Abstract
Introduction
Post-mastectomy radiotherapy (PMRT) improves local control rates and survival in patients with adverse prognostic features. The dose coverage to target volumes is critical to yield maximum benefit to treated patients, increasing local control and reducing risk of toxicity. This study aims to assess patterns of breast cancer relapse in patients treated with mastectomy, breast reconstruction and PMRT.
Methods
Breast cancer patients treated with PMRT between 1992 and 2017 were retrospectively reviewed. Clinical and pathological characteristics of patients were collected. Recurrences were defined as “in field,” “marginal” or “out of field.” Survival analyses were performed in relation to progression-free survival (PFS) and overall survival (OS). Correlation between baseline features was explored.
Results
Data of 140 patients are collected. After a median follow-up time of 72 months, median PFS and OS of 63 and 74 months were detected, respectively. Neoadjuvant chemotherapy, lympho-vascular space invasion (LVI) and size of primary tumor were all significantly associated with worst PFS and OS. Ten patients developed local recurrence: 30% "in field," 30% marginal recurrences, 20% "out of field" and 20% both “in field” and “out of field.” No recurrence was detected under the expander, 80% above the device and 20% patients relapsed on IMN chain. The mean distant relapse-free survival was 39 months. Overall, 39 of 140 patients developed distant metastases.
Conclusions
The onset of local–regional relapses occurred mainly above the expander/prosthesis, underlying the importance of inclusion of the subcutaneous tissues within the target volume. In order to refine new contouring recommendations for PMRT and breast reconstruction, future prospective studies are needed.
Similar content being viewed by others
Introduction
Higher T and N stage, younger age at diagnosis, estrogen receptor (ER)-negative disease and presence of extracapsular extension are crucial factors associated with post-mastectomy risk of locoregional recurrence [1, 2]. Post-mastectomy radiotherapy (PMRT) showed to improve local control rates and survival in patients with adverse prognostic features (e.g., T4 tumors, positive margins, > 3 positive nodes, estrogen receptor-negative disease or young age) [2]. According to the literature, standard indications for PMRT are stage III disease, T3N0 (if high-risk features) and node-positive disease (debated the number of positive nodes) with remarkable benefit in patients with > 3 axillary involved lymph nodes [3]. However, the optimal definition of target volumes and dose coverage are still debated in order to increase locoregional control and reduce unnecessary risk of toxicity. Indeed, the excessive inclusion of normal tissue in the field of treatment may increase risk of acute and late toxicity. In this regard, recent development of common contouring guidelines [4,5,6] helped to uniform target volumes definition and to reduce impact of interobserver variability, a potential confounding factor influencing dose coverage, dose to organs at risk and patient outcome after PMRT [8], especially in terms of quality assessment of clinical trials [9, 10]. Moreover, suboptimal coverage of the microscopic disease sites may lead to higher risk of recurrence. Advances in breast cancer radiation therapy (RT) have led to the introduction of 4D planning, intensity-modulated RT (IMRT) and volumetric arc therapy increasing dose homogeneity and normal tissue sparing [11].
The aforementioned critical considerations are of utmost importance especially if modern oncoplastic techniques (e.g., prepectoral or subpectoral tissue expander placement) prompt clinicians to tailor treatment based on clinical judgment. In this situation, the appropriate spatial identification of relapse patterns after PMRT has to be implemented in clinical practice. Therefore, this study aims to assess patterns of breast cancer relapse in a monocentric large series of patients treated with mastectomy, breast reconstruction and PMRT, in order to identify which target volumes are at higher or lower risk of recurrence after treatment, allow effective target coverage and organs at risk sparing.
Materials and methods
Between 1992 and 2017, clinical records of patients with locally advanced breast cancer treated at the Radiation Oncology Unit, Oncology Department, of the Azienda Ospedaliero-Universitaria Careggi, University of Florence, were retrospectively reviewed. We included (1) stage IIA–IIIC breast cancer patients (2) aged > 18 years old (3) treated with mastectomy, axillary lymphadenectomy, post-surgical breast reconstruction with prepectoral or subpectoral tissue expander placement and PMRT. Regional nodal irradiation (RNI) was performed at clinician discretion according to baseline risk features. Data about age, disease stage at diagnosis (according to AJCC classification VIII edition), grading, estrogen and progesterone receptor (PgR) status, Ki-67 proliferative index, human epidermal growth factor receptor 2 (HER2) status, multifocality, multicentricity, presence of lympho-vascular space invasion (LVI) and treatments administered were collected and reported. Data about relapse detection site were retrieved from clinical reports, recurrences were defined as “in field,” “marginal” or “out of field” according to their position relative to chest wall treatment field and nodal target volumes, defined according to European SocieTy for Radiotherapy and Oncology (ESTRO) guidelines [7, 12]. All recurrences clinically detected within 1 cm from chest wall or within regional lymph nodes (when RNI was performed) were defined as “in field.” Recurrences detected between 1 and 2 cm from chest wall were defined as “marginal.” Finally, any relapse that occurred outside 2 cm from the treatment field within regional lymph nodes (when RNI was not performed) or outside from regional lymph nodes was defined as “out of field.” Data about site of recurrences in relation to tissue expander were collected. Survival analyses were performed in relation to progression-free survival (PFS) and overall survival (OS). The last follow-up and the cause of death were collected. Correlation between baseline features (use of neoadjuvant chemotherapy, grading, size of the primary tumor and LVI) was explored. Univariate analysis was conducted through log-rank test. A multivariable Cox proportional regression model was used to identify independent factors of specific events. All statistical analyses were performed using Medcalc® software.
Surgical treatment
All patients underwent mastectomy and axillary lymphadenectomy, defined as the dissection of at least ten level I and II axillary nodes, according to the European Organisation for Research and Treatment of Cancer (EORTC) breast cancer group manual [13]. Post-mastectomy breast reconstruction with prepectoral or subpectoral expander placement was performed.
Radiation therapy
Concerning PMRT, all included patients received a total dose of 50 Gy in 25 fractions. Patients underwent CT simulation with a breast board and supine position. Patients were scanned with 5-mm slices from mid-neck to mid-abdomen. The chest wall clinical target volume (CTV) was delineated on transverse CT images, and the planning target volume (PTV) was obtained by adding a 5-mm margin to the CTV. A two opposed tangential fields technique was used. A boost to surgical scar was administered in selected cases (e.g., tumor infiltrating the pectoral fascia). RNI was performed when at least four positive nodes were found at the pathological examination. Axillary nodal levels III–IV were treated with a nondivergent anterior photon field including the head of the clavicle medially and the coracoid process laterally. Inferiorly, the border was matched to the superior limit of the chest wall. Two techniques were used to cover internal mammary nodes (IMN), either a modified wide-tangent technique with the upper fields widened to include the internal mammary nodes, or a separate photon field angled to match the tangent fields used to cover chest wall CTV [14, 15].
Systemic treatment
Adjuvant or neoadjuvant systemic therapies were administered in all patients. Chemotherapy included both anthracyclines and taxane regimens, trastuzumab for HER2-positive patients was used since 2005. Postoperative hormonal treatment was administered in ER-positive patients.
Results
The individual characteristics of 140 patients are summarized in Table 1. Average age was 48.7 years (range, 30–71 years). The treatment characteristics of all 140 patients are listed in Table 2. Fifty-three out of 115 patients (46.1%) received letrozole, 51 patients (36.4%) received neoadjuvant chemotherapy, 105 (75%) adjuvant chemotherapy and, in 18 patients (12.9%), neo- and adjuvant treatments were performed. The whole cohort of patients enrolled in the analysis received radical mastectomy. Expander-based breast reconstruction was performed in 128 patients (91.4%), and 12 patients (8.6%) received definitive prosthesis. Notably, 105 patients underwent prepectoral breast reconstruction, and 35 patients underwent a subpectoral tissue expander placement. A Chi-square test (X2) of independence was performed to examine the relation between breast reconstruction type and the incidence of relapse. The relation between these variables was not statistically significant, X2 is 0.1436 and p-value is 0.7 (Table 3). One hundred and four patients received PMRT with RNI. After a median follow-up time of 72 months, median PFS and OS of 63 (range 54–75, 1; 95% CI) and 74 months (range 65–84.07; 95%CI) were detected, respectively (Figs. 1a and 2a). At univariate analysis, neoadjuvant chemotherapy, higher tumor grade, larger size of primary tumor and LVI were all significantly associated with worst PFS. Mean PFS was 78.01 months without neoadjuvant chemotherapy and 71.8 in the primary systemic therapy population (p = 0.0006); 110.48 months for G1 tumors, 81.5 for G2 and 66.9 for G3 tumors (p = 0.0078); 86.6 months for T1, 75.87 for T2 and 65.12 for T3 (p = 0.0001) and 87.3 months without LVI and 68.35 with LVI (p = 0.0039) (Fig. 1b–e). At the multivariate analysis, all the aforementioned factors showed to be independently associated with PFS (p = 0.005, p = 0.01, p = 0.02 and p = 0.02 for use neoadjuvant chemotherapy, tumor grading, LVI and tumor size, respectively). At univariate analysis, use of neoadjuvant chemotherapy, LVI and size of primary tumor were all significantly associated with worst OS. Mean OS was 85.7 months without neoadjuvant chemotherapy versus 74.95 in the population performing primary systemic therapy (p = 0.0282); 94.3 months without LVI versus 87.3 with LVI (p = 0.0023) and 90.4 months for T1, 88.12 for T2 and 81.26 for T3 (p 0.0011) (Fig. 2b–d). At the multivariate analysis, none of the factors was independently associated with OS. Extent of extracapsular extension (ECE), ER status and HER-2 status are not statistically associated with PFS or OS at univariate nor multivariate analysis.
Progression-free survival (PFS). Mean PFS: 75.7 months (range 66,2–85,3; 95% CI). Median PFS: 63 months (range 54–75, 1; 95% CI) (a). PFS in patients not undergoing neoadjuvant chemotherapy (blue) and performing preoperative treatments (green) (b). Impact of grading on PFS (c). Impact of primary tumor size (T) on PFS (d). PFS in patients with (green) or without (blue) lympho-vascular space invasion (LVI) (e)
Overall survival (OS). Mean OS: 90.2 months (range 9–322; 95% CI). Median OS: 74 months (range 65–84.07; 95%CI) (a). OS in patients not undergoing neoadjuvant chemotherapy (blue) and patients undergoing preoperative treatments (green) (b). Impact of primary tumor size (T) on PFS (c). OS with (green) or without (blue) lympho-vascular space invasion (LVI) (d)
Patterns of local–regional recurrences
Overall, 10 patients (7.1%) developed local recurrence; average time to recurrence was 27 months (range 3–87). Average size of recurrence was 18.5 mm (range 6–30). Tables 4 and 5 present characteristics of patients with local–regional recurrences. Notably, the majority of the population with recurrence disease had T2 tumor (80%), N2 stage (60%) and stage IIIA (70%) at diagnosis. Most patients had aggressive biological features at diagnosis, like a Ki67 index ≥ 20% (60%) or a G3 tumor grading (80%). Recurrences were defined as "in field," "out of field" and marginal recurrences in 3 (30%), 2 (20%) and 3 patients (30%), respectively. Two patients had both "in field" and "out of field" recurrences. Concerning the site of relapse in relation to tissue expander, no recurrence was detected under the device, 8 patients (80%) had relapse above the device, 2 (20%) patients relapsed on IMN chain. After recurrence, four patients received local re-irradiation for an average total dose of 36.25 Gy (range 30–50 Gy), three patients received endocrine therapy alone, two patients underwent surgery and one patient was treated with chemotherapy. Three relapsed patients were still alive with no evidence of disease, one is alive with distant metastases, four died for metastatic disease and two died for other causes. The mean distant relapse-free survival (DRFS) was 39 months (range, 3–215 months). Overall, 39 of 140 patients (27.9%) developed distant metastases, and data are summarized in supplementary material (Table 5). Most patients with metastatic disease (51.3%) were treated with chemotherapy. At the time of the analysis, ten patients (25.6%) were still alive with metastatic disease, twenty-eight patients (71.8%) died for disease progression and one patient (2.6%) died for other causes.
Discussion
Over the past decades, the introduction of modern oncoplastic techniques has revolutionized breast surgical approaches. Modern advances in breast reconstruction have led to the transition from the traditional submuscular procedure to the new prepectoral implant-based strategy. Therefore, many experience have aimed to define the optimal breast reconstruction type for patients treated with mastectomy [16]. Recently, this new technique is increasing in the high-volume centers owing to its more minimal approach. Indeed, a retrospective analysis of 146 patients showed that immediate prepectoral breast reconstruction was effective and safe with five locoregional recurrences after 4 years of follow-up and a low acute and early-late complication rates [17]. According to our data, 25% and 75% of patients received subpectoral and prepectoral breast reconstruction, respectively. We did not find a statistically significant correlation between prepectoral procedures and the incidence of relapse. In line with the previous literature, our findings may support the adoption of the subcutaneous implant-based reconstruction as an effective procedure in terms of locoregional control. However, further studies comparing the prepectoral approach with the previous standard of care are strongly needed in order to evaluate long-term oncological and surgical outcomes.
As expected, our results also confirmed the low rate of local–regional recurrence after PMRT and reported both in field and marginal/out of field recurrence. In this regard, many authors have focused their efforts on the investigation about correct target definition and dose coverage in this setting of patients [18]. To the best of our knowledge, a consensus recommendation for the optimal coverage of treated areas in breast contouring is still missing. ESTRO guidelines aim to minimize target volumes while RADCOMP recommendations, regarding patients potentially at greater risk of recurrence, delineated an enlargement of the treated area [7, 19]. However, relapses were significantly associated with biological aggressiveness of disease, suggesting that suboptimal target coverage may not be the only factor influencing risk recurrence. Of note, local recurrences in the present series were exclusively located above the expander, suggesting that target coverage of the subcutaneous tissue within skin and implanted device is critical for treatment outcome (Fig. 3 [20]). Moreover, under-prosthetic relapse is an extremely rare event, and dose coverage of implants, ribs and intercostal muscles should not be considered a critical issue from this point of view. Skin bolus remains an option in selected cases with a high risk of recurrence, to minimize build-up influence on target coverage. Randomized trials and meta-analyses confirmed the benefit of PMRT in terms of local–regional control (reducing the recurrence rate) and overall survival (OS) in patients with high-risk breast cancer [21,22,23,24]. Although the aforementioned results led to a growth in PMRT indications [23], this treatment is related to an increase in the complication rate (lymphedema, brachial plexopathy, radiation pneumonia, rib fractures, cardiac toxicity and radiation-induced malignancies) [25,26,27,28,29,30,31,32,33,34,35,36,37,38,39] and a greater risk of breast reconstruction failure [40,41,42,43]. For this reason, minimizing the exposure of organs at risk and selecting the target volumes at risk for local recurrence are a critical issue in this setting.
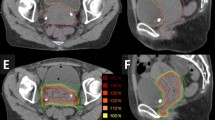
Site of chest wall recurrences: Relapse occurs above tissue expander (60%), above tissue expander and at the level of axillary nodes region (magenta) (20%) and internal mammary chain (green) (20%); modified from Gee et al. [20] (a). Spatial distribution of chest wall recurrences (b)
The limitations of the present experience are the retrospective nature of the analysis and the limited sample size.
Notwithstanding the limits of the present study, our data are in line with the current indication from ESTRO/ACROP guidelines recommending that after tissue expander reconstruction, only tissue in above the device should be included in the target volume, to reduce treatment complications. Only patients with under-pectoral placement of device with particular risk factors (e.g., large primary tumor, pectoral muscle or chest wall infiltration or poor response to neoadjuvant chemotherapy) may benefit from irradiation of under-prosthetic tissues [44].
Conclusion
In conclusion, our real-life retrospective experience described the recurrence at the level of the chest wall as an extremely rare event in the case of PMRT. Both in field and marginal/out of field recurrences were detected, suggesting that some patients may recur due to the biological aggressiveness of disease rather than suboptimal coverage of target volumes. The onset of local–regional relapses occurred mainly above the expander/prosthesis, underlying the importance of inclusion of the subcutaneous tissues within the target volume. In order to refine new contouring recommendations for PMRT and breast reconstruction, future prospective studies are needed. However, the design of the ideal study to assess the relationship between treatment volumes and patterns of recurrences remains a challenge.
Data availability
Not applicable.
References
Recht A, Gray R, Davidson NE et al (1999) Locoregional failure 10 years after mastectomy and adjuvant chemotherapy with or without tamoxifen without irradiation: experience of the Eastern cooperative oncology group. J Clin Oncol 17(6):1689–1700. https://doi.org/10.1200/JCO.1999.17.6.1689
Harms W, Budach W, Dunst J et al (2016) DEGRO practical guidelines for radiotherapy of breast cancer VI: therapy of locoregional breast cancer recurrences. Strahlenther Onkol 192(4):199–208. https://doi.org/10.1007/s00066-015-0939-7
EBCTCG (Early Breast Cancer Trialists’ Collaborative Group), McGale P, Taylor C et al (2014) Effect of radiotherapy after mastectomy and axillary surgery on 10-year recurrence and 20-year breast cancer mortality: meta-analysis of individual patient data for 8135 women in 22 randomised trials. Lancet 383(9935):2127–2135. https://doi.org/10.1016/S0140-6736(14)60488-8
Atean I, Pointreau Y, Ouldamer L et al (2013) A simplified CT-based definition of the supraclavicular and infraclavicular nodal volumes in breast cancer. Cancer Radiother 17(1):39–43. https://doi.org/10.1016/j.canrad.2012.11.007
Faculty of Radiation Oncology (2015) Quality Guidelines for Volume Delineation in Radiation Oncology, 23 October 2015. Available from URL: http://www.ranzcr.com/college/document-library/quality-guidelines-for-volume-delineation-in-radiation-oncology
Offersen BV, Boersma LJ, Kirkove C et al (2015) ESTRO consensus guideline on target volume delineation for elective radiation therapy of early stage breast cancer. Radiother Oncol 114(1):3–10. https://doi.org/10.1016/j.radonc.2014.11.030
White JT, Arthur D, Buchholz T et al (2017) Breast Cancer Atlas for Radiation Therapy Planning: Consensus Definitions, Accessed 21 Feb 2017. Available from URL: http://www.rtog.org/LinkClick
Li XA, Tai A, Arthur DW et al (2009) Variability of target and normal structure delineation for breast cancer radiotherapy: an RTOG multi-institutional and multiobserver study. Int J Radiat Oncol Biol Phys 73(3):944–951. https://doi.org/10.1016/j.ijrobp.2008.10.034
Francolini G, Thomsen MS, Yates ES et al (2017) Quality assessment of delineation and dose planning of early breast cancer patients included in the randomized Skagen Trial 1. Radiother Oncol 123(2):282–287. https://doi.org/10.1016/j.radonc.2017.03.011
Eldesoky AR, Francolini G, Thomsen MS et al (2017) Dosimetric assessment of an Atlas based automated segmentation for loco-regional radiation therapy of early breast cancer in the Skagen trial 1: a multi-institutional study. Clin Transl Radiat Oncol 2:36–40. https://doi.org/10.1016/j.ctro.2017.01.004
Harsolia A, Kestin L, Grills I et al (2007) Intensity-modulated radiotherapy results in significant decrease in clinical toxicities compared with conventional wedge-based breast radiotherapy. Int J Radiat Oncol Biol Phys 68(5):1375–1380. https://doi.org/10.1016/j.ijrobp.2007.02.044
Kaidar-Person O, Vrou Offersen B, Hol S et al (2019) ESTRO ACROP consensus guideline for target volume delineation in the setting of postmastectomy radiation therapy after implant-based immediate reconstruction for early stage breast cancer. Radiother Oncol 137:159–166. https://doi.org/10.1016/j.radonc.2019.04.010
Julien JP, Jassem J, Rutgers E, EORTC Breast Cancer Cooperative Group (2004) Manual for Clinical Research in Breast Cancer. 5th Greenwich Medical Media, London, UK
Donker M, van Tienhoven G, Straver ME et al (2014) Radiotherapy or surgery of the axilla after a positive sentinel node in breast cancer (EORTC 10981–22023 AMAROS): a randomised, multicentre, open-label, phase 3 non-inferiority trial. Lancet Oncol 15(12):1303–1310. https://doi.org/10.1016/S1470-2045(14)70460-7
Whelan TJ, Olivotto IA, Parulekar WR et al (2015) Regional nodal irradiation in early-stage breast cancer. N Engl J Med 373(4):307–316. https://doi.org/10.1056/NEJMoa1415340
Vidya R, Iqbal FM (2017) A guide to prepectoral breast reconstruction: a new dimension to implant-based breast reconstruction. Clin Breast Cancer 17(4):266–271. https://doi.org/10.1016/j.clbc.2016.11.009
Bernini M, Meattini I, Saieva C et al (2021) Pre-pectoral breast reconstruction: early and long-term safety evaluation of 146 unselected cases of the early pre-pectoral era of a single-institution, including cases with previous breast irradiation and post-mastectomy radiation therapy. Breast Cancer. https://doi.org/10.1007/s12282-021-01314-0
Chang JS, Byun HK, Kim JW et al (2017) Three-dimensional analysis of patterns of locoregional recurrence after treatment in breast cancer patients: validation of the ESTRO consensus guideline on target volume. Radiother Oncol 122:24–29
Bekelman J, Cahlon O, McDonald S (2017) Pragmatic randomized trial of proton vs. photon therapy for patients with non-metastatic breast cancer: a radiotherapy comparative effectiveness (RADCOMP) consortium trial (Accessed 21 Feb 2017). Available from URL: https://clinicaltrials.gov/ct2/show/NCT02603341.f
Gee HE, Moses L, Stuart K et al (2019) Contouring consensus guidelines in breast cancer radiotherapy: comparison and systematic review of patterns of failure. J Med Imaging Radiat Oncol 63(1):102–115. https://doi.org/10.1111/1754-9485.1280
Ragaz J, Jackson SM, Le N et al (1997) Adjuvant radiotherapy and chemotherapy in node-positive premenopausal women with breast cancer. N Engl J Med 337(14):956–962. https://doi.org/10.1056/NEJM199710023371402
Overgaard M, Hansen PS, Overgaard J et al (1997) Postoperative radiotherapy in high-risk premenopausal women with breast cancer who receive adjuvant chemotherapy. N Engl J Med 337(14):949–955. https://doi.org/10.1056/NEJM199710023371401
Clarke M, Collins R, Darby S et al (2005) Effects of radiotherapy and of differences in the extent of surgery for early breast cancer on local recurrence and 15-year survival: an overview of the randomised trials. Lancet 366(9503):2087–2106. https://doi.org/10.1016/S0140-6736(05)67887-7
EBCTCG (Early Breast Cancer Trialists’ Collaborative Group), McGale P, Taylor C et al (2014) Effect of radiotherapy after mastectomy and axillary surgery on 10-year recurrence and 20-year breast cancer mortality: meta-analysis of individual patient data for 8135 women in 22 randomised trials. Lancet 383(9935):2127–2135. https://doi.org/10.1016/S0140-6736(14)60488-8
National Comprehensive Cancer Network. Breast Cancer. Guidelines Version 1.2019. https://www.nccn.org/professionals/physician_gls/pdf/breast.pdf Accessed 9 June 2019
Recht A, Edge SB, Solin LJ et al (2001) Postmastectomy radiotherapy: clinical practice guidelines of the American society of clinical oncology. J Clin Oncol 19(5):1539–1569. https://doi.org/10.1200/JCO.2001.19.5.1539
Recht A, Comen EA, Fine RE et al (2016) Postmastectomy radiotherapy: an American society of clinical oncology, American society for radiation oncology, and society of surgical oncology focused guideline update. Pract Radiat Oncol 6(6):e219–e234. https://doi.org/10.1016/j.prro.2016.08.009
Yavas G, Yavas C, Akyurek N (2013) Postmastectomy radiation therapy in locally advanced breast cancer. Exp Oncol 35(4):258–266
Gross NJ (1977) Pulmonary effects of radiation therapy. Ann Intern Med 86(1):81–92. https://doi.org/10.7326/0003-4819-86-1-81
Marks LB (1994) The pulmonary effects of thoracic irradiation. Oncology (Williston Park, NY) 8(6):89–106
Metz JM, Schultz DJ, Fox K et al (1999) Long-term outcome after postmastectomy radiation therapy for breast cancer patients at high risk for local-regional recurrence. Cancer J Sci Am 5(2):77–83
Cuzick J, Stewart H, Peto R et al (1987) Overview of randomized trials of postoperative adjuvant radiotherapy in breast cancer. Cancer Treat Rep 71(1):15–29
Shapiro CL, Recht A (2001) Side effects of adjuvant treatment of breast cancer. N Engl J Med 344(26):1997–2008. https://doi.org/10.1056/NEJM200106283442607
Højris I, Overgaard M, Christensen JJ, Overgaard J (1999) Morbidity and mortality of ischaemic heart disease in high-risk breast-cancer patients after adjuvant postmastectomy systemic treatment with or without radiotherapy: Analysis of DBCG 82b and 82c randomised trials. Lancet 354(9188):1425–1430. https://doi.org/10.1016/S0140-6736(99)02245-X
Nixon AJ, Manola J, Gelman R et al (1998) No long-term increase in cardiac-related mortality after breast-conserving surgery and radiation therapy using modern techniques. J Clin Oncol 16(4):1374–1379. https://doi.org/10.1200/JCO.1998.16.4.1374
Vallis KA, Pintilie M, Chong N et al (2002) Assessment of coronary heart disease morbidity and mortality after radiation therapy for early breast cancer. J Clin Oncol 20(4):1036–1042. https://doi.org/10.1200/JCO.2002.20.4.1036
Darby SC, Ewertz M, McGale P et al (2013) Risk of ischemic heart disease in women after radiotherapy for breast cancer. N Engl J Med 368(11):987–998. https://doi.org/10.1056/NEJMoa1209825
Grantzau T, Overgaard J (2016) Risk of second non-breast cancer among patients treated with and without postoperative radiotherapy for primary breast cancer: a systematic review and meta-analysis of population-based studies including 522,739 patients. Radiother Oncol 121(3):402–413. https://doi.org/10.1016/j.radonc.2016.08.017
Diamandidou E, Buzdar AU, Smith TL et al (1996) Treatment-related leukemia in breast cancer patients treated with fluorouracil-doxorubicin-cyclophosphamide combination adjuvant chemotherapy: the university of Texas M.D. Anderson cancer center experience. J Clin Oncol 14(10):2722–2730. https://doi.org/10.1200/JCO.1996.14.10.2722
Barry M, Kell MR (2011) Radiotherapy and breast reconstruction: a meta-analysis. Breast Cancer Res Treat 127(1):15–22. https://doi.org/10.1007/s10549-011-1401-x
Nava MB, Pennati AE, Lozza L et al (2011) Outcome of different timings of radiotherapy in implant-based breast reconstructions. Plast Reconstr Surg 128(2):353–359. https://doi.org/10.1097/PRS.0b013e31821e6c10
Whitfield GA, Horan G, Irwin MS et al (2009) Incidence of severe capsular contracture following implant-based immediate breast reconstruction with or without postoperative chest wall radiotherapy using 40 Gray in 15 fractions. Radiother Oncol 90(1):141–147. https://doi.org/10.1016/j.radonc.2008.09.023
Behranwala KA, Dua RS, Ross GM et al (2006) The influence of radiotherapy on capsule formation and aesthetic outcome after immediate breast reconstruction using biodimensional anatomical expander implants. J Plast Reconstr Aesthet Surg 59(10):1043–1051. https://doi.org/10.1016/j.bjps.2006.01.051
Kaidar-Person O, Offersen BV, Hol S et al (2019) ESTRO ACROP consensus guideline for target volume delineation in the setting of postmastectomy radiation therapy after implant-based immediate reconstruction for early stage breast cancer. Radiother Oncol 137:159–166. https://doi.org/10.1016/j.radonc.2019.04.010
Acknowledgements
Nothing to declare.
Funding
Open access funding provided by Università degli Studi di Firenze within the CRUI-CARE Agreement. Not applicable.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by VS, ID and MV. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Salvestrini, V., Valzano, M., Meattini, I. et al. Anatomical assessment of local recurrence site in breast cancer patients after breast reconstruction and post-mastectomy radiotherapy: implications for radiation volumes and techniques. Radiol med (2024). https://doi.org/10.1007/s11547-024-01812-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11547-024-01812-z