation. We believe that the system can support the planning and execution of the access approach to the round window, as well as the optimization of the electrode insertion angle and intraoperative 3D electrode array monitoring in the cochlea. The aim of study here is to evaluate incidence of electrode misalignment and electrode insertion angle into the cochlear in the hybrid CBCT-laser-fluoroscopic-guided cochlear implant surgery in comparison of those in the conventional approach.
Materials and methods
The local ethics committee approved this retrospective study (Nr: 2021-15,837).
Patient cohort
The study cohort consisted of 50 cases (47 patients) of conventional cochlear implantation and nine cases (nine patients) of cochlear implantation by CT-laser-guided surgery.
Conventional surgery group A: Patients’ age in the conventional surgery cohort ranged from 9 to 80 years at the time of surgery. Nineteen patients were female, and 28 were male. Implantations were conducted between June 2013 and January 2018. Twenty-one right-sided and 29 left-sided implantations were performed.
Hybrid CBCT-laser-fluoroscopic-guided surgery group B: Patients’ ages in the laser-guided surgery cohort ranged from 19 to 64 years at the time of surgery. Two patients were female, and seven patients were male. Implantations were conducted between August 2017 and March 2018. Four right-sided and five left-sided implantations were performed.
Examination technique
The surgical approach in group B was conducted using a novel robotically controlled C-arm-system for flexible intraoperative imaging and navigation, and the ARTIS Pheno (Siemens Healthineers, Forchheim, Germany) CBCT imaging was performed before and after cochlear surgery.
Intraoperatively, the primary CBCT reconstructions were preformed directly after anesthetic induction and head fixation (with self-adhesive fixation tapes) using a workstation (SyngoVia V10B, Siemens Healthineers, Forchheim, Germany).
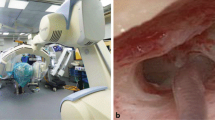
Based on these multiplanar reconstructions, the surgical approach was performed by mastoidectomy in the usual way. The optimized multiplanar insertion angle of the electrode array into the cochlea was also planned and determined jointly by an experienced ear surgeon and a neuroradiologist in an interdisciplinary manner. The jointly verified electrode insertion angle was drilled with a thin drill channel based on the multiplanar image data set using the SyngoVia “needle guidance” application in the appropriate automatically calculated fluoroscopic angles (e.g., bulls eye view) and additional laser navigated projection for the surgeon (Fig. 1). The general setup is shown in Fig. 2. The application visualized the insertion angle in a double oblique reconstruction, which showed the cochlear turns in relation to the round window membrane.
Illustration of the hybrid CBCT- laser-fluoroscopic-guided procedure. A CBCT-laser-fluoroscopic-guided insertion of the drill channel. Shown is the drill with the centered laservization for the access path determined by CBCT B Corresponding fluoroscopic bullseye view of the navigated insertion. Centered in the white circle the drill is pictured. C Fluoroscopic second view from another viewing angle of the navigated insertion. The drill can be delimited here in its longitudinal extension
In addition, the facial nerve was controlled by means of neuromonitoring. Corresponding to the angulation of the drill channel, the cochlear electrode was inserted into the round window under real-time fluoroscopy. Electrodes from MED-El® (Starnberg, Germany) were used for all implantations. Access was through the round window in both groups. No intraoperative complications occurred in both groups.
After complete implantation of the electrode array, a second CBCT scan was immediately performed to check the intratympanic electrode position.
The CBCT (ARTIS Pheno, Siemens Healthineers, Forchheim, Germany) scan used a 30 × 40 detector with a 3D volume size of 13 × 9.3 inches and automated tube voltage selection (CARE kV). The quality reference tube voltage was 90 kV. Intraoperative laser-fluoroscopic imaging was based on three automatically calculated projections (needle guidance application) with microfocus and overlaid navigation paths (Fig. 1).
Images were obtained using a rotation time = 4 s, section thickness = 0.25 mm, and section interval = 0.1 mm. Reconstruction was performed using the Hr60 kernel.
Postoperative CBCT controls were performed immediately after cochlear implantation with the surgical access route not yet closed. This allowed immediate correction of electrode misalignments.
In group A, the postoperative radiological assessment was conducted by computed tomography (MSCT) in 32 cases and by cone beam CT in 18 cases.
The MSCT scan used the Somatom Force (dual-source 192-dectetor row scanner; Siemens Healthineers, Forchheim, Germany) with single-energy automated tube voltage selection (CARE kV). The quality reference tube voltage was 120 kV, providing an acquisition of 100 kV and a tube current of 375 mAs.
Images were obtained using a beam collimation = 0.5 mm, rotation time = 0.25 s, FOV = 240 mm, section thickness = 0.5 mm, section interval = 0.25 mm, and pitch = 0.8. Reconstruction was performed using the Hr60 kernel.
The cone beam CT was performed by the CBCT 3D Accuitomo 170 (J Morita Mfg Corp., Kyoto, Japan) using 90-kV tube voltage and 5-mA current, with a high-resolution mode with a rotation of 180 degrees. A voxel size of 0.125 mm and an ROI of 80 × 80 × 80 mm are observed. Images were reconstructed with filtered back-projection using the G_001 reconstruction algorithm.
Image evaluation
All obtained images in both groups were reconstructed using a workstation (SyngoVia V10B, Siemens Healthineers, Forchheim, Germany) and examined by two experienced neuroradiologists.
First, the electrode array positions were assessed in the cochlear with respect to the presence of misalignments (Table 1).
Second, we determined the optimal insertion vector aligned with the basal turn of the cochlea/scala tympani. Due to anatomical conditions, this angle would mostly lead inoperably through the middle cranial fossa. The deviations (angle alpha and beta) in our study are calculated in relation to this optimal insertion vector (Fig. 3).
Visualization of the different insertion angles (exemplary representation of a patient of conventional surgery group A). White arrow represents the optimal insertion vector (theoretical; through the middle cranial fossa). Green arrow represents the theoretical hybrid CBCT-laser-fluoroscopic-guided insertion vector. Red arrow represents the actual surgical insertion vector. (A) angle of deviation of the conventional surgery vector from the optimal insertion vector (angle alpha). (B) Angle of deviation of the hybrid CBCT-laser-fluoroscopic-guided insertion vector from the optimal insertion vector (angle beta). (C) Cutaneous distance between actual surgical vector and the theoretical hybrid CBCT-laser-fluoroscopic-guided vector
Angle alpha describes the degree of deviation of the conventional surgery cochlear electrode insertion from the optimal insertion vector. Angle beta represents the degree of deviation from the hybrid CBCT-laser-fluoroscopic-guided insertion from optimal insertion vector. (Table 2).
Statistical analysis
The statistical calculations were performed using SPSS (IBM SPSS Statistics, version 20.0 for Macintosh; SPSS, Inc., Chicago, IL, USA).
All continuous variables are expressed as arithmetic mean ± standard deviation (SD). A significance level of 5% was used.
The different insertion angles were evaluated with paired t test and unpaired t test. Normal distribution of the data sets was analyzed with Kolmogorov–Smirnov test. Chi-square test was used to analyze the frequencies of electrode misalignment for the different surgical methods.
Results
The operation time ranged from 82 to 124 min (mean 106 ± 12) in group A, and from 175 to 237 min in the group B (mean 198 ± 19 min; vs. group A, P < 0.001, t test).
Cochlear electrode array misalignment
The incidence of misalignment of electrodes yielded no statistical significance comparing group A and group B (P = 0.720). In the group A, electrode array misalignment was detected postoperatively in 14 of 50 cases (28.0%); ten cases needed a revision. In four cases, the patients experienced a subjective hearing improvement after implantation, so the patients declined revision surgery. In one patient, the electrode array was misaligned initially as well as in the revision. In ten cases, a primary displacement from scala tympani to scala vestibuli was detected; in two cases, such a misalignment was preceded by fold-over (one case) or buckling (one case) of the electrode array. In one case, vestibular displacement was accompanied by complete dislocation of the electrode. In two other cases, the cochlear implant and the electrodes, respectively, were inserted incompletely (Fig. 4). In this group B, primary electrode array misalignment was detected intraoperatively in two patients (22.2%): In one of them, a primary misalignment to the scala vestibuli most likely caused by considerable sclerosis of the cochlea occurred, in the other, a primary displacement to the sacculus was detected. In both, the displacement was corrected in the same session. The follow-up scans did not reveal any displacements (Table 1).
Cochlear insertion angles
In group A, the actual angle of deviation from the optimal insertion vector was 47.5 ± 2.6 degrees (angle alpha). In the group B, the angle of deviation from the optimal insertion vector was 17.9 ± 2.5 degrees (angle beta). The angles of the both groups were significantly different (P < 0.001)(Fig. 5). Additionally, the theoretic possible CBCT-laser-guided insertion angle of group A was 17.6 ± 2.8 degrees, which is significant different from angle alpha (P < 0.001, t-test), but similar to angle beta (Fig. 5).
Bar charts of comparison of different insertion angels. Comparison of actual insertion angle from conventional surgery group (alpha angle) with theoretical insertion angle (theor) of hybrid CBCT-laser-fluoroscopic-guided surgery method (postoperative control CBCT) (P < 0.001). Comparison of actual insertion angle of conventional surgery group A with actual insertion angle from hybrid CBCT-laser-fluoroscopic-guided surgery (beta angle) (P < 0.001). Comparison of theoretical CBCT-laser-fluoroscopic-guided surgery insertion angle from conventional surgery group with actual insertion angle from hybrid CBCT-laser-fluoroscopic-guided surgery group B (P = 0.763). asterisks indicate significance, NS Nonsignificant
Discussion
In our study, we analyzed a hybrid CBCT-laser-fluoroscopic-guided surgery. The study revealed that this procedure has the potential to improve outcomes even further than intraoperative radiology alone. An essential difference to the technique presented by Bárdosi et al. [11] is the real-time CBCT fluoroscopic laser navigation in our study.
This means that potential disadvantages such as a coregistration of a preoperatively prepared CT data set with the associated risk of potential false registration can be dispensed with. Given the success of navigation support in sinus surgery [11, 12], thoracoscopy [13, 14], lumbar spine surgery [15], and cardiac implantation of electronic devices [16], we evaluated its usefulness for the cochlear implantation.
The study demonstrates that the real-time CBCT fluoroscopic laser navigation can be possible and beneficial in cochlear implantation as well. In particular, the surgeon has the possibility of a real-time double check of his surgical access route to the round window membrane by means of laser navigation and, if necessary, supplementary corresponding navigated fluoroscopy to immediately detect deviations from the planned access route (Fig. 1). The likelihood for correct placement of the electrode in the scala tympani is the highest in a surgical round window approach [1]. The electrodes of all patients in this study were inserted through the round window approach. The difficulty of electrode implantation through the round window is dependent on the patient’s anatomy; a larger angle between the line connecting the leading edge of the facial nerve to the midpoint of the round window and the median sagittal line, which can be measured in preoperative CT scans, is associated with a greater difficulty in round window implantation [17]. Poor mastoid aeration and lower position of the tegmen are also associated with higher difficulty in accessing the round window [18]. The real-time CBCT fluoroscopic laser navigation is capable of detecting deviations from the correct angle of insertion during surgery.
Electrode malposition can also be detected during surgery because the advancement of electrode array is immediately visualized on the monitor. The incidence of electrode misalignment is reported to be below 3% in the literature [19, 20]. However, our clinical experience and the data in the present study suggest that the number of undetected cases is considerably higher, due to a lack of standardization in postoperative imaging [21]. This is also reflected in the fact that there is a wide range of misalignment rates especially for scalar translocation, which is the most common type of misalignment, between different studies with up to 54% scalar translocation rates [21]. The results of our study with 28.0% overall misalignment in group A and 22.2% overall misalignment in group B are in this range.
Electrode kinking was found in 2.3% of cases as an intraoperative complication in one study [19]. The tip fold-over, which was not immediately clinically identifiable, occurred in 1.98% of cases in a study with 303 cochlea implantations [20].
These previous reports suggest that early intraoperative detection of electrode misalignment and thus real-time CBCT fluoroscopic laser navigation can be beneficial. Only if, in conjunction with the impedance measurement and the surgical assessment, this seems necessary for an improvement of the hearing results. Intraoperative detection of electrode malposition allows the electrode position to be changed in the same session.
Study limitations
Our study focused on intraoperative and postoperative electrode misalignment as primary outcome. Outcome analysis with respect to clinical parameters such as length of hospitalization, postoperative complications such as infection and audiometric evaluation for the different study cohorts were not the primary target of this study. These analyses need to be further investigated in larger long-term multicenter studies.
The patient cohort of group B is limited in numbers due to the proof of concept. Therefore, the incidence of misalignment of electrodes might be large even in group B. We have also not yet focused on optimizing the prolonged temporal procedures for group B. Further investigations about the merits of this navigated access route in comparison with the conventional access routes remain to be conducted in larger numbers.
We have also not yet focused on optimizing the prolonged temporal procedures for group B. Further investigations about the merits of this navigated access route in comparison with the conventional access routes remain to be conducted in larger numbers.
Conclusions
Even if the comparison of both surgical procedures in our study did not lead to statistically significant different results, we consider that the hybrid CBCT-laser-fluoroscopic-guided surgical approach in cochlear implant surgery can be beneficial because electrode misalignments as one of the important causes of postoperative implant malfunction might be reduced.
In the present study, using hybrid CBCT-laser-fluoroscopic-guided surgery with an intraoperative C-arm CBCT, the authors were able to identify a new, optimized navigated access route into the cochlear for the electrode insertion with a steeper angle toward the round window, reducing the risk of electrode misalignment.
In addition, real-time multiplanar cross-sectional imaging allows immediate control of intracochlear electrode position and, if necessary, direct correction, and it reduces radiation dose by eliminating the need for additional postoperative CT scans.
Abbreviations
- CBCT:
-
Cone beam computed tomography
- CT:
-
Computed tomography
- mAs:
-
Milliampere-second
- MSCT:
-
Multislice computed tomography
- kV:
-
kilovoltage
References
O’Connell BP, Hunter JB, Wanna GB (2016) The importance of electrode location in cochlear implantation. Laryngoscope Investig Otolaryngol 1(6):169–174. https://doi.org/10.1002/lio2.42
Qiu J, Chen Y, Tan P, Chen J, Han Y, Gao L, Lu Y, Du B (2011) Complications and clinical analysis of 416 consecutive cochlear implantations. Int J Pediatr Otorhinolaryngol 75(9):1143–1146. https://doi.org/10.1016/j.ijporl.2011.06.006
Sabban D, Parodi M, Blanchard M, Ettienne V, Rouillon I, Loundon N (2018) Intra-cochlear electrode tip fold-over. Cochlear Implants Int 19(4):225–229. https://doi.org/10.1080/14670100.2018.1427823
Migirov L, Muchnik C, Kaplan-Neeman R, Kronenberg J (2006) Surgical and medical complications in paediatric cochlear implantation: a review of 300 cases. Cochlear Implants Int 7(4):194–201. https://doi.org/10.1179/cim.2006.7.4.194
Verbist BM, Joemai RM, Briaire JJ, Teeuwisse WM, Veldkamp WJ, Frijns JH (2010) Cochlear coordinates in regard to cochlear implantation: a clinically individually applicable 3 dimensional CT-based method. Otol Neurotol 31(5):738–744. https://doi.org/10.1097/MAO.0b013e3181d8d3c4
Garaycochea O, Manrique-Huarte R, Manrique M (2020) Intra-operative radiological diagnosis of a tip roll-over electrode array displacement using fluoroscopy, when electrophysiological testing is normal: the importance of both techniques in cochlear implant surgery. Braz J Otorhinolaryngol 86(Suppl 1):38–40. https://doi.org/10.1016/j.bjorl.2017.05.003
Viccaro M, Covelli E, De Seta E, Balsamo G, Filipo R (2009) The importance of intra-operative imaging during cochlear implant surgery. Cochlear Implants Int 10(4):198–202. https://doi.org/10.1179/cim.2009.10.4.198
Hassan AM, Patel R, Redleaf M (2015) Intra-operative skull X-ray for misdirection of the cochlear implant array into the vestibular labyrinth. J Laryngol Otol 129(9):923–927. https://doi.org/10.1017/S0022215115001966
Viccaro M, De Seta E, Covelli E, Marvaso V, Filipo R (2008) Another reason for intra-operative imaging during cochlear implantation. J Laryngol Otol 122(4):e12. https://doi.org/10.1017/S0022215108001989
Jiang Y, Gu P, Li B, Gao X, Sun B, Song Y, Wang G, Yuan Y, Wang C, Liu M, Han D, Dai P (2017) Analysis and management of complications in a cohort of 1065 minimally invasive cochlear implantations. Otol Neurotol 38(3):347–351. https://doi.org/10.1097/MAO.0000000000001302
Bárdosi Z, Plattner C, Özbek Y, Hofmann T, Milosavljevic S, Schartinger V, Freysinger W (2020) CIGuide: in situ augmented reality laser guidance. Int J Comput Assist Radiol Surg 15(1):49–57. https://doi.org/10.1007/s11548-019-02066-1
Khan M, Kosmecki B, Reutter A, Ozbek C, Keeve E, Olze H (2012) A noncontact laser-guided system for endoscopic computer-assisted sinus surgery. Surg Innov 19(3):308–315. https://doi.org/10.1177/1553350611426565
Hsieh MJ, Fang HY, Lin CC, Wen CT, Chen HW, Chao YK (2018) Single-stage localization and removal of small lung nodules through image-guided video-assisted thoracoscopic surgery. Eur J Cardiothorac Surg 53(2):353–358. https://doi.org/10.1093/ejcts/ezx309
Cheng YF, Chen HC, Ke PC, Hung WH, Cheng CY, Lin CH, Wang BY (2020) Image-guided video-assisted thoracoscopic surgery with Artis Pheno for pulmonary nodule resection. J Thorac Dis 12(4):1342–1349. https://doi.org/10.21037/jtd.2020.02.12
Richter PH, Gebhard F, Salameh M, Schuetze K, Kraus M (2017) Feasibility of laser-guided percutaneous pedicle screw placement in the lumbar spine using a hybrid-OR. Int J Comput Assist Radiol Surg 12(5):873–879. https://doi.org/10.1007/s11548-017-1529-1
Zardo P, Busk H, Hadem J, Baraki H, Kensah G, Kutschka I (2016) A novel video-assisted approach to excimer laser-guided cardiac implantable electronic devices lead extraction. Innovations (Phila) 11(3):210–213. https://doi.org/10.1097/IMI.0000000000000277
Xie L-H, Tang J, Miao W-J, Tang X-L, Li H, Tang A-Z (2018) Preoperative evaluation of cochlear implantation through the round window membrane in the facial recess using high-resolution computed tomography. Surg Radiol Anat 40(6):705–711
Park E, Amoodi H, Kuthubutheen J, Chen JM, Nedzelski JM, Lin VY (2015) Predictors of round window accessibility for adult cochlear implantation based on pre-operative CT scan: a prospective observational study. J Otolaryngol-Head Neck Surg 44(1):1–7
Hsieh HS, Wu CM, Zhuo MY, Yang CH, Hwang CF (2015) Intraoperative facial nerve monitoring during cochlear implant surgery: an observational study. Medicine (Baltimore) 94(4):e456. https://doi.org/10.1097/MD.0000000000000456
Zuniga MG, Rivas A, Hedley-Williams A, Gifford RH, Dwyer R, Dawant BM, Sunderhaus LW, Hovis KL, Wanna GB, Noble JH, Labadie RF (2017) Tip fold-over in cochlear implantation: case series. Otol Neurotol 38(2):199–206. https://doi.org/10.1097/MAO.0000000000001283
Dong Y, Briaire JJ, Siebrecht M, Stronks HC, Frijns JHM (2021) Detection of translocation of cochlear implant electrode arrays by intracochlear impedance measurements. Ear Hear 42(5):1397–1404. https://doi.org/10.1097/AUD.0000000000001033
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Waldeck, S., Schmidt, S., von Falck, C. et al. New hybrid multiplanar cone beam computed tomography-laser-fluoroscopic-guided approach in cochlear implant surgery. Int J CARS 17, 1837–1843 (2022). https://doi.org/10.1007/s11548-022-02703-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11548-022-02703-2