Abstract
Aim of this study was to systematically review the literature to assess efficacy and safety of stereotactic radiotherapy (SRT) in combination with immunotherapy for the treatment of melanoma brain metastases (MBM). The literature was searched using PubMed, Scopus, and Embase. Studies comparing SRT plus immunotherapy versus SRT or immunotherapy alone were deemed eligible for inclusion. Two studies showed improved overall survival after SRT plus immunotherapy in melanoma cancer patients with brain metastases. Three studies reported data on LC and DFS showing as SRT plus immunotherapy did not improve local control and DFS rates. G3-G4 toxicity was reported in only one study (20% in the SRT plus immunotherapy group versus 23% in the immunotherapy group). Despite SRT plus concurrent immunotherapy seems associated with possible survival advantage and low ≥ G3 late toxicity rates, the quality of evidence is very low. Therefore, in patients with brain metastases from melanoma, SRT plus immunotherapy should be evaluated on an individual basis after discussion by a multidisciplinary team.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Immune checkpoint inhibitors and targeted therapies have demonstrated to enable long-term disease control in a high percentage of patients with metastatic melanoma; however, prognosis of patients with melanoma brain metastases (MBM) remain poor, with 17–22 weeks median overall survival from the time of diagnosis [1,2,3].
Data on the efficacy of immunotherapy in patients with MBM are limited having generally been excluded from clinical trials. In phase II studies, where patients with metastatic melanoma and at least one brain metastasis were included, both anti-CTLA-4 (ipilimumab) and anti-PD1 (nivolumab and pembrolizumab) antibodies achieved response rates of 10–24% for ipilimumab [4], 20–22% for nivolumab and pembrolizumab [5, 6], and 46–57% for the ipilimumab plus nivolumab combination. [6, 7]
At the same time, SRT became largely available and nowadays it was increasingly used in MBM. In fact, stereotactic radiotherapy (SRT) in patients with MBM plays a key role due to excellent local control rates, minimal invasiveness, and possibility to repeat the treatment in case of new lesions. The effects of radiotherapy (RT) on both tumour cells and non-malignant tissues implicate a complex interaction with the immune system, resulting in both immunostimulatory and immunosuppressive outcomes. Advantages of RT are the enhancement of the immune-checkpoint inhibitors antitumour effects through increased production of cytokines and endogenous danger signals, tumour microenvironment changes, promotion of tumour-associated antigens presentation on antigen- presenting cells, and stimulus to T cell repertoire diversification [8]. Based on these biological mechanisms, RT plus concurrent immunotherapy could be a combined modality treatment theoretically associated with improved patient outcome. However, synergy between RT and immunotherapy should be clinically and radiologically supervised, due to potentially increased risk of either RT-induced or immune-mediated toxicity. In particular, RT-induced toxicity could be increased by endothelial apoptosis and neuroinflammation produced by immunotherapy. The risk of brain toxicity is associated with tumour size, exposure to higher doses of RT, and concurrent use of chemotherapy. The safety of this combined approach has been extensively analysed in a recent review including data from a large number of retrospective studies [9]. The review suggests that the combination of intracranial RT and immunotherapy has an acceptable safety profile. In addition to these findings, Kroeze et al. showed that cranial SRT is well tolerated when combined with most immune or targeted therapies [10].
The present systematic review was performed to assess the efficacy and the safety of SRT in combination with immunotherapy for the treatment of MBM compared to SRT alone or immunotherapy alone in terms of overall survival, local control, disease free survival, melanoma specific survival, and late ≥ G3 toxicity.
Materials and methods
Development of clinical question
The clinical question was developed based on the P.I.C.O. framework as: population (P), intervention (I), comparison (C), and outcomes (O). The clinical question was: (P) in melanoma brain metastases, is SRT plus immunotherapy (I) superior when compared to SRT alone or immunotherapy alone (C), in relation to the outcomes (O) of benefit and harm? Supplementary 1 reports the development of GRADE (Grades of Recommendation, Assessment, Development and Evaluation) Recommendation.
Search strategy and selection of evidence
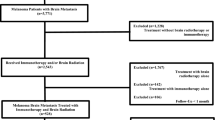
The systematic review was conducted in accordance with the PRISMA guidelines [11]. We performed a comprehensive literature search using PubMed, Scopus, and Embase (up to July 2020) to identify the full articles evaluating efficacy and safety of SRT plus immunotherapy in brain metastases from melanoma (Fig. 1). ClinicalTrials.gov was searched for ongoing or recently completed trials, and PROSPERO was searched for ongoing or recently completed systematic reviews (Supplementary 2). Electronic search was supplemented by manually searching the references of included studies and review articles. The studies were identified using the following medical subject headings (MeSH) and keywords: “melanoma”, “immunotherapy”, “radiotherapy”, “toxicity”, “brain metastases”. The search strategy was: (“melanoma” [Mesh] OR “melanoma” [All fields]) AND (“radiotherapy” [Mesh] OR “radiation therapy” [All fields]), AND “immunotherapy” [Mesh] OR “immunotherapy” [All fields]) AND “toxicity” [Mesh] OR “toxicity” [All fields] AND “neoplasm metastases” [Mesh] OR “brain metastases” [All fields]). The search was restricted to papers published in English. In order to avoid the missing of relevant studies, we chose this strategy burdened by high sensitivity and low specificity. We analysed only clinical studies presented as full texts and reporting on patients with MBM who underwent SRT plus immunotherapy. Conference papers, surveys, letters, editorials, book chapters, case reports, and reviews were excluded. Time restriction (2010– July 2020) of the publication was considered. Studies were identified through a search process performed by three independent reviewers (VL, LDR, BF), and uncertainty regarding eligibility was resolved by a multidisciplinary committee (ADS and RP—Dermato-oncologist expert in melanoma, MB—Radiation Oncologist expert in SRT, FB—Medical and Radiation Oncologist expert in immunotherapy, ER—Medical Oncologist expert in immunotherapy). Eligible citations were retrieved for full-text review. An external expert committee defined the outcomes of benefit and harm (CG, CeL, CaL, AGM, MM). A multidisciplinary master board (GS—Medical Oncologist expert in skin cancer, LT—Radiation Oncologist expert in skin cancer, KP—Dermato-oncologist expert in dermato-oncology) coordinated the project and performed the final independent check and the definitive approval of the review. The GRADEpro Guideline Development Tool (GDT) (McMaster University, 2015) was used to create Summary of Findings tables in Cochrane systematic reviews (Supplementary 3). The quality assessment showed high clinical and methodological heterogeneity and risks of bias in the included studies, making quantitative synthesis inappropriate. Therefore, meta-analysis outcomes were not reported.
Identification of outcomes
The external expert committee identified the following outcomes of benefit: overall survival (OS, defined as the time from baseline to death from any cause or last follow-up), melanoma specific survival (MSS, defined as the time from baseline to death from disease cause or last follow-up), disease free survival (DFS, defined as time from baseline to clinical or radiological progression) and local control (LC, defined as time from baseline to cancer detected in the treated site at any time after initial treatment). The identified outcome of harm included acute and late ≥ G3 toxicity. All these outcomes were considered as “critical” for the decision-making process.
Quality of evidence evaluation
Certainty of evidence for all selected outcomes was performed according to the GRADE approach, considering study limitations, imprecision, indirectness, inconsistency, and publication biases. Certainty level starts at higher pre-specified level for randomized controlled trials, but levels of certainty can be downgraded if limitations in one of the above-mentioned domains are detected. Evidence was classified as having high, moderate, low, and very low level of certainty.
Benefit/harm balance and clinical recommendation
Based on the summary of evidence, the following judgments about the benefit-to-risk ratio between intervention and comparison were stated: favourable, uncertain/favourable, uncertain, uncertain/unfavourable, and unfavourable (both for intervention or comparison). The strength of the recommendation is considered as strong positive, conditional positive, uncertain, conditional negative, or strong negative.
Results
Search strategy results and details of the identified relevant studies
Seventy potentially relevant studies were identified through the database searches after duplicates and title removal. After screening of title and abstract, 53 papers were excluded. Of the remaining 17 papers, 13 were excluded through the full text examination for the following reasons: (1) the majority of patients received whole brain RT prior to SRT and those who received SRT were not separated in a way allowing for data extraction; (2) a small minority of patients received SRT but data on their primary or secondary outcomes measures was not reported and therefore could not be extracted for the analysis; (3) few patients (< 10) were enrolled; (4) a comparison group was lacking (Fig. 1). At the end of the process, four original manuscripts were selected for analysis.
Studies characteristics
The systematic review was performed on a total of 367 patients with melanoma brain metastases included in four studies published from 2010 to 2020 [12,13,14,15]. Patient demographics, treatment characteristics, OS, LC, DFS, MSS, and toxicity data were recorded (Table 1). The results of excluded studies are presented in Supplementary Table 4. The median follow-up time ranged between 0.2 and 58.4 months (median: 6.9 months).
The median age for patients treated with SRT plus immunotherapy was 56.6 years (range: 27–87 years) and for SRT or immunotherapy alone was 54.5 years (range: 27–91 years). The most commonly used immunotherapy drugs were pembrolizumab and nivolumab, which were used in two out of four studies [12, 13]. Ipilimumab was administered in three studies [13,14,15]. The most commonly used SRT average dose was 20 Gy (range: 14–24 Gy). In the included studies, SRT was administered via Gamma Knife in one study [14] and Cyberknife in another study [12], while in the other two studies the treatment machines was not specified [13, 15]. Ninety-one patients received whole brain irradiation after SRT [13, 15]. One hundred sixty-four patients presented extracranial disease at the time of brain metastases diagnosis. One hundred forty-two patients had solitary brain metastases. In the SRT plus immunotherapy group and in the SRT or immunotherapy group, 90 and 145 patients underwent prior systemic therapy, respectively.
Studies description
The retrospective study of Trommer-Nestler et al [12]. compared SRT plus immunotherapy to SRT alone in patients with MBM. Twenty-six patients were treated either with SRT alone (13 patients; 20 lesions) or in combination with anti-PD-1 (13 patients; 28 lesions). Simultaneous treatment was defined as the somministration of the first dose of PD-1 inhibitor at least one week before SRT treatment and for at least six weeks after irradiation (n = 13). Non-simultaneous treatment (n = 13) was defined as the delivery of SRT at least three months after the last cycle of immunotherapy and at least six months before the first cycle. Patients with simultaneous treatment received at least three cycles of anti-PD-1 therapy with either pembrolizumab at a dose of 2 mg/kg every three weeks (n = 10) or nivolumab at a dose of 3 mg/kg every two weeks (n = 3). Eight (62%) of the combined treated patients were positive for BRAF mutation, five (38%) for NRAS mutation, and one for c-KIT mutation. In the SRT group, 10 (77%) patients were positive for BRAF mutation, one for NRAS mutation, and one for c-KIT mutation. A median of two (range: 1–5) lesions per patient were treated in the SRT plus anti-PD-1 group and a median of one (range: 1–3) in the SRT group. The median lesion size for the SRT plus anti-PD-1 group was 0.6 cm3 (range: 0.1–7.4 cm3) and 0.1 cm3 (range: 0.01–2.7 cm3) in the SRT group.
A prospective non-randomized study [13] compared the role of SRT plus immunotherapy versus immunotherapy alone in patients with MBM. Simultaneous treatment was defined as the delivery of radiotherapy treatment 30 days before the first systemic therapy dose to 30 days after the first dose of the same therapy line. Non-simultaneous treatment was defined as the delivery of SRT ≥ 30 days before the start of the same therapy line. No significant differences in gender, LDH, BRAF status, age, performance Karnofsky status (KPS), tumour volume, number of lesions, and incidence of extracranial disease were found between the two groups. Fifty-eight (62%) of the simultaneously treated patients and ninety-six (57%) of the immunotherapy group were positive for BRAF mutation. Thirty-nine (42%) patients in the combined group and sixty-four (38%) in the immunotherapy group had more than three lesions.
In line with this study, Mathew et al. [14] compared the role of SRT plus ipilimumab to SRT alone in patients with MBM, and found no significant differences in age, KPS, number of lesions, tumour volume, and incidence of extracranial disease between the two groups. A median of three (range: 1–9) lesions per patient was treated in the SRT plus ipilimumab group and in the SRT group. The median lesion size in the SRT plus ipilimumab group and in the SRT group was 0.6 cm3 (0.1–4.6 cm3) and 1.7 cm3 (0.2–8 cm3), respectively.
Finally, Silk et al. [15] retrospectively evaluated the role of SRT in combination with immunotherapy versus SRT alone in patients with brain metastases of melanoma. There were no significant differences between the SRT plus ipilimumab group and the comparison group. Seventeen (51.5%) of the simultaneously treated patients and three (25%) of the SRT group were positive for BRAF mutation. Fifteen (45.4%) patients in the combined group and twenty-one (56.7%) in the SRT group had 1–3 lesions.
Outcomes of harm
All four studies reported data on toxicity (Table 2). G3-G4 toxicity was reported in only one study (20% in the SRT plus immunotherapy group versus 23% in the immunotherapy group). Overall, of the 136 patients treated with SRT plus immunotherapy combination12-15, G2 intracranial haemorrhage was reported in eight (5.9%) patients14,15, headache in six (4.4%; G1: 2.9%, G2: 1.5%)12, nausea in two (1.5%; G1: 0.75%, G2: 0.75%)12, vertigo in four (2.9%; G1: 2.15%, G2: 0.75%)12, and fatigue in three (2.15%)0.12. Moreover, thyroid disorders were detected in five patients (3.7%; G1: 2.2%, G2: 1.5%)12,13 and gastrointestinal G2 toxicity in two patients (2.9%)0.12 Of 231 patients who were treated with SRT or immunotherapy alone, G2 intracranial haemorrhage was reported in 11 patients (4.8%)14,15, G2 radiation necrosis in three (1.3%)15, G1 headache in three (1.3%), G1 nausea in one (0.4%), G1 vertigo in two (0.8%), fatigue in three (1.3%, G1: 0.9% and G2: 0.4%)12, and thyroid disorders in one (G1: 0.4%)0.13.
The certainty of evidence was considered as “very low” for each outcome of harm for the following reasons: indirectness for population including both target therapy and immunotherapy13, imprecision for sample size12-15, and finally to possible selection bias due to a sub-group analysis. [13].
Outcomes of benefit
Three studies reported OS rates [13, 15] and two of them showed improved OS after SRT plus immunotherapy in melanoma cancer patients with brain metastases [13, 15]. Three studies reported data on LC and DFS showing as SRT plus immunotherapy did not improve local control [12,13,14] and DFS rates [13, 14]. Finally, no studies reported data about MSS. The Summary of Findings table for outcomes of benefit is reported in Table 3. The certainty of evidence was judged as “very low” for each outcome of benefit for the following reasons: indirectness for population including both target therapy and immunotherapy [13], imprecision for sample size [12,13,14,15], and finally to possible selection bias due to a sub-group analysis. [13]
Evidence to decision framework
In the MBM setting, the proposed intervention (SRT plus immunotherapy) did not increase the incidence of side effects (treatment-associated brain toxicity) compared to the control one (SRT or immunotherapy alone). Moreover, SRT plus immunotherapy has proven effective in improving OS without benefit in terms of LC and DFS.
Benefit/harm balance and final recommendation
The panel voted for the benefit/harm as uncertain. The strength of the recommendation was voted as conditionally weak by all five panel members. Hence, the final recommendation of the panel was: “In patients with melanoma brain metastases, the combination of SRT plus immunotherapy should be evaluated on an individual basis through discussion by a multidisciplinary team”.
Discussion
The modern approach to cancer patients is based on personalized treatment. Integration between different therapies, especially if loco-regional and systemic, can offer remarkable clinical benefit to patient in terms of oncological outcomes and particularly of OS.
In our systematic review, we examined whether SRT plus immunotherapy is superior to SRT alone in terms of benefit and harm balance in patients with MBM. The expert panel suggested the use of SRT plus immunotherapy in patients with MBM after discussion about each patient by the multidisciplinary team. This underlines the lack of data regarding the effect of timing and type of immunotherapy on the outcome after SRT of MBM. The results of the present systematic review suggest that (1) immunotherapy and SRT have an almost additive effect considering the improved OS recorded in the combined modality treatment group, and (2) local response is greater and faster after SRT plus concurrent immunotherapy compared to SRT or immunotherapy alone. Heterogeneity in treatment protocols (SRT total dose, schedule, isodose prescription) and in the definition of "simultaneous" SRT plus immunotherapy combination, small patients population included in the selected studies, and selection biases could have affected the possibility to accurately define the effective role of SRT plus immunotherapy in this setting [12,13,14,15]. Therefore, we reported only a qualitative literature analysis.
Despite this limitations, two studies showed that the combination of SRT plus immunotherapy in MBM patients improves OS [13,14,15] without significant improvement of LC [12,13,14] and DFS rates [13, 14]. Moreover, one study showed a significant improvement of OS also in patients receiving immunotherapy after SRT [15]. This finding is supported by a small number of retrospective clinical studies (not included in the present review) evaluating the timing effect in the combination of SRT and immunotherapy for MBM [16,17,18,19]. Kiess et al. reported improved OS in patients treated with SRT before or during ipilimumab compared to those treated with SRT after ipilimumab [16]. Similarly, Schoenfeld et al. reported improved OS after SRT delivered before ipilimumab compared to SRT after ipilimumab. [17].
However, even if confirmed by the latter studies, the results of our analysis present paradoxical aspects. Indeed, an improvement in OS without prolonged LC and DFS is hard to be explained. Since this review is based on retrospective studies, selection bias could be hypothesized and in particular a preferential referral of patients with better performance status or with less relevant comorbidities to the combined modality treatment [18].
Nevertheless, these results are similar to the ones recorded in other oncological settings, showing an increased effect of immunotherapy given after RT. For example, the PACIFIC trial evaluated activity and safety of durvalumab administered after combined chemoradiation in patients affected by stage III NSCLC. In that trial, median time to death or distant metastasis was longer with durvalumab compared to placebo (23.2 months vs 14.6 months; p < 0.001) [19]. Radiotherapy may be a synergistic effect with immunotherapy. Indeed, RT can cause a transient alteration in the blood–brain barrier [20], resulting in an uptake of immunotherapy. Moreover, the combination of RT and immunotherapy may increase systemic antitumour response [20] and it could lead to an abscopal effect, correlating with prolonged survival [21].
In any case, if the positive effect of the SRT plus immunotherapy combination was confirmed, it would be useful to define which type of immune checkpoint inhibitor is more effective when combined to SRT. Lehrer et al. [22]. suggested that the association of anti-PD-1 and SRT results in a greater and faster tumour shrinkage compared to anti-CTLA-4 plus SRT. However, these results may have been influenced by a higher number of lesions in the anti-PD-1 plus SRT group [23].
In the present systematic review, SRT plus immunotherapy combination was found to have tolerable toxicity profile. In the SRT plus immunotherapy group (136 patients) intracranial haemorrhage was recorded only in eight patients [14, 15], headache in six, nausea in two, vertigo in four, and fatigue in four [12]. In the SRT or immunotherapy alone group (231 patients), intracranial haemorrhage was recorded in eleven [14, 15], radiation necrosis in three patients [15] headache in three, nausea in one, vertigo in two, and fatigue in three [12]. In patients included in this analysis, adverse events severity was never scored as higher than Grade 2 according to the CTCAE scale.
Limitations of our systematic review include the small number of studies, especially for the secondary outcome measures. In addition, none of them was a randomized controlled trial and all studies were retrospective. Toxicity data were extrapolated from retrospective studies with different adverse effects categorization and in some cases with short follow-up. More generally, a median follow-up of 6.9 months (range: 0.2–58.4 months) is insufficient to allow a proper assessment of late toxicity. However, longer follow-up is unlikely to lead to the detection of further differences in late adverse events between patients treated with SRT plus immunotherapy compared to single treatment groups.
Conclusions
The final recommendation released by the panel was: “In patients with brain metastases from melanoma, SRT plus immunotherapy should be evaluated on individual basis after discussion by a multidisciplinary team”. The communication with the patient should include the following topics: prognosis with and without treatment, limited power of evidence on the benefit derived from treatment combination, and treatment related risks including haemorrhage and radiation necrosis.
References
Katalin L, Serge MC, Udo SG, Benjamin F (2018) Editorial: radiation and the immune system: current knowledge and future perspectives. Front Immunol 8:1933. https://doi.org/10.3389/fimmu.2017.01933
Spagnolo F, Picasso V, Lambertini M, Ottaviano V, Dozin B, Queirolo P (2016) Survival of patients with metastatic melanoma and brain metastases in the era of MAP-kinase inhibitors and immunologic checkpoint blockade antibodies: a systematic review. Cancer Treat Rev 45:38–45. https://doi.org/10.1016/j.ctrv.2016.03.003
Davies MA, Liu P, McIntyre S, Kim KB, Papadopoulos N, Hwu WJ, Hwu P, Bedikian A (2011) Prognostic factors for survival in melanoma patients with brain metastases. Cancer 117:1687–1696. https://doi.org/10.1002/cncr.25634
Margolin K, Ernstoff MS, Hamid O, Lawrence D, McDermott D, Puzanov I et al (2012) Ipilimumab in patients with melanoma and brain metastases: an open-label, phase 2 trial. Lancet Oncol 13:459–465. https://doi.org/10.1016/S1470-2045(12)70090-6
Goldberg SB, Gettinger SN, Mahajan A, Chiang AC, Herbst RS, Sznol M et al (2016) Pembrolizumab for patients with melanoma or non-small-cell lung cancer and untreated brain metastases: early analysis of a non-randomised, open-label, phase 2 trial. Lancet Oncol S1470e2045:30053–30055. https://doi.org/10.1016/S1470-2045(16)30053-5
Long GV, Atkinson V, Lo S, Sandhu S, Guminski AD, Brown MP et al (2018) Combination nivolumab and ipilimumab or nivolumab alone in melanoma brain metastases: a multicentre randomised phase 2 study. Lancet Oncol 19:672–681. https://doi.org/10.1016/S1470-2045(18)30139-6
Tawbi H, Forsyth P, Algazi A, Hamid O, Hodi S, Moschos SJ (2018) Efficacy and safety of Nivolumab plus ipilimumab in patients with melanoma metastatic to the brain: results of the phase II study checkmate 204. Combined Nivolumab and Ipilimumab in Melanoma Metastatic to the Brain. N Engl J Med 379:722–730. https://doi.org/10.1056/NEJMoa1805453
Bernstein MB, Krishnan S, Hodge JW, Chang JY (2016) Immunotherapy and stereotactic ablative radiotherapy (ISABR): a curative approach? Nat Rev Clin Oncol 13:516–524. https://doi.org/10.1038/nrclinonc.2016.30
Hwang WL, Pike LRG, Royce TJ, Mahal BA, Loeffler JS (2018) Safety of combining radiotherapy with immune-checkpoint inhibition. Nat Rev Clin Oncol 15:477–494. https://doi.org/10.1038/s41571-018-0046-7
Kroeze SG, Fritz C, Hoyer M et al (2017) Toxicity of concurrent stereotactic radiotherapy and targeted therapy or immunotherapy: a systematic review. Cancer Treat Rev 53:25–37. https://doi.org/10.1016/j.ctrv.2016.11.013
Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP et al (2009) The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. Ann Intern Med 151:65–94. https://doi.org/10.7326/0003-4819-151-4-200908180-00136
Trommer-Nestler M, Marnitz S, Kocher M et al (2018) Robotic Stereotactic radiotherapy in Melanoma Patients with Brain Metastases under Simultaneous Anti-PD-1 Treatment. Int J Mol Sci 19:2653. https://doi.org/10.3390/ijms19092653
Tetu P, Allayous C, Oriano B et al (2019) Impact of radiotherapy administered simultaneously with systemic treatment in patients with melanoma brain metastases within MelBase, a French multicentric prospective cohort. Eur J Cancer 112:38–46. https://doi.org/10.1016/j.ejca.2019.02.009
Mathew M, Tam M, Ott PA et al (2013) Ipilimumab in melanoma with limited brain metastases treated with stereotactic radiotherapy. Melanoma Res 23:191–195. https://doi.org/10.1097/CMR.0b013e32835f3d90
Silk AW, Bassetti MF, West BT, Tsien CI, Lao CD (2013) Ipilimumab and radiation therapy for melanoma brain metastases. Cancer Med 2:899–906. https://doi.org/10.1002/cam4.140
Kiess AP, Wolchok JD, Barker CA et al (2015) Stereotactic radiotherapy for melanoma brain metastases in patients receiving ipilimumab: safety profile and efficacy of combined treatment. Int J Radiat Oncol Biol Phys 92:368–375. https://doi.org/10.1016/j.ijrobp.2015.01.004
Schoenfeld JD, Mahadevan A, Floyd SR et al (2015) Ipilmumab and cranial radiation in metastatic melanoma patients: a case series and review. J Immunother Cancer 3:50. https://doi.org/10.1186/s40425-015-0095-8
Greto D, Scoccianti S, Compagnucci A et al (2016) Gamma Knife Radiosurgery in the management of single and multiple brain metastases. Clin Neurol Neurosurg 141:43–47. https://doi.org/10.1016/j.clineuro.2015.12.009
Antonia SJ, Villegas A, Daniel D et al (2017) PACIFIC investigators. durvalumab after chemoradiotherapy in stage III non-small-cell lung cancer. N Engl J Med 377:1919–1929. https://doi.org/10.1056/NEJMoa1709937
Cao Y, Tsien CI, Shen Z et al (2005) Use of magnetic resonance imaging to assess blood-brain/blood-glioma barrier opening during conformal radiotherapy. J Clin Oncol 23:4127–4136
Desideri I, Francolini G, Scotti V et al (2019) Beneft of ablative versus palliative-only radiotherapy in combination with nivolumab in patients afected by metastatic kidney and lung cancer. Clin Transl Oncol 21:933–938. https://doi.org/10.1007/s12094-018-02005-7
Lehrer EJ, Peterson J, Brown PD et al (2019) Treatment of brain metastases with stereotactic radiotherapy and immune checkpoint inhibitors: An international meta-analysis of individual patient data. Radiother Oncol 130:104–112. https://doi.org/10.1016/j.radonc.2018.08.025
Franceschini D, Franzese C, Navarria P et al (2016) Radiotherapy and immunotherapy: can this combination change the prognosis of patients with melanoma brain metastases? Cancer Treat Rev 50:1–8. https://doi.org/10.1016/j.ctrv.2016.08.003
Funding
Open access funding provided by Università Cattolica del Sacro Cuore within the CRUI-CARE Agreement. The authors did not receive support from any organization for the submitted work.
Author information
Authors and Affiliations
Contributions
KP and LT coordinated the project and contributed equally to this trial. CG, CeL, CaL, AGM, MM defined the outcomes of benefit and harm. VL, LDR, LT, KP were responsible for the conception and design of the study. VL, LDR, BF, RP, ER, ADS, MB, FB contributed to data collection of. All authors were responsible for data interpretation of. VL, LDL, ADS, and KP wrote the manuscript. All authors reviewed and approved the manuscript for submission.
Corresponding author
Ethics declarations
Conflict of interest
All authors certify that they have no affiliations with or involvement in any organization or entity with any financial interest or non-financial interest in the subject matter or materials discussed in this manuscript.
Human and animal rights
Not applicable.
Ethical approval
Not applicable.
Consent Informed
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Luca Tagliaferri and Ketty Peris have equally contributed to this work.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Lancellotta, V., Del Regno, L., Di Stefani, A. et al. The role of stereotactic radiotherapy in addition to immunotherapy in the management of melanoma brain metastases: results of a systematic review. Radiol med 127, 773–783 (2022). https://doi.org/10.1007/s11547-022-01503-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11547-022-01503-7