Abstract
Introduction
This study aims to explore the similarities in functional connectivity (FC) patterns in individuals when listening to different music genres and, in comparison, to the spoken word, using a novel data-driven approach. Our model and findings can potentially be utilized for evaluating the neurological effects of therapeutic music interventions.
Materials and methods
Twelve healthy volunteers listened to seven different sound tracks while undergoing functional magnetic resonance imaging (fMRI) scans: music of the volunteer’s choice with positive emotional attachment, two selections of unfamiliar classical music, one classical piece repeated with visual guidance and three spoken language tracks. FC network graphs were created, and selected graph properties were evaluated toward their commonalities across sound tracks. For comparison, FC patterns represented by the graph adjacency matrices were directly compared for high and low BOLD activation during listening.
Results
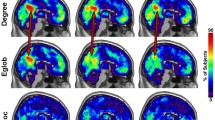
Graph properties averaged across subjects showed similar values for the same sound track compared to different sound tracks (p < 0.003). For high BOLD activation involving most areas in the auditory cortex, FC patterns for the same sound track correlated highly (0.74 ± 0.11), whereas FC patterns for different sound tracks did not (0.09 ± 0.07; p < 6e−5). For low BOLD activation involving additional brain regions, correlation of FC patterns for the sound tracks was still higher (0.43 ± 0.07) than for different sound tracks (0.09 ± 0.05; p < 8e−6).
Conclusion
Similar music creates similar functional activation and connectivity patterns in the brain of healthy individuals as does listening to the spoken word. Direct comparison of FC patterns yielded higher correlations than indirect comparisons of graph properties derived from corresponding FC networks.





Similar content being viewed by others
References
Trehub SE, Hannon EE (2006) Infant music perception: domain-general or domain-specific mechanisms? Cognition 100(1):73–99
Kawakami A, Furukawa K, Okanoya K (2014) Music evokes vicarious emotions in listeners. Front Psychol 5:431
Schlaug G (2009) Part VI introduction: listening to and making music facilitates brain recovery processes. Ann N Y Acad Sci 1169:372–373
Chen JL, Penhune VB, Zatorre RJ (2008) Listening to musical rhythms recruits motor regions of the brain. Cereb Cortex 18(12):2844–2854
Koelsch S, Skouras S (2014) Functional centrality of amygdala, striatum and hypothalamus in a “small-world” network underlying joy: an fMRI study with music. Hum Brain Mapp 35(7):3485–3498
Limb CJ (2006) Structural and functional neural correlates of music perception. Anat Rec A Discov Mol Cell Evol Biol 288(4):435–446
Trost W, Ethofer T, Zentner M, Vuilleumier P (2012) Mapping aesthetic musical emotions in the brain. Cereb Cortex 22(12):2769–2783
Panksepp J, Bernatzky G (2002) Emotional sounds and the brain: the neuro-affective foundations of musical appreciation. Behav Process 60(2):133–155
Jancke L, Alahmadi N (2016) Detection of independent functional networks during music listening using electroencephalogram and sLORETA-ICA. NeuroReport 27(6):455–461
Thaut MH, McIntosh GC, Rice RR (1997) Rhythmic facilitation of gait training in hemiparetic stroke rehabilitation. J Neurol Sci 151(2):207–212
Thaut MH, McIntosh GC, Rice RR, Miller RA, Rathbun J, Brault JM (1996) Rhythmic auditory stimulation in gait training for Parkinson’s disease patients. Mov Disord 11(2):193–200
Thaut MH, Leins AK, Rice RR, Argstatter H, Kenyon GP, McIntosh GC, Bolay HV, Fetter M (2007) Rhythmic auditory stimulation improves gait more than NDT/Bobath training in near-ambulatory patients early poststroke: a single-blind, randomized trial. Neurorehabil Neural Repair 21(5):455–459
Thaut MH, Gardiner JC, Holmberg D, Horwitz J, Kent L, Andrews G, Donelan B, McIntosh GR (2009) Neurologic music therapy improves executive function and emotional adjustment in traumatic brain injury rehabilitation. Ann N Y Acad Sci 1169:406–416
Raglio A, Galandra C, Sibilla L, Esposito F, Gaeta F, Di Salle F, Moro L, Carne I, Bastianello S, Baldi M, Imbriani M (2016) Effects of active music therapy on the normal brain: fMRI based evidence. Brain Imaging Behav 10(1):182–186
Pereira CS, Teixeira J, Figueiredo P, Xavier J, Castro SL, Brattico E (2011) Music and emotions in the brain: familiarity matters. PLoS ONE 6(11):e27241
Karmonik C, Brandt A, Anderson J, Brooks F, Lytle J, Silverman E, Frazier JT (2016) Music listening modulates functional connectivity and information flow in the human brain. Brain Connect 6(8):632–641
Rubinov M, Sporns O (2010) Complex network measures of brain connectivity: uses and interpretations. Neuroimage 52(3):1059–1069
Trost W, Fruhholz S, Cochrane T, Cojan Y, Vuilleumier P (2015) Temporal dynamics of musical emotions examined through intersubject synchrony of brain activity. Soc Cogn Affect Neurosci 10(12):1705–1721
Li K, Guo L, Nie J, Li G, Liu T (2009) Review of methods for functional brain connectivity detection using fMRI. Comput Med Imaging Graph 33(2):131–139
Medaglia JD, Lynall ME, Bassett DS (2015) Cognitive network neuroscience. J Cogn Neurosci 27(8):1471–1491
Sporns O (2018) Graph theory methods: applications in brain networks. Dialogues Clin Neurosci 20(2):111–121
Chen ZJ, He Y, Rosa-Neto P, Germann J, Evans AC (2008) Revealing modular architecture of human brain structural networks by using cortical thickness from MRI. Cereb Cortex 18(10):2374–2381
Montag C, Reuter M, Axmacher N (2011) How one’s favorite song activates the reward circuitry of the brain: personality matters! Behav Brain Res 225(2):511–514
Acknowledgements
This study was funded by a Grant from the Ting Tsung and Wei Fong Chao Foundation and the Center for Performing Arts Medicine of Houston Methodist Hospital.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
No author has any potential conflict of interests that are directly or indirectly related to the research.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Appendix A: Technical details for algorithms to quantify similarity in FC patterns
Appendix A: Technical details for algorithms to quantify similarity in FC patterns
A.1: Selection of nodes for FC networks
To sensitize the FC analysis to only brain regions participating in listening to the auditory pieces, only voxels that exhibited a BOLD effect as determined by the standard fMRI analysis were included when composing the graph adjacency matrices that corresponded to the FC patterns. For that purpose, first an fMRI BOLD activation map was calculated (first-level GLM analysis) for each auditory piece and then one activation map per sound track for all subjects was created employing the Student’s t test (second-level GLM analysis). Two thresholds were chosen, t = 5.0 (to create the focused activation mask) and t = 2.4 (extended activation mask). The two t values were chosen so that for focused activation, the mask consisted mainly of voxels in the auditory cortex (sensory auditory processing) while for the extended activation mask, additional voxels in the frontal, temporal, parietal lobes as well as in the cerebellum, brainstem and basal ganglia were included where activation was statistically significant (additional higher cognitive processing, p < 0.05).
A.2: Pipeline for quantifying similarity of FC patterns
A schematic is provided to outline the data processing pipeline further described in this section (Fig. 1). The pipeline consisted of the following steps:
- (1)
First-level GLM analysis yielded task-specific averaged fMRI activation maps (seven maps for four music pieces and three spoken-word pieces).
- (2)
Second-level GLM analysis (t test) yielded one common averaged fMRI activation map. Two thresholds (low p value of 0.024 and high p value of p < 0.05) yielded two masks for ‘Focused Activation’ and ‘Extended Activation’, respectively.
- (3)
The mask for each condition served to select voxels for inclusion in the FC network.
- (4)
From all the voxels included in the mask, the BOLD signal time courses were extracted for each dataset and the corresponding individual symmetric graph adjacency matrix was calculated with Pearson correlation coefficient values as entries. These correlation coefficients represented a measure of similarity of the BOLD signal time courses of two voxels.
- (5a)
Analysis path for comparing graph properties Using the igraph package of the R-project, graph networks were created from the adjacency matrices. First, entries lower in the adjacency matrix representing a low correlation (smaller than 0.5) were set to zero (i.e., removed). Second, undirected graphs with nodes as voxels and edge weights using the remaining entries in the adjacency matrices were created. Self-loops in the graphs were removed, i.e., the diagonal elements of the adjacency matrix were set to zero. For each graph, the following properties were calculated using standard setting of the software (igraph functions given in parenthesis): maximum degree (max(degree())), edge density (edge_density()), mean value of betweenness (mean(betweenness()), modularity (modularity()), cluster coefficient (transivity()), diameter (diameter()), number of communities (length(cluster_fast_greedy()) and number of connected nodes (sum(sizes(cluster_fast_greedy()))). These graph properties were selected as they provide a description of the individual graphs on different levels, from simple connectivity (maximum degree and edge density), over information flow (betweeness) to clusters or communities (modularity, cluster coefficient and number of communities). A vector was created, comprising scaled and centered values of these properties, averaged over all subjects for each sound track. A correlation matrix (the functional connectivity or FC matrix) was calculated for these vectors. The entries of this FC matrix provide a measure for inter-track similarity and intra-track similarity based on the graph properties.
- (5b)
Analysis path for comparing FC connectivity patterns directly The upper triangular matrix of each individual adjacency matrix was linearized into a vector. This vector was included as a new row in a new matrix (termed group adjacency matrix). The number of rows of this matrix then corresponded to the number of individual listening task and the number of columns corresponded to V*(V − 1)/2 (i.e., the number of entries in the upper triangular matrix) where V is the number of voxels in the mask. The Pearson correlation coefficient (CC) between each row was calculated and displayed in a matrix (termed ‘FC matrix’). Entries in this matrix quantify the bipartite similarity of FC patterns between subjects.
Across all subjects, the means of entries in the FC matrix were calculated, thereby creating two averaged FC matrices, one for the ‘focused activation’ and one for the ‘extended activation’ condition. The entries of these matrices provide a measure for inter-track similarity and intra-track similarity based on the graph adjacency matrices.
A.3: Statistical analysis
Standard first-level GLM analysis was used to calculate the individual BOLD fMRI activation maps. Second-level GLM analysis was then employed to define the masks for focused and extended activation. Thresholds in the t values of the corresponding t test (t = 5.0 (high activation) and t = 2.4 (low activation)) were chosen to identify voxels for each mask. The intra-CC and inter-CC values were obtained by calculating the Pearson correlation coefficient between the linearized upper triangular adjacency matrices.
Rights and permissions
About this article
Cite this article
Karmonik, C., Brandt, A., Elias, S. et al. Similarity of individual functional brain connectivity patterns formed by music listening quantified with a data-driven approach. Int J CARS 15, 703–713 (2020). https://doi.org/10.1007/s11548-019-02077-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11548-019-02077-y