Abstract
Purpose
Poorly differentiated invasive non-mucinous pulmonary adenocarcinoma (IPA), based on the novel grading system, was related to poor prognosis, with a high risk of lymph node metastasis and local recurrence. This study aimed to build the radiomic and quantitative-semantic models of low-dose computed tomography (LDCT) to preoperatively predict the poorly differentiated IPA in nodules with solid component, and compare their diagnostic performance with radiologists.
Materials and methods
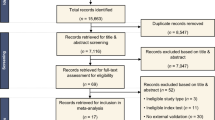
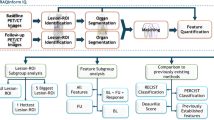
A total of 396 nodules from 388 eligible patients, who underwent LDCT scan within 2 weeks before surgery and were pathologically diagnosed with IPA, were retrospectively enrolled between July 2018 and December 2021. Nodules were divided into two independent cohorts according to scanners: primary cohort (195 well/moderate differentiated and 64 poorly differentiated) and validation cohort (104 well/moderate differentiated and 33 poorly differentiated). The radiomic and quantitative-semantic models were built using multivariable logistic regression. The diagnostic performance of the models and radiologists was assessed by area under curve (AUC) of receiver operating characteristic (ROC) curve, accuracy, sensitivity, and specificity.
Results
No significant differences of AUCs were found between the radiomic and quantitative-semantic model in primary and validation cohorts (0.921 vs. 0.923, P = 0.846 and 0.938 vs. 0.911, P = 0.161). Both the models outperformed three radiologists in the validation cohort (all P < 0.05).
Conclusions
The radiomic and quantitative-semantic models of LDCT, which could identify the poorly differentiated IPA with excellent diagnostic performance, might provide guidance for therapeutic decision making, such as choosing appropriate surgical method or adjuvant chemotherapy.




Similar content being viewed by others
Abbreviations
- AI:
-
Artificial intelligence
- AIC:
-
Akaike’s information criterion
- AUC:
-
Area under the curve
- CT:
-
Computed tomography
- CTR:
-
Consolidation/tumor ratio
- DCA:
-
Decision curve analysis
- IASLC:
-
International Association for the Study of Lung Cancer Pathology Committee
- IBSI:
-
Imaging Biomarker Standardization Initiative
- ICC:
-
Intraclass correlation coefficient
- IPA:
-
Invasive non-mucinous pulmonary adenocarcinoma
- LASSO:
-
Least absolute shrinkage and selection operator
- LDCT:
-
Low-dose computed tomography
- mRMR:
-
Minimum redundancy-maximum relevance
- RFS:
-
Recurrence-free survival
- ROC:
-
Receiver operating characteristic
- TRIPOD:
-
Transparent Reporting of a Multivariable Prediction Model for Individual Prognosis or Diagnosis
- WHO:
-
World Health Organization
References
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A et al (2021) Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 71(3):209–249. https://doi.org/10.3322/caac.21660
Moreira AL, Ocampo PSS, Xia Y, Zhong H, Russell PA, Minami Y et al (2020) A grading system for invasive pulmonary adenocarcinoma: a proposal from the international association for the study of lung cancer pathology committee. J Thorac Oncol 15(10):1599–1610. https://doi.org/10.1016/j.jtho.2020.06.001
Deng C, Zheng Q, Zhang Y, Jin Y, Shen X, Nie X et al (2021) Validation of the novel international association for the study of lung cancer grading system for invasive pulmonary adenocarcinoma and association with common driver mutations. J Thorac Oncol 16(10):1684–1693. https://doi.org/10.1016/j.jtho.2021.07.006
Rokutan-Kurata M, Yoshizawa A, Ueno K, Nakajima N, Terada K, Hamaji M et al (2021) Validation study of the international association for the study of lung cancer histologic grading system of invasive lung adenocarcinoma. J Thorac Oncol 16(10):1753–1758. https://doi.org/10.1016/j.jtho.2021.04.008
Hou L, Wang T, Chen D, She Y, Deng J, Yang M et al (2022) Prognostic and predictive value of the newly proposed grading system of invasive pulmonary adenocarcinoma in Chinese patients: a retrospective multicohort study. Mod Pathol 35(6):749–756. https://doi.org/10.1038/s41379-021-00994-5
Yanagawa N, Shiono S, Abiko M, Katahira M, Osakabe M, Ogata SY (2016) The clinical impact of solid and micropapillary patterns in resected lung adenocarcinoma. J Thorac Oncol 11(11):1976–1983. https://doi.org/10.1016/j.jtho.2016.06.014
Nitadori J, Bograd AJ, Kadota K, Sima CS, Rizk NP, Morales EA et al (2013) Impact of micropapillary histologic subtype in selecting limited resection vs lobectomy for lung adenocarcinoma of 2cm or smaller. J Natl Cancer Inst 105(16):1212–1220. https://doi.org/10.1093/jnci/djt166
Baig MZ, Razi SS, Weber JF, Connery CP, Bhora FY (2020) Lobectomy is superior to segmentectomy for peripheral high grade non-small cell lung cancer ≤2 cm. J Thorac Dis 12(10):5925–5933. https://doi.org/10.21037/jtd-20-1530
Tsao MS, Marguet S, Le Teuff G, Lantuejoul S, Shepherd FA, Seymour L et al (2015) Subtype classification of lung adenocarcinoma predicts benefit from adjuvant chemotherapy in patients undergoing complete resection. J Clin Oncol 33(30):3439–3446. https://doi.org/10.1200/jco.2014.58.8335
Weng CF, Huang CJ, Huang SH, Wu MH, Tseng AH, Sung YC et al (2020) New international association for the study of lung cancer (IASLC) pathology committee grading system for the prognostic outcome of advanced lung adenocarcinoma. Cancers (Basel). https://doi.org/10.3390/cancers12113426
Lederlin M, Puderbach M, Muley T, Schnabel PA, Stenzinger A, Kauczor HU et al (2013) Correlation of radio- and histomorphological pattern of pulmonary adenocarcinoma. Eur Respir J 41(4):943–951. https://doi.org/10.1183/09031936.00056612
Miao Y, Zhang J, Zou J, Zhu Q, Lv T, Song Y (2017) Correlation in histological subtypes with high resolution computed tomography signatures of early stage lung adenocarcinoma. Transl Lung Cancer Res 6(1):14–22. https://doi.org/10.21037/tlcr.2017.02.06
Chen LW, Yang SM, Wang HJ, Chen YC, Lin MW, Hsieh MS et al (2021) Prediction of micropapillary and solid pattern in lung adenocarcinoma using radiomic values extracted from near-pure histopathological subtypes. Eur Radiol 31(7):5127–5138. https://doi.org/10.1007/s00330-020-07570-6
Yang SM, Chen LW, Wang HJ, Chen LR, Lor KL, Chen YC et al (2018) Extraction of radiomic values from lung adenocarcinoma with near-pure subtypes in the International Association for the Study of Lung Cancer/the American Thoracic Society/the European Respiratory Society (IASLC/ATS/ERS) classification. Lung Cancer 119:56–63. https://doi.org/10.1016/j.lungcan.2018.03.004
He B, Song Y, Wang L, Wang T, She Y, Hou L et al (2021) A machine learning-based prediction of the micropapillary/solid growth pattern in invasive lung adenocarcinoma with radiomics. Transl Lung Cancer Res 10(2):955–964. https://doi.org/10.21037/tlcr-21-44
Li M, Ruan Y, Feng Z, Sun F, Wang M, Zhang L (2021) Preoperative CT-based radiomics combined with nodule type to predict the micropapillary pattern in lung adenocarcinoma of size 2 cm or less: a multicenter study. Front Oncol 11:788424. https://doi.org/10.3389/fonc.2021.788424
Travis WD, Brambilla E, Nicholson AG, Yatabe Y, Austin JHM, Beasley MB et al (2015) The 2015 world health organization classification of lung tumors: impact of genetic, clinical and radiologic advances since the 2004 classification. J Thorac Oncol 10(9):1243–1260. https://doi.org/10.1097/jto.0000000000000630
Kadota K, Kushida Y, Kagawa S, Ishikawa R, Ibuki E, Inoue K et al. (2019) Cribriform subtype is an independent predictor of recurrence and survival after adjustment for the eighth edition of TNM staging system in patients with resected lung adenocarcinoma. J Thorac Oncol 14(2):245–54. https://doi.org/10.1016/j.jtho.2018.09.028
Warth A, Muley T, Kossakowski C, Stenzinger A, Schirmacher P, Dienemann H et al (2015) Prognostic impact and clinicopathological correlations of the cribriform pattern in pulmonary adenocarcinoma. J Thorac Oncol 10(4):638–644. https://doi.org/10.1097/jto.0000000000000490
Gao Y, Hua M, Lv J, Ma Y, Liu Y, Ren M et al (2022) Reproducibility of radiomic features of pulmonary nodules between low-dose CT and conventional-dose CT. Quant Imag Med Surg 12(4):2368–2377. https://doi.org/10.21037/qims-21-609
Liu J, Xu H, Qing H, Li Y, Yang X, He C et al (2021) Comparison of radiomic models based on low-dose and standard-dose CT for prediction of adenocarcinomas and benign lesions in solid pulmonary nodules. Front Oncol 10:634298. https://doi.org/10.3389/fonc.2020.634298
Kim YJ, Lee HJ, Kim KG, Lee SH (2019) The effect of CT Scan parameters on the measurement of CT radiomic features: a lung nodule phantom study. Comput Math Methods Med 2019:8790694. https://doi.org/10.1155/2019/8790694
de Koning HJ, van der Aalst CM, de Jong PA, Scholten ET, Nackaerts K, Heuvelmans MA et al (2020) Reduced lung-cancer mortality with volume CT screening in a randomized trial. N Engl J Med 382(6):503–513. https://doi.org/10.1056/NEJMoa1911793
Li Y, Liu J, Yang X, Xu H, Qing H, Ren J et al (2022) Prediction of invasive adenocarcinomas manifesting as pure ground-glass nodules based on radiomic signature of low-dose CT in lung cancer screening. Br J Radiol 95(1133):20211048. https://doi.org/10.1259/bjr.20211048
Lee HY, Choi YL, Lee KS, Han J, Zo JI, Shim YM et al (2014) Pure ground-glass opacity neoplastic lung nodules: histopathology, imaging, and management. AJR Am J Roentgenol 202(3):W224–W233. https://doi.org/10.2214/ajr.13.11819
Wang Z, Zhu W, Lu Z, Li W, Shi J (2021) Invasive adenocarcinoma manifesting as pure ground glass nodule with different size: radiological characteristics differ while prognosis remains the same. Transl Cancer Res 10(6):2755–2766. https://doi.org/10.21037/tcr-21-78
Hattori A, Suzuki K, Takamochi K, Wakabayashi M, Aokage K, Saji H et al (2021) Prognostic impact of a ground-glass opacity component in clinical stage IA non-small cell lung cancer. J Thorac Cardiovasc Surg 161(4):1469–1480. https://doi.org/10.1016/j.jtcvs.2020.01.107
Lin YH, Chen CK, Hsieh CC, Hsu WH, Wu YC, Hung JJ et al (2020) Lymphadenectomy is unnecessary for pure ground-glass opacity pulmonary nodules. J Clin Med. https://doi.org/10.3390/jcm9030672
Wang Q, Zhou X, Wang C, Liu Z, Huang J, Zhou Y et al (2019) WGAN-based synthetic minority over-sampling technique: improving semantic fine-grained classification for lung nodules in CT images. IEEE Access 7:18450–18463. https://doi.org/10.1109/ACCESS.2019.2896409
Mu G, Chen Y, Wu D, Zhan Y, Zhou XS, Gao Y (2019) relu cascade of feature pyramid networks for CT pulmonary nodule detection. In: Suk H-I, Liu M, Yan P, Lian C (eds) Machine learning in medical imaging. Springer International Publishing, Cham, pp 444–452
van Griethuysen JJM, Fedorov A, Parmar C, Hosny A, Aucoin N, Narayan V et al (2017) Computational radiomics system to decode the radiographic phenotype. Cancer Res 77(21):e104–e107. https://doi.org/10.1158/0008-5472.can-17-0339
Zwanenburg A, Vallieres M, Abdalah MA, Aerts H, Andrearczyk V, Apte A et al (2020) The image biomarker standardization initiative: standardized quantitative radiomics for high-throughput image-based phenotyping. Radiology 295(2):328–338. https://doi.org/10.1148/radiol.2020191145
Hanchuan P, Fuhui L, Ding C (2005) Feature selection based on mutual information criteria of max-dependency, max-relevance, and min-redundancy. IEEE Trans Pattern Anal Mach Intell 27(8):1226–1238. https://doi.org/10.1109/TPAMI.2005.159
Sauerbrei W, Boulesteix AL, Binder H (2011) Stability investigations of multivariable regression models derived from low- and high-dimensional data. J Biopharm Stat 21(6):1206–1231. https://doi.org/10.1080/10543406.2011.629890
MacMahon H, Naidich DP, Goo JM, Lee KS, Leung ANC, Mayo JR et al (2017) Guidelines for management of incidental pulmonary nodules detected on CT images: from the Fleischner society 2017. Radiology 284(1):228–243. https://doi.org/10.1148/radiol.2017161659
Saji H, Okada M, Tsuboi M, Nakajima R, Suzuki K, Aokage K et al (2022) Segmentectomy versus lobectomy in small-sized peripheral non-small-cell lung cancer (JCOG0802/WJOG4607L): a multicentre, open-label, phase 3, randomised, controlled, non-inferiority trial. Lancet 399(10335):1607–1617. https://doi.org/10.1016/s0140-6736(21)02333-3
Hosmer DW, Hosmer T, Le Cessie S, Lemeshow S (1997) A comparison of goodness-of-fit tests for the logistic regression model. Stat Med 16(9):965–980. https://doi.org/10.1002/(sici)1097-0258(19970515)16:9%3c965::aid-sim509%3e3.0.co;2-o
Vickers AJ, Elkin EB (2006) Decision curve analysis: a novel method for evaluating prediction models. Med Decis Making 26(6):565–574. https://doi.org/10.1177/0272989x06295361
Moons KG, Altman DG, Reitsma JB, Ioannidis JP, Macaskill P, Steyerberg EW et al (2015) Transparent Reporting of a multivariable prediction model for Individual Prognosis or Diagnosis (TRIPOD): explanation and elaboration. Ann Intern Med 162(1):W1-73. https://doi.org/10.7326/M14-0698
Nicholson AG, Tsao MS, Beasley MB, Borczuk AC, Brambilla E, Cooper WA et al (2022) The 2021 WHO classification of lung tumors: impact of advances since 2015. J Thorac Oncol 17(3):362–387. https://doi.org/10.1016/j.jtho.2021.11.003
Huang KY, Ko PZ, Yao CW, Hsu CN, Fang HY, Tu CY et al (2017) Inaccuracy of lung adenocarcinoma subtyping using preoperative biopsy specimens. J Thorac Cardiovasc Surg. https://doi.org/10.1016/j.jtcvs.2017.02.059
Song SH, Park H, Lee G, Lee HY, Sohn I, Kim HS et al (2017) Imaging phenotyping using radiomics to predict micropapillary pattern within lung adenocarcinoma. J Thorac Oncol 12(4):624–632. https://doi.org/10.1016/j.jtho.2016.11.2230
Xu Y, Ji W, Hou L, Lin S, Shi Y, Zhou C et al (2021) Enhanced CT-based radiomics to predict micropapillary pattern within lung invasive adenocarcinoma. Front Oncol 11:704994. https://doi.org/10.3389/fonc.2021.704994
He L, Huang Y, Ma Z, Liang C, Liang C, Liu Z (2016) Effects of contrast-enhancement, reconstruction slice thickness and convolution kernel on the diagnostic performance of radiomics signature in solitary pulmonary nodule. Sci Rep 6:34921. https://doi.org/10.1038/srep34921
Fujikawa R, Muraoka Y, Kashima J, Yoshida Y, Ito K, Watanabe H et al (2022) Clinicopathologic and genotypic features of lung adenocarcinoma characterized by the international association for the study of lung cancer grading system. J Thorac Oncol 17(5):700–707. https://doi.org/10.1016/j.jtho.2022.02.005
Takahashi S, Tanaka N, Okimoto T, Tanaka T, Ueda K, Matsumoto T et al (2012) Long term follow-up for small pure ground-glass nodules: implications of determining an optimum follow-up period and high-resolution CT findings to predict the growth of nodules. Jpn J Radiol 30(3):206–217. https://doi.org/10.1007/s11604-011-0033-8
Nakazono T, Sakao Y, Yamaguchi K, Imai S, Kumazoe H, Kudo S (2005) Subtypes of peripheral adenocarcinoma of the lung: differentiation by thin-section CT. Eur Radiol 15(8):1563–1568. https://doi.org/10.1007/s00330-004-2595-7
Xia T, Cai M, Zhuang Y, Ji X, Huang D, Lin L et al (2020) Risk factors for the growth of residual nodule in surgical patients with adenocarcinoma presenting as multifocal ground-glass nodules. Eur J Radiol 133:109332. https://doi.org/10.1016/j.ejrad.2020.109332
Gao F, Li M, Ge X, Zheng X, Ren Q, Chen Y et al (2013) Multi-detector spiral CT study of the relationships between pulmonary ground-glass nodules and blood vessels. Eur Radiol 23(12):3271–3277. https://doi.org/10.1007/s00330-013-2954-3
Aerts HJ (2016) The potential of radiomic-based phenotyping in precision medicine: a review. JAMA Oncol 2(12):1636–1642. https://doi.org/10.1001/jamaoncol.2016.2631
Tomaszewski MR, Gillies RJ (2021) The biological meaning of radiomic features. Radiology 298(3):505–516. https://doi.org/10.1148/radiol.2021202553
Vicini S, Bortolotto C, Rengo M, Ballerini D, Bellini D, Carbone I et al (2022) A narrative review on current imaging applications of artificial intelligence and radiomics in oncology: focus on the three most common cancers. Radiol Med 127(8):819–836. https://doi.org/10.1007/s11547-022-01512-6
Mackin D, Fave X, Zhang L, Fried D, Yang J, Taylor B et al (2015) Measuring computed tomography scanner variability of radiomics features. Invest Radiol 50(11):757–765. https://doi.org/10.1097/rli.0000000000000180
Berenguer R, Pastor-Juan MDR, Canales-Vázquez J, Castro-García M, Villas MV, Mansilla Legorburo F et al (2018) Radiomics of CT features may be nonreproducible and redundant: influence of CT acquisition parameters. Radiology 288(2):407–415. https://doi.org/10.1148/radiol.2018172361
Peng X, Yang S, Zhou L, Mei Y, Shi L, Zhang R et al (2022) Repeatability and reproducibility of computed tomography radiomics for pulmonary nodules: a multicenter phantom study. Invest Radiol 57(4):242–253. https://doi.org/10.1097/rli.0000000000000834
Li Y, Reyhan M, Zhang Y, Wang X, Zhou J, Zhang Y et al (2022) The impact of phantom design and material-dependence on repeatability and reproducibility of CT-based radiomics features. Med Phys 49(3):1648–1659. https://doi.org/10.1002/mp.15491
Cardenas CE, Yang J, Anderson BM, Court LE, Brock KB (2019) Advances in auto-segmentation. Semin Radiat Oncol 29(3):185–197. https://doi.org/10.1016/j.semradonc.2019.02.001
Liu L, Wolterink JM, Brune C, Veldhuis RNJ (2021) Anatomy-aided deep learning for medical image segmentation: a review. Phys Med Biol. https://doi.org/10.1088/1361-6560/abfbf4
Funding
This study was supported by the National Natural Science Foundation of China (82202141), Sichuan Science and Technology Program (2021YFS0075, 2021YFS0225) and the Chengdu Science and Technology Program (2021-YF05-01507-SN).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. YL and JL involved in conceptualization, data curation, formal analysis, methodology, and writing—original draft; XY, FX, and LW performed the data curation and validation. The study was founded by JL and PZ. Writing—review & editing was performed by HQ, JR, and PZ. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
The study was approved by the Institutional Review Board of Sichuan Cancer Hospital & Institute, and the patient consent was waived because anonymized information was used for this retrospective study.
Informed consent
Patient consent was waived because anonymized information was used for this retrospective study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Li, Y., Liu, J., Yang, X. et al. Radiomic and quantitative-semantic models of low-dose computed tomography for predicting the poorly differentiated invasive non-mucinous pulmonary adenocarcinoma. Radiol med 128, 191–202 (2023). https://doi.org/10.1007/s11547-023-01591-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11547-023-01591-z