Abstract
Characterizing potato cultivars is essential for diagnosing the specific production and management requirements. Employing techniques that facilitate rapid, objective, and cost-effective identification of various aspects of plant physiology is crucial for generating this valuable information. In this study, our goal was to identify different potato cultivars across three distinct phenological phases utilizing reflectance spectroscopy within the visible and near-infrared ranges. Reflectance spectra were measured using a portable spectrometer on leaves obtained from five different potato cultivars. We constructed classification models to determine the accurate classification percentages for each cultivar within specific phenological stages. General trends in the reflectance spectra were observed, which were consistent across all five cultivars within the measured range. During the vegetative phase, there was a uniform reflectance of around 5% in the green region. This value experienced a slight decrease during the flowering phase and subsequently rose to approximately 12% during the senescence phase. In the near-infrared (NIR) range, a reduction in reflectance from 50 to 30% was noted during the flowering phase, followed by a cultivar-dependent increase towards senescence. The specific wavelengths identified in the spectra enabled the accurate classification of plants from each cultivar across the vegetative, flowering, and senescence phases. The classification rates were notably high, with a success rate of 87% for Var. Paola, 96% for Var. Paysandú, 93% for Var. Violeta, and 100% for cv. 448 and 440. The Var. Paola exhibited superior correct classification rates, ranging from 85% for Paysandú and Violeta, 90% for cv. 440, to 100% for cv. 448. The characterization of potato cultivars using spectroscopy techniques yields valuable information that serves as fundamental input for precision agriculture systems.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Spectroscopy measures how electromagnetic radiation interacts with matter as a function of wavelength, with absorption or emission of radiant energy. Visible (Vis) and near-infrared spectroscopy (NIR) are modern tools used for various applications in different areas of daily life (Kortum 2012), such as medicine, chemistry, and industry; moreover, in recent decades, interest has increased with the development of sensors that can be integrated into agricultural tools and equipment (Cavaco et al. 2022). In modern agricultural systems, this technique has been used in early detection of plant stress (Zahir et al. 2022), soil studies (Wetterlind et al. 2013), food quality (with emphasis on fruit quality), nutrient quantification, measuring nutritional parameters in grains, forage quality, and mineral contents in fertilizers and compost (García-Sánchez et al. 2017). Techniques based on hyperspectral data in the Vis/NIR ranges are being incorporated more quickly in the agricultural productive sector than techniques based on spectral images because the latter have limitations in spectral resolution, their precision is often limited, and they cannot effectively detect biotic and/or abiotic stress (Lu et al. 2020).
Importantly, specific applications of this technique are linked to each range of the measured spectrum. For example, the Vis range is defined as the wavelengths between 380 and 750nm, and, specifically in plants, its absorption is mainly associated with the presence and concentration of the principal photosynthetic pigments (chlorophylls, carotenes, xanthophylls, anthocyanins, and phycobilins) (Blackburn 2006). The spectral information of the Vis range is widely used to detect physiological changes caused by different types of biotic or abiotic stress since these kinds of changes lead to variation in the spectral signature in the Vis, caused by the breakdown of chlorophyll pigments and changes in the levels of carotenoids and other pigments through degradation of the cellular structure (Zubler and Yoon 2020). Otherwise, the spectra in the NIR are quantitatively and qualitatively related to the chemical composition because the energy absorbed in this region by the plant causes vibration in covalent bonds C-H, O-H, and N-H, which are important components of organic matter (Beć and Huck 2019). The tissue presents a variable spectrum in the NIR because of differences in the organic composition and spectral information for this range in terms of compounds and structure, which, along with their concentrations, must be studied in plants (Beć et al. 2020). Additionally, plants do not use the infrared spectrum in photosynthesis, so there is no overlap of the spectral signatures in these two ranges.
Spectral characterization is related to the description of frequency measurements in a range of the electromagnetic spectrum and the study of their interaction with matter, whether with percentages of absorption, reflection, or transmission. The main applications of Vis and/or NIR spectroscopy in potato crops (Solanum tuberosum L.) have been used to determine the internal components of tubers, such as the amount of dry matter, moisture content, carbohydrates (mainly starch), protein content, determination of minor components (fats and acrylamides), estimation of total and individual carotenoids (López et al. 2013), and disease detection (Zhou et al. 2015; Fernández et al. 2020). In addition, other authors have focused on relating spectral signatures to external properties of tubers or plants, which are important to production and quality, such as damage (Evans and Muir 1999), texture (Boeriu et al. 1998), and sprouting capacity (Jeong et al. 2008)
However, currently, there are few reports focused on the spectral characterization of healthy potato plants at different phenological stages. This knowledge is necessary to generate a baseline that can be used as a basic input for subsequent applied research, where detailed information can be provided on the spectral changes that occur in plants subjected to different types of biotic or abiotic stress. Potato is the fourth most important crop in the world, after rice, wheat, and maize, and is an indispensable product for the food and nutritional security of the population (Thongam et al. 2017; Seminario-Cunva et al. 2018; Singh et al. 2019). Solanum tuberosum L., Solanum tuberosum L. subspecies andigena, and Solanum phureja are also cultivated within the Solanum genus. Phureja group (creoles) is a particularly important food in Andean countries such as Colombia and Peru (Ghislain et al. 1999). In this study, five potato varieties developed from the Phureja group were selected as model organisms, which will help to understand the functioning of the spectral response in other similar varieties and species. On the other hand, in this crop, some diseases (e.g. Phytophthora infestans) and water stress are critical factors for harvest yield (Jacques et al. 2020; Vilvert et al. 2022), where spectral information can be used in future research projects. Specifically, the main objectives of this article were to characterize the spectral signatures of five potato varieties (at the leaf scale) in three of the main phenological stages of growth and to identify potato varieties using reflectance spectroscopy in the visible (Vis) and near-infrared (NIR) ranges.
Methodology
Study Site and Plant Material
This study was conducted under greenhouse conditions at the Paysandú agricultural station (2500 mamsl) of the Universidad Nacional de Colombia (UNAL), located in the Santa Elena district of Medellín. Five varieties developed from the Phureja group (Solanum tuberosum, Phureja group) were used, obtained from clones: S. tuberosum var. Paola, S. tuberosum var. Paysandu, S. tuberosum var. Violeta, S. tuberosum cv. 448, and S. tuberosum cv. 440. A completely randomized design was used with 15 plants per treatment (cultivars evaluated), which were maintained under greenhouse conditions with an average temperature of 14 °C and relative humidity between 80 and 90%. Some general characteristics of the cultivars are described in Table 1:
Spectral Response Measurements
The spectral response measurements were taken at three phenological stages according to BBCH coding for potato (Meier 2018): “formation of lateral shoots” (22 202), “flowering” (66 606), and “senescence” (91 901); three measurements were taken on the plants of all varieties at each phenological stage every 48 h.
The reflectance measurement system used in this study had a portable Hr2000 spectrometer, with an HL-2000-HP tungsten halogen light source and a bifurcated fiber optic cable (Ocean Optics, FL, USA). Spectra were measured in reflectance mode in the range of 400–1000 nm. This system has an optical resolution of approximately 0.55 nm in the Vis and 6 nm between 700 and 1000 nm. The integration time was 9 ms, and 10 averages were taken for each observation, with the curve smoothed to a value of 2.5 nm. Each sample corresponded to a field of view around 2 mm in diameter, which were measured between 9:00 a.m and 11:00 a.m. according to a modified protocol developed by Marín-Ortiz et al. (2020).
Data Analysis
A completely randomized design was used to compare the spectral response of the five potato varieties at three different phenological stages. Three measurements (reflectance spectra) were taken in the middle third of each leaf (leaf three, leaflet three) on the main stem of 15 clones measured per cultivar. A total of 2025 reflectance spectra were measured, with 225 recorded per measurement day and 675 obtained per phenological stage. As part of the data preprocessing, spectra with noise, distortion, or reading errors were removed. To visualize the overall differences between the reflectance spectra, average spectra were calculated for the five cultivars at the three phenological stages. The Relief algorithm (Urbanowicz et al. 2018) was then used to identify specific wavelengths that could classify plants of each cultivar at a specific phenological stage, as well as those that could classify plants by cultivar. Finally, linear discriminant analyses were carried out as a supervised classification method for qualitative variables, in which the observations were classified into three groups per cultivar (bud formation, flowering, and senescence). All analyses were performed using R software v.4.0.5 (RStudio Team, Boston, MA, USA).
Results
Spectral Characterization of Five Potato Varieties at Three Different Phenological Stages
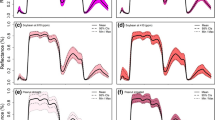
The average reflectance spectra from healthy leaves of the five potato varieties at three different phenological stages are plotted in Fig. 1. The general behavior of the average reflectance spectra of the five cultivars tended to be zero for most of the Vis range, with a peak maximum at 550 nm (green). The highest reflectance in the green fluctuated between 3.5 and 5.5% during the vegetative phase, decreasing slightly to 2–3% in the flowering phase and increasing to 8–10% in the maturation phase. The spectral response in the measured NIR range (750–1000 nm) was homogeneous during the vegetative phase for the evaluated cultivars, which presented reflectance values around 50%, except for the Violeta cultivar, which had values close to 37% (Fig. 1e). In this spectral range, there was also a decrease in reflectance during the flowering phase, decreasing to 30–35% and increasing again in the maturation phase to an approximate value of 38%.
Selection of Specific Wavelengths in Three Phenological Phases
Based on the weight values calculated with the Relief algorithm, spectral wavelengths with relatively high weights (high discriminative power) were found for the change from the vegetative phase to flowering in the studied material, mainly in the spectral ranges of blue, green, red, and the measured NIR (750–1000 nm). In the transition from flowering to senescence, wavelengths with a high discriminative power were also found in the first three spectral ranges, with greater relevance in the regions of green and red, particularly the red edge, between 720 and 750 nm for the Violeta, 448 and 440 varieties; however, there was a noticeable decrease in weight values in the measured NIR range at this stage (Table 2 or Fig. 2).
The linear discriminant analysis (LDA) of the selected wavelengths to separate the vegetative (green dots), flowering (purple dots), and senescence (brown dots) phenological phases in improved varieties and cultivars of potatoes: a Paola, b Paysandú, c 448, d 440, e Violeta. The selected wavelengths for performing the discriminant analyses (LDA) are indicated at the tips of the arrows for each cultivar’s graph
A large number of linear discriminant analyses (LDAs) were developed using different combinations of variables classified with the Relief algorithm. Figure 2 shows the graphs of the LDAs with the wavelengths that separated the plants of five cultivars in the vegetative, flowering, and senescence phases. In the Paola cultivar, the wavelengths that discriminated the phenological phases were located in the blue range of the visible spectrum, at 440 nm, 480 nm, and 500 nm. On the other hand, for Paysandú, the differentiating wavelengths were in the green (516 nm) and red, at 705 nm and 730 nm. Cultivars 448 and 440 presented the same discriminant wavelengths for the phenological phases in the short near-infrared region, measured at 785 nm, 880 nm, and 995 nm. Finally, the discriminant wavelengths for the Violeta cultivar were in the blue (470 nm and 490 nm) and red edge (750 nm and 780 nm). These wavelengths identified the plants of each cultivar in each phenological phase, with a correct classification of 87% for Paola, 96% for Paysandú, 100% for 448 and 440, and 93% for Violeta.
Discrimination of Varieties and Cultivars
A pairwise comparison was carried out between the evaluated varieties in each phenological phase to obtain differentiating wavelengths (Table 3). In general, a greater number of wavelengths with high values in the comparison weights between the Violeta cultivar and the other genetic materials were observed in the vegetative phase. Although these wavelengths with higher assigned weights were also found in the violet (430 nm) and green (500–540 nm), the red range (615–750 nm) and measured NIR (745–1000 nm) had wider ranges with higher values in the weights in this phase (except for the Violeta-Paola comparison, which presented low values). On the other hand, the other varieties differed from each other in wavelengths that were mainly in the red (625–750 nm), with some particular exceptions in the green and measured NIR (var. Paola-var. 448 and var. Paola-var. 440).
Otherwise, in the flowering phase, varieties and cultivars differed mainly in wavelengths in the violet (430 nm and 440 nm), blue (445 nm, 455 nm, 470 nm, 480–500 nm), and red (610–645 nm, 660–690 nm, 705 nm, and 710 nm), with relatively high values in the weight values in the NIR only in the comparison between the Violeta and 448 varieties (780–100 nm). Finally, there were few wavelengths with high weights in the comparisons between varieties in the senescence phase, restricted only to short ranges mainly in blue (450–500 nm), yellow and orange (570–610 nm), and red (685 nm, 705 nm, 720 nm, and 740 nm).
The specific discriminatory wavelengths of the vegetative phase indicated in Table 3 were used in LDA, in which the varieties and cultivars were predefined groups (Fig. 3). In general, a high percentage of correct classification was obtained for the cultivar Paola, with values ranging from 85% for Paysandú and Violeta, 90% for material 440, and 100% for the cultivar 448 (Fig. 3a–d). Paysandú had a low classification percentage with 440, while it achieved high percentages with Violeta and 448 (Fig. 3e, f). Finally, a 100% correct classification was achieved between Violeta with 448 and 440 (Fig. 3h, i).
Linear discriminant analysis (LDA) of selected wavelengths to separate improved potato varieties and cultivars. The specific discriminating wavelengths of the vegetative phase indicated in Table 2 were used
Discussion
Reflectance spectroscopy is a fast and non-destructive method for the characterization of potato varieties in different phenological stages. In general, the evaluated varieties showed typical reflectance in the measured spectral range of healthy potato leaves in the vegetative phase, as reported by other researchers in other potato varieties (Couture et al. 2018; Fernández et al. 2020), i.e., values less than 5% in blue and red, between 5–10% in green, and 50–60% in the measured infrared. However, our results differ from the average spectrum reported by Zhao et al. (2021) in leaf-scale reflectance measurements under field conditions, which reached 35% reflectance in blue and approximately 30% in green (with higher values throughout the Vis range). These differences in the results can be explained by the measurement methodology; these researchers collected upper canopy leaves (new leaves) from different crops, which possibly had lower photosynthetic activity and a low chlorophyll concentration (Panda et al. 2019), so their absorbance was low (high reflectance) in the visible range.
When changes between different phenological stages were examined based on the average spectrum for each cultivar, similar spectral patterns were obtained in the Vis (400–750 nm) and NIR (751–1000 nm) regions, but they showed a notable variation between phenological stages. The results indicate that there are spectral regions within the measured range that vary specifically in response to the phenological change of the evaluated potato varieties. In recent decades, results have been published from studies on the relationship between variations in different physiological parameters and their spectral response in the Vis (Marín-Ortiz et al. 2020). In the visible region, the minimum reflectance was between 400–500 nm and 600–700 nm because of strong absorption by chlorophylls and carotenes, and its sharp increase at approximately 780 nm was associated with the higher reflective surface in the spongy mesophyll structure (Peñuelas and Filella 1998). Additionally, reflectance in the spectral range between 780 and 1000 nm (also known as the “infrared valley”) is characterized as being constant with high reflectance. However, in most spectra, a decrease was observed towards the end of the measured range, close to 980 nm, which may be associated with low absorption of leaf water content. The results of the characterization of the spectral response of the evaluated varieties and cultivars showed a decrease in reflectance in the measured spectral range from the vegetative phase to flowering. These results are consistent with those obtained by other researchers in potatoes, where it has been proven that the chlorophyll concentration increases to maximum levels in the tuber-forming stage with flowers and then gradually decreases in the senescence stage when the leaves turn yellow (Liu et al. 2020). This is mainly due to nutrient transfer from leaves and stems to tubers during these periods.
Taking into account the genetic relationship between the parents of the Paola, Paysandú, and Violeta varieties (Table 1), it is important to highlight the high percentages of correct classification (above 85%) with the selected wavelengths for most comparisons between varieties, except for 440 with 71% (Table 3, Fig. 3). Even among highly related varieties, such as Paysandú and Violeta, it is possible to identify specific wavelength bands that vary, allowing acceptable discrimination. This can be observed particularly in the Paola and Paysandú varieties, which were developed from the cross of S. phureja x LP-Cal-10 and could be discriminated with a percentage of correct classification of 97% using some wavelengths in the Vis and NIR. Although there are few publications aimed at identifying the spectral response to identify the reflectance pattern in potato varieties, some authors have managed to determine some optimal spectral ranges for discriminating potato varieties. In this sense, our results agree with those obtained by Aboelghar et al. (2013), who performed several linear discriminant analyses to identify the optimal wavelength bands that could be used to identify four potato varieties. These authors found a wide band of optimal wavelengths in the visible range (350–712 nm) to identify the Rosetta cultivar, a band in the near-infrared range (713–1345 nm) for the Diamond cultivar, and a narrow band (~712–717 nm) for the Everest and Mondial varieties. The results obtained in these studies suggested that there are changes in the spectral response of varieties that undergo improvement processes to obtain different cultivars with the desired characteristics.
The characterization of the spectral response of high-quality potato cultivars is important to developing methodologies for monitoring biochemical and biophysical parameters that could be incorporated into precision agriculture systems (Cohen and Alchanatis 2018). Additionally, the identification of spectral signatures of healthy plants could be used as a basic input in automatic systems mounted on drones or satellites (Aasen et al. 2018) that have sensors that read wavelengths (or bands) for characterizing the specific cultivar. This would allow the development of intelligent models that quickly obtain information of interest from automatic monitoring, such as detection of stress in plants from biotic or abiotic factors, phenological state of plants, and estimation of cultivated area (Zubler and Yoon 2020). This methodology has also been suggested for identifying plant varieties in early stages of vegetative material propagation with direct reading of planting units, which is useful in identifying economically important cultivars of different species and varieties (Buitrago et al. 2018; Huang et al. 2020). This methodology provides producers with a robust and useful tool for making rapid decisions, improving crop productivity and reducing the likelihood of economic losses.
Conclusions
In this study, the spectral response of five potato varieties developed from the Phureja group was characterized to identify their spectral footprint at the leaf scale in three main phenological stages of growth using Vis-NIR reflectance spectroscopy. This technique classified the phenological states of the evaluated cultivars with high percentages of correct classification. Reflectance decreased in the transition from the vegetative phase to flowering and increased towards leaf wilting in the measured spectral range (400–1000 nm). Spectral signatures for the cultivars in each phenological stage were obtained using the RELIEF classification algorithm, which determined the best set of characteristics to obtain good discriminatory power of the relevant wavelengths in the LDA.
The spectral response difference between potato cultivar clones and their respective physiological stages can be quickly identified using reflectance spectroscopy in the VIS-NIR range. Specifically, the newly developed cultivars (440 and 448) exhibited spectral signatures that clearly differentiated them from other cultivars in all phenological stages. Spectral characterization allowed for the precise and objective differentiation of potato varieties and cultivars with very close parental relationships in different phenological phases. This information is a basic input that could be incorporated into precision agriculture systems to improve productivity and maximize yields in production systems.
References
Aasen H, Honkavaara E, Lucieer A, Zarco-Tejada P (2018) Quantitative remote sensing at ultra-high resolution with UAV spectroscopy: a review of sensor technology, measurement procedures, and data correction workflows. Remote Sens 10(7):1091. https://doi.org/10.3390/rs1007109
Aboelghar M, Arafat S, Farag E (2013) Hyper spectral measurements as a method for potato crop characterization. Int J Adv Remote Sens GIS. 2(1):122-129. http://technical.cloud-journals.com/index.php/IJARSG/article/view/Tech-97
Beć KB, Grabska J, Huck CW (2020) Near-infrared spectroscopy in bio-applications. Molecules 25(12):2948. https://doi.org/10.3390/molecules25122948
Beć KB, Huck CW (2019) Breakthrough potential in near-infrared spectroscopy: spectra simulation. a review of recent developments. Front Chem 7. https://doi.org/10.3389/fchem.2019.00048
Blackburn GA (2006) Hyperspectral remote sensing of plant pigments. J Exp Bot 58(4):855–867. https://doi.org/10.1093/jxb/erl123
Boeriu CG, Yuksel D, der Vuurst Van, de Vries R, Stolle-Smits T, Van Dijk C (1998) Correlation between near infrared spectra and texture profiling of steam cooked potatoes. J Near Infrared Spectrosc 6:A291–A297. https://doi.org/10.1255/jnirs.210
Buitrago MF, Skidmore AK, Groen T, Hecker CA (2018) Connecting infrared spectra with plant traits to identify species. ISPRS J Photogramm Remote Sens 139:183–200. https://doi.org/10.1016/j.isprsjprs.2018.03.013
Cavaco AM, Utkin AB, Marques da Silva J, Guerra R (2022) Making sense of light: the use of optical spectroscopy techniques in plant sciences and agriculture. Appl Sci 12(3):997. https://doi.org/10.3390/app12030997
Cohen Y, Alchanatis V (2018) Spectral and spatial methods for hyperspectral and thermal image-analysis to estimate biophysical and biochemical properties of agricultural crops. CRC Press, EEUU
Couture JJ, Singh A, Charkowski AO, Groves RL, Gray SM, Bethke PC, Townsend PA (2018) Integrating spectroscopy with potato disease management. Plant Dis 102(11):2233–2240. https://doi.org/10.1094/PDIS-01-18-0054-RE
Evans SD, Muir AY (1999) Reflectance spectrophotometry of bruising in potatoes. I. Ultraviolet to near infrared. Int Agrophys 13:203–209
Fernández CI, Leblon B, Haddadi A, Wang J, Wang K (2020) Potato late blight detection at the leaf and canopy level using hyperspectral data. Can J Remote Sens 46(4):390–413. https://doi.org/10.1080/07038992.2020.1769471
García-Sánchez F, Galvez-Sola L, Martínez-Nicolás JJ, Muelas-Domingo R, Nieves M (2017) Using near-infrared spectroscopy in agricultural systems. InTech. Spectroscopy. https://doi.org/10.5772/67236
Ghislain M, Zhang DP, Fajardo D, Huamán Z, Hijmans RJ (1999) Marker-assisted sampling of the cultivated Andean potato Solanum phureja collection using RAPD markers. Genet Resour Crop Evol 46:547–555. https://doi.org/10.1023/A:1008724007888
Huang Y, Yang Y, Sun Y, Zhou H, Chen K (2020) Identification of apple varieties using a multichannel hyperspectral imaging system. Sensors 20(18):5120. https://doi.org/10.3390/s20185120
Jacques MM, Gumiere SJ, Gallichand J, Celicourt P, Gumiere T (2020) Impacts of water stress severity and duration on potato photosynthetic activity and yields. Front Agron 2:590312. https://doi.org/10.3389/fagro.2020.590312
Jeong J, Ok H, Hur O, Kim C (2008) Prediction of sprouting capacity using near-infrared spectroscopy in potato tubers. Am J Potato Res. 85:309–314. https://doi.org/10.1007/s12230-008-9010-x
Kortum G (2012) Reflectance spectroscopy. Principles, methods, applications. Springer Berlin, Heidelberg. https://doi.org/10.1007/978-3-642-88071-1
Liu N, Zhao R, Qiao L, Zhang Y, Li M, Sun H, Xing Z, Wang X (2020) Growth stages classification of potato crop based on analysis of spectral response and variables optimization. Sensors 20(14):3995. https://doi.org/10.3390/s20143995
López A, Arazuri S, García I, Mangado J, Jarén C (2013) A review of the application of near-infrared spectroscopy for the analysis of potatoes. J Agric Food Chem 61(23):5413–5424. https://doi.org/10.1021/jf401292j
Lu B, Dao PD, Liu J, He Y, Shang J (2020) Recent advances of hyperspectral imaging technology and applications in agriculture. Remote Sens 12(16):2659. https://doi.org/10.3390/rs12162659
Marín-Ortiz JC, Gutierrez-Toro N, Botero-Fernández V, Hoyos-Carvajal LM (2020) Linking physiological parameters with visible/near-infrared leaf reflectance in incubation period of vascular wilt disease. Saudi J Biol Sci 27(1):88–99. https://doi.org/10.1016/j.sjbs.2019.05.007
Meier U (2018) Growth stages of mono- and dicotyledonous plants. Electronic version published jointly by BBA, BSA, IGZ, IVA, AgrEvo, BASF, Bayer and Novartis. https://doi.org/10.5073/20180906-074619
Panda D, Mandal L, Barik J, Barik J, Mishra SS, Padhan B (2019) Improvement of growth, photosynthesis and antioxidant defense in rice (Oryza sativa L.) grown in fly ash-amended soil. Proc Natl Acad Sci 89:853–860. https://doi.org/10.1007/s40011-18-0996-7
Peñuelas J, Filella I (1998) Visible and near-infrared reflectance techniques for diagnosing plant physiological status. Trends Plant Sci 3(4):151–156. https://doi.org/10.1016/s1360-1385(98)01213-8
Seminario-Cunva JF, Villanueva-Guevara R, Valdez-Yopla MH (2018) Rendimiento de cultivares de papa (Solanum tuberosum L.) amarillos precoces del grupo Phureja. Agron Mesoamericana 29(3):639–653. https://doi.org/10.15517/ma.v29i3.32623
Singh SP, Rawal S, Dua VK, Roy S, Sharma SK (2019) Evaluation of post emergence herbicide bentazon in potato crop. Int J Chem Stud 7(3):2816-2820. https://www.chemijournal.com/archives/2019/vol7issue3/PartAS/7-3-179-286.pdf
Thongam B, Kadam AS, Singh AA, Singh YH (2017) Influence of planting dates on growth and yield of potato (Solanum tuberosum L.). J Pharmacogn Phytochem 6(6): 1243-1246. https://www.phytojournal.com/archives/2017/vol6issue6/PartR/6-6-37-819.pdf
Urbanowicz RJ, Meeker M, La Cava W, Olson RS, Moore JH (2018) Relief-based feature selection: introduction and review. J Biomed Inform 85(189):203. https://doi.org/10.1016/j.jbi.2018.07.014
Vilvert E, Stridh L, Andersson B, Olson Å, Aldén L, Berlin A (2022) Evidence based disease control methods in potato production: a systematic map protocol. Environ Evid 11:6. https://doi.org/10.1186/s13750-022-00259-x
Wetterlind J, Stenberg B, Rossel RA (2013) Soil analysis using visible and near infrared spectroscopy. Methods Mol Biol 953:95–107. https://doi.org/10.1007/978-1-62703-152-3_6
Zahir SADM, Omar AF, Jamlos MF, Azmi MAM, Muncan J (2022) A review of visible and near-infrared (Vis-NIR) spectroscopy application in plant stress detection. Sensors Actuators A Phys 338:113468. https://doi.org/10.1016/j.sna.2022.113468
Zhao R, An L, Song D, Li M, Qiao L, Liu N, Sun H (2021) Detection of chlorophyll fluorescence parameters of potato leaves based on continuous wavelet transform and spectral analysis. Spectrochim Acta Part A: Mol Biomol Spectrosc 259:119768. https://doi.org/10.1016/j.saa.2021.119768
Zhou Z, Zeng S, Li X, Zheng J (2015) Nondestructive detection of blackheart in potato by visible/near infrared transmittance spectroscopy. J Spectrosc 2015:1–9. https://doi.org/10.1155/2015/786709
Zubler AV, Yoon JY (2020) Proximal methods for plant stress detection using optical sensors and machine learning. Biosensors 10(12):193. https://doi.org/10.3390/bios10120193
Acknowledgements
The authors acknowledge the funding provided by the “Universidad Nacional de Colombia” under the “Convocatoria de Estancias Posdoctorales en la Universidad Nacional de Colombia sede Medellín 2021.”
Funding
Open Access funding provided by Colombia Consortium
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection, analysis and first draft of the manuscript were performed by Juan Carlos Marín-Ortiz. All authors commented on previous versions of the manuscript. The management of the project and writing of the manuscript was carried out by Carlos Felipe Barrera-Sánchez. The critical review for important intellectual content was carried out by Oscar De Jesús Córdoba-Gaona, Lilliana María Hoyos-Carvajal and Verónica Botero-Fernández. All authors approved the version to be published.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Marín-Ortiz, J.C., Hoyos-Carvajal, L.M., Botero-Fernández, V. et al. Characterizing Diploid and Tetraploid Potato Cultivars with Reflectance Spectroscopy. Potato Res. (2024). https://doi.org/10.1007/s11540-023-09674-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11540-023-09674-0