Abstract
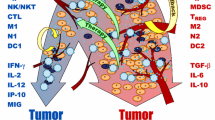
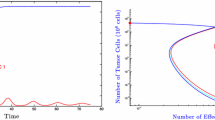
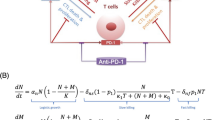
Cellular exhaustion in various immune cells develops in response to prolonged stimulation and overactivation during chronic infections and in cancer. Marked by an upregulation of inhibitory receptors and diminished effector functions, exhausted immune cells are unable to fully eradicate the antigen responsible for the overexposure. In cancer settings, this results in a relatively small but constant tumor burden known as a localized tumor-immune stalemate. In recent years, studies have elucidated key aspects of the development and progression of cellular exhaustion and have re-addressed previous misconceptions. Biological publications have also provided insight into the functional capabilities of exhausted cells. Complementing these findings, the model presented here serves as a mathematical framework for the establishment of cellular exhaustion and the development of the localized stalemate against a solid tumor. Analysis of this model indicates that this stalemate is stable and can handle small perturbations. Additionally, model analysis also provides insight into potential targets of future immunotherapy efforts.



Similar content being viewed by others
Data Availibility
All data used in this paper was published in Miller et al. (2019). No new data was generated.
References
Akondy RS, Fitch M, Edupuganti S, Yang S, Kissick H, Li K, Youngblood B, Abdelsamed H, McGuire D, Cohen K, Alexe G, Nagar S, McCausland M, Gupta S, Tata P, Haining W, McElrath M, Zhang D, Hu B, Greenleaf W, Goronzy J, Mulligan M, Hellerstein M, Ahmed R (2017) Origin and differentiation of human memory cd8 t cells after vaccination. Nature 552:362–367
Alfei F, Kanev K, Hofmann M, Wu M, Ghoneim H, Roelli P, Utzschneider D, von Hoesslin M, Cullen J, Fan Y, Eisenberg V, Wohlleber D, Steiger K, Merkler D, Delorenzi M, Knolle P, Cohen C, Thimme R, Youngblood B, Zehn D (2019) Tox reinforces the phenotype and longevity of exhausted t cells in chronic viral infection. Nature 571:265–269
Altan-Bonnet G, Mora T, Walczak A (2020) Quantitative immunology for physicists. Phys Rep 849:1–83
Baral S, Raja R, Sen P, Dixit N (2019) Towards multiscale modeling of the cd8+ t cell response to viral infections. Wiley Interdiscip Rev Syst Biol Med 11:e1446
Beltra J, Manne S, Abdel-Hakeem M, Kurachi M, Giles J, Chen Z, Casella V, Ngiow S, Khan O, Huang Y, Yan P, Nzingha K, Xu W, Amaravadi R, Xu X, Karakousis G, Mitchell T, Schuchter L, Huange A, Wherry E (2020) Developmental relationships of four exhausted cd8+ t cell subsets reveals underlying transcriptional and epigenetic landscape control mechanisms. Immunity 52:825–841
Boer RD, Homann D, Perelson A (2003) Different dynamics of cd4+ and cd8+ t cell responses during and after acute lymphocytic choriomeningitis virus infection. J Immunol 171:3928–3935
Catakovic K, Klieser E, Neureiter D, Geisberger R (2017) T cell exhaustion: from pathophysiological basics to tumor immunotherapy. Cell Commun Signal 15:1
Dolina J, Braeckel-Budimir NV, Thomas G, Salek-Ardakani S (2021) Cd8+ t cell exhaustion in cancer. Front Immunol 12:715234
D’Souza W, Lefrancois L (2004) An in-depth evaluation of the production of il-2 by antigen-specific cd8 t cells in vivo. Eur J Immunol 34:2977–2985
Eftimie R, Bramson J, Earn D (2011) Interactions between the immune system and cancer: a brief review of non-spatial mathematical models. Bull Math Biol 73:2–32
Eftimie R, Gillard J, Cantrell D (2016) Mathematical models for immunology: current state of the art and future research directions. Bull Math Biol 78:2091–2134
Gadhamsetty S, Maree A, Beltman J, de Boer R (2014) A general functional response of cytotoxic t lymphocyte-mediated killing of target cells. Biophys J 106:178–1791
Gadhamsetty S, Maree A, Beltman J, de Boer R (2017) A sigmoid functional response emerges when cytotoxic t lymphocytes start killing fresh target cells. Biophys J 112:1221–1235
Gaffen S, Liu K (2004) Overview of interleukin-2 function, production and clinical applications. Cytokine 28:109–123
Hanna B, Llao-Cid L, Iskar M, Roessner P, Klett L, Wong J, Paul Y, Ioannou N, Ozturk S, Mack N, Kalter V, Colomer D, Campo E, Bloehdorn J, Stilgenbauer S, Dietrich S, Schmidt M, Gabriel R, Rippe K, Feuerer M, Ramsay A, Lichter P, Zapatka M, Seiffert M (2021) Interleukin-10 receptor signaling promotes the maintenance of a pd-1int tcf-1+ cd8+ t cell population that sustains anti-tumor immunity. Immunity 54:2825–2841
Hoves S, Trapani J, Voskoboinik I (2009) The battlefield of perforin/granzyme cell death pathways. J Leukoc Biol 87:237–243
Hudson W, Gensheimer J, Hashimoto M, Wieland A, Valanparambil R, Li P, Lin J, Konieczny B, Im S, Freeman G, Leonard W, Kissick H, Ahmed R (2019) Proliferating transitory t cells with an effector-like transcriptional signature emerge from pd-1+ stem-like cd8+ t cells during chronic infection. Immunity 51:1043–1058
Im S, Hashimoto M, Gerner M, Lee J, Kissick H, Burger M, Shan Q, Hale J, Lee J, Nasti T, Sharpe A, Freeman G, Germain R, Nakaya H, Xue H, Ahmed R (2016) Defining cd8+ t cells that provide the proliferative burst after pd-1 therapy. Nature 537:417–421
Jiang Y, Li Y, Zhu B (2015) T-cell exhaustion in the tumor microenvironment. Cell Death Dis 6:e1792–e1792
Jiang W, He Y, He W, Wu G, Zhou X, Sheng Q, Zhong W, Lu Y, Ding Y, Lu Q, Ye F, Hua H (2021) Exhausted cd8+ t cells in the tumor immune microenvironment: new pathways to therapy. Front Immunol 11:622509
Kaech S, Ahmed R (2001) Memory cd8+ t cell differentiation: initial antigen encounter triggers a developmental program in naïve cells. Nat Immunol 2:415–422
Khan O, Giles J, McDonald S, Manne S, Ngiow S, Patel K, Werner M, Huang A, Alexander K, Wu J, Attanasio J, Yan P, George S, Bengsch B, Staupe R, Donahue G, Xu W, Amaravadi R, Xu X, Karakousis G, Mitchell T, Schuchter L, Kaye J, Berger S, Wherry E (2019) Tox transcriptionally and epigenetically programs cd8+ t cell exhaustion. Nature 571:211–218
Kim P, Lee P, Levy D (2010) Emergent group dynamics governed by regulatory cells produce a robust primary t cell response. Bull Math Biol 72:611–644
Kim P, Lee P, Levy D (2011) A theory of immunodominance and adaptive regulation. Bull Math Biol 73:1645–1665
Kim P, Lee P, Levy D (2013) Basic principles in modeling adaptive regulation and immunodominance. In: Mathematical Methods and Models in Biomedicine. Springer
Liang C, Huang S, Zhao Y, Chen S, Li Y (2021) Tox as a potential target for immunotherapy in lymphocytic malignancies. Biomark Res 9:1–8
McLane L, Abdel-Hakeem M, Wherry E (2019) Cd8 t cell exhaustion during chronic viral infection and cancer. Annu Rev Immunol 37:457–495
Miller B, Sen D, Abosy RA, Bi K, Virkud Y, LaFleur M, Yates K, Lako A, Felt K, Naik G, Manos M, Gjini E, Kuchroo J, Ishizuka J, Collier J, Griffin G, Maleri S, Comstock D, Weiss S, Brown F, Zimmer APM, Manguso R, Hodi F, Rodig S, Sharpe A, Haining W (2019) Subsets of exhausted cd8+ t cells differentially mediate tumor control and respond to checkpoint blockade. Nat Immunol 20:326–336
Milzman J, Sheng W, Levy D (2021) Modeling lsd1-mediated tumor stagnation. Bull Math Biol 83:1–29
Mohri H, Perelson A, Tung K, Ribeiro R, Ramratnam B, Markowitz M, Kost R, Hurley A, Weinberger L, Cesar D, Hellerstein M, Ho D (2001) Increased turnover of t lymphocytes in hiv-1 infection and its reduction by antiretroviral therapy. J Exp Med 194:1277–1287
Nguyen L, Ohashi P (2015) Clinical blockade of pd1 and lag3-potential mechanisms of action. Nat Rev Immunol 15:45–56
Pardoll D (2012) The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer 12:252–264
Pauken K, Wherry E (2015) Overcoming t cell exhaustion in infection and cancer. Trends Immunol 36:365–376
Philip M, Schietinger A (2022) Cd8+ t cell differentiation and dysfunction in cancer. Nat Rev Immunol 22:209–223
Sears J, Waldron K, Wei J, Chang C (2021) Targeting metabolism to reverse t-cell exhaustion in chronic viral infections. Immunology 162:135–144
Seo W, Jerin C, Nishikawa H (2021) Transcriptional regulatory network for the establishment of cd8+ t cell exhaustion. Exp Mol Med 53:202–209
Smith-Garvin J, Koretzky G, Jordan M (2009) T cell activation. Annu Rev Immunol 27:591–619
Song G, Tian T, Zhang X (2020) A mathematical model of cell-mediated immune response to tumor. Math Biosci Eng 18:373–385
Sprent J (2005) Direct stimulation of naïve t cells by antigen-presenting cell vesicles. Blood Cells Mol Dis 35:17–20
Voskoboinik I, Whisstock J, Trapani J (2015) Perforin and granzymes: function, dysfunction and human pathology. Nat Rev Immunol 15:388–400
Wherry E (2011) T cell exhaustion. Nat Immunol 12:492–499
Wherry E, Kurachi M (2015) Molecular and cellular insights into t cell exhaustion. Nat Rev Immunol 15:486–499
Wherry E, Ha S, Kaech S, Haining W, Sarkar S, Kalia V, Subramaniam S, Blattman J, Barber D, Ahmed R (2007) Molecular signature of cd8+ t cell exhaustion during chronic viral infection. Immunity 27:670–684
Wilson S, Levy D (2013) Functional switching and stability of regulatory t cells. Bull Math Biol 75:1891–1911
Wing J, Tanaka A, Sakaguchi S (2019) Human foxp3+ regulatory t cell heterogeneity and function in autoimmunity and cancer. Immunity 50:302–316
Wodarz D (2005) Mathematical models of immune effector responses to viral infections: virus control versus the development of pathology. Comput Appl Math 184:301–319
You R, Artichoker J, Fries A, Edwards A, Combes A, Reeder G, Samad B, Krumel M (2021) Active surveillance characterizes human intratumoral t cell exhaustion. J Clin Invest 131
Zhang Z, Liu S, Zhang B, Qiao L, Zhang Y, Zhang Y (2020) T cell dysfunction and exhaustion in cancer. Front Cell Dev Biol 8:17
Acknowledgements
We thank Dr. Brian Miller and Dr. Debattama Sen and co-authors of Miller et al. (2019) for providing access to their published data. The research of D. Levy was partially funded by the Simons Foundation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical Appoval
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Simmons, T., Levy, D. Modeling the Development of Cellular Exhaustion and Tumor-Immune Stalemate. Bull Math Biol 85, 106 (2023). https://doi.org/10.1007/s11538-023-01207-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11538-023-01207-7