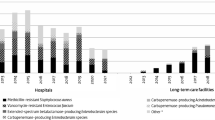
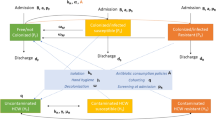
Abstract
Methicillin-resistant Staphylococcus aureus (MRSA) and third-generation cephalosporin-resistant Escherichia coli (3GCREc) are community and hospital-associated pathogens causing serious infections among populations by infiltrating into hospitals and surrounding environment. These main multi-drug resistant or antimicrobial resistance (AMR) bacterial pathogens are threats to human health if not properly tackled and controlled. Tackling antimicrobial resistance (AMR) is one of the issues for the World Health Organization (WHO) to design a comprehensive set of interventions which also helps to achieve the end results of the developing indicators proposed by the same organization. A deterministic mathematical model is developed and studied to investigate the impact of the WHO policy on integrated antimicrobial stewardship activities to use effective protection measures to control the spread of AMR diseases such as MRSA and 3GCREc in hospital settings by incorporating the contribution of the healthcare workers in a hospital and the environment in the transmission dynamics of the diseases. The model also takes into account the parameters describing various intervention measures and is used to quantify their contribution in containing the diseases. The impact of combinations of various possible control measures on the overall dynamics of the disease under study is investigated. The model analysis suggests that the contribution of the interventions: screening and isolating the newly admitted patients, improving the hygiene in hospital settings, decolonizing the pathogen carriers, and increasing the frequency of disinfecting the hospital environment are effective tools to contain the disease from invading the population. The study revealed that without any intervention, the diseases will continue to be a major cause of morbidity and mortality in the affected communities. In addition, the study indicates that a coordinated implementation of the integrated control measures suggested by WHO is more effective in curtailing the spread of the diseases than piecemeal strategies. Numerical experiments are provided to support the theoretical analysis.












Similar content being viewed by others
Notes
Out of the total 11.85% reduction, 9.3% is the reduction in prevalence of the CA strain.
\( 81 \simeq [0.093\times 0.033 + 0.0255\times 0.2]\times 10,000\))
This amounts to saving nearly 132 (\(\simeq [0.1858\times 0.033+0.0355\times 0.2]\times 10,000\)) lives per 10,000 humans per day.
References
Ayscue P, Lanzas C, Ivanek R et al (2009) Modeling on-farm E. coli O157:H7 population dynamics. Foodborne Pathog Dis 6:461–470. https://doi.org/10.1089/fpd.2008.0235
Bani-Yaghoub M, Gautam R, Dopfer D et al (2012) Effectiveness of environmental decontamination in control of infectious diseases. Epidemiol Infect 140(3):542–553. https://doi.org/10.1017/S0950268811000604
Berge T, Lubuma JMS, Moremedi GM et al (2016) A simple mathematical model for Ebola in Africa. J Biol Dyn 11(1):42–74. https://doi.org/10.1080/17513758.2016.1229817
Berge T, Chapwanya M, Lubuma JMS et al (2018) A mathematical model for Ebola epidemic with self-protection measures. J Biol Dyn 26(1):107–131. https://doi.org/10.1142/S0218339018500067
Bloomfield SF, Cookson B, Falkiner F et al (2007) Methicillin-resistant Staphylococcus aureus, Clostridium difficile, and extended-spectrum beta-lactamase-producing Escherichia coli in the community: assessing the problem and controlling the spread. Am J Infect Control 35(2):86–8. https://doi.org/10.1016/j.ajic.2006.10.003
Blower SM, Dowlatabadi H (1994) Sensitivity and uncertainty analysis of complex models of disease transmission: an HIV model as an example. Int Stat Rev 62(2):229–243. https://doi.org/10.2307/1403510
Boyce JM (2007) Environmental contamination makes an important contribution to hospital infection. J Hosp Infect 65:50–54. https://doi.org/10.1016/S0195-6701(07)60015-2
Boyce JM, Potter-Bynoe G, Chenevert C et al (1997) Environmental contamination due to methicillin-resistant Staphylococcusaureus: possible infection control implications. Infect Control Hosp Epidemiol 18(9):622–627. https://doi.org/10.2307/30141488
Browne C, Webb GF (2015) A nosocomial epidemic model with infection of patients due to contaminated rooms. Discrete Contin Dyn Syst Ser 12:761–787. https://doi.org/10.3934/mbe.2015.12.761
Busenberg S, Cooke K (1993) Vertically transmitted disease: models and dynamics, vol 23. Springer, Berlin
CDC (2003) Methicillin-resistant Staphylococcus aureus infections among competitive sports participants-Colorado, Indiana, Pennsylvania, and Los Angeles county, 2000–2003. MMWR Morb Mortal Wkly Rep 52(33):793–795
Chamchod F, Ruan S (2012) Modeling methicillin-resistant Staphylococcus aureus in hospitals: transmission dynamics, antibiotic usage and its history. Theor Biol Med Model 9:25. https://doi.org/10.1186/1742-4682-9-25
Clancy D (2005) A stochastic sis infection model incorporating indirect transmission. J Appl Probab 42(3):725–737. https://doi.org/10.1239/jap/1127322023
Cooper BS, Medley GF, Stone SP, et al (2004) Methicillin-resistant Staphylococcus aureus in hospitals and the community: stealth dynamics and control catastrophes. Proc Natl Acad Sci USA 101(27):10,223–10,228. https://doi.org/10.1073/pnas.0401324101
D’Agata EMC, Webb GF, Horn MA et al (2009) Modelling the invasion of community-acquired methicillin-resistant Staphylococcus aureus into hospitals. Clin Infect Dis 48(3):274–284. https://doi.org/10.1086/595844
Davis SL, Rybak MJ, Amjad M et al (2006) Characteristics of patients with healthcare-associated infection due of SCCmec type IV methcillin-resistant Staphylococcus aureus. Infect Control Hosp Epidemiol 27(3):1025–1031. https://doi.org/10.1086/507918
Delaney JAC, Schneider-Lindner V, Brassard P, et al (2008) Mortality after infection with methicillin-resistant Staphylococcus aureus (MRSA) diagnosed in the community. BMC Med 6(2). https://doi.org/10.1186/1741-7015-6-2
Di Ruscio F, Guzzetta G, Bjørnholt JV, et al (2009) Quantifying the transmission dynamics of MRSA in the community and healthcare settings in a low-prevalence country. Proc Natl Acad Sci USA 116(29):14,599–14,605. https://doi.org/10.1073/pnas.1900959116
Diekmann O, Heesterbeek J (2000) Mathematical epidemiology of infectious diseases: model building. Analysis and interpretation. Wiley, Baffin lane
Ding W, Webb GF (2017) Optimal control applied to community-acquired methicillin-resistant Staphylococcus aureus in hospitals. J Biol Dyn 11:65–78. https://doi.org/10.1080/17513758.2016.1151564
Donkor ES, Codjoe FS (2019) Methicillin resistant Staphylococcus aureus and extended spectrum Betstlactamase producing enterobacteriaceae: a therapeutic challenge in the 21th century. Open Microbiol J 13:94–100. https://doi.org/10.2174/1874285801913010094
Döpfer D, Geue L, Schares S et al (2012) Dynamics of shiga-toxin producing E. coli (STEC) and their virulence factors in cattle. Prev Vet Med 103(1):22–30. https://doi.org/10.1016/j.prevetmed.2011.08.007
Endimiani A, Rossano A, Kunz D et al (2012) First countrywide survey of third-generation cephalosporin-resistant Escherichia coli from broilers, swine, and cattle in Switzerland. Diagn Microbiol Infect Dis 73(1):31–38. https://doi.org/10.1016/j.diagmicrobio.2012.01.004
European Centre for Disease Prevention and Control (ECDC) (2020) Antimicrobial resistance in the EU/EEA (ears-net)-annual epidemiological report for 2019. Technical report, ECDC, Stockholm
Grundmann H, Aires-de-Sousa M, Boyce J et al (2006) Emergence and resurgence of meticillin-resistant Staphylococcus aureus as a public-health threat. Lancet 368(9538):874–885. https://doi.org/10.1016/S0140-6736(06)68853-3
Hall IM, Barrass I, Leach S et al (2012) Transmission dynamics of methicillin-resistant Staphylococcus aureus in a medical intensive care unit. J R Soc Interface 9(75):2639–2652. https://doi.org/10.1098/rsif.2012.0134
Herold B, Immergluck LC, Maranan MC et al (1998) Community-acquired methicillin-resistant Staphylococcus aureus in children with no identified predisposing risk. JAMA 279(8):593–598. https://doi.org/10.1001/jama.279.8.593
Hoare A, Regan DG, Wilson DP (2008) Sampling and statistical analyses tools SaSAT for computational modelling. Math Biosci 5(8):593–598. https://doi.org/10.1186/1742-4682-5-4
Jarvis WR, Schlosser J, Chinn RY et al (2006) National prevalence of methicillin-resistant Staphylococcus aureus in inpatients at US health care facilities. Am J Infect Control 35(10):631–637. https://doi.org/10.1016/j.ajic.2007.10.009
Jevons MP (1961) “Celbenin’’-resistant staphylococci. J Br Med 1:124–125
Kamgang JC, Sallet G (2008) Computation of threshold conditions for epidemiological models and global stability of the disease-free equilibrium (DFE). Math Biosci 213(1):1–12. https://doi.org/10.1016/j.mbs.2008.02.005
Kassa S, Njagarah JBH, Terefe YA (2020) Analysis of the mitigation strategies for COVID-19: from mathematical modelling perspective. Chaos Solitons Fractals 138:109968. https://doi.org/10.1016/j.chaos.2020.109968
Kassa SM, Njagarah JBH, Terefe YA (2021) Modelling COVID-19 mitigation and control strategies in the presence of migration and vaccination: the case of South Africa. Afrika Matematika. 32:1295–1322. https://doi.org/10.1007/s13370-021-00900-x
Klein E, Smith DL, Laxminarayan R (2007) Hospitalisations and deaths caused by methicillinresistant Staphylococcus aureus, USA, 1999–2005. Emerg Infect Dis 13(12):1840–1846. https://doi.org/10.3201/eid1312.070629
Klevens RM, Morrison MA, Nadle J et al (2007) Invasive methicillin-resistant Staphylococcus aureus infections in the USA. JAMA 298(1):1763–71. https://doi.org/10.1001/jama.298.15.1763
Kluytmans-VandenBergh MFQ, Kluytmans JAW (2006) Community-acquired methicillin-resistant Staphylococcus aureus: current perspectives. Clin Microbiol Infect 12(Suppl 1):9–15. https://doi.org/10.1111/j.1469-0691.2006.01341.x
Knight G, Davies N, Colijn C et al (2019) Mathematical modelling for antibiotic resistance control policy: do we know enough? BMC Infect Dis 19:1011. https://doi.org/10.1186/s12879-019-4630-y
Lakhundi S, Zhang K (2018) Methicillin-resistant Staphylococcus aureus: molecular characterization, evolution, and epidemiology. Clin Microbiol Rev 31(4):e00,020–18. https://doi.org/10.1128/CMR.00020-18
Levin BR (2001) Minimizing potential resistance: a population dynamics view. Clin Infect Dis 33(Suppl 3):S161–S169. https://doi.org/10.1086/321843
Liu WC, Jenkins C, Shaw DJ et al (2005) Modelling the epidemiology of verocytotoxin-producing E. coli serogroups in young calves. Epidemiol Infect 133(3):449–458. https://doi.org/10.1017/S09502688040036
Liu WC, Shaw DJ, Matthews L et al (2007) Modelling the epidemiology and transmission of verocytotoxin- producing E. coli serogroups O26 and O103 in two different calf cohorts. Epidemiol Infect 135(8):1316–1323. https://doi.org/10.1017/S0950268806007722
Marino S, Hogue IB, Ray CJ et al (2008) A methodology for performing global uncertainty and sensitivity analysis in systems biology. J Theor Biol 254(1):178–196. https://doi.org/10.1016/j.jtbi.2008.04.011
McBryde ES, McElwain DL (2006) A mathematical model investigating the impact of an environmental reservoir on the prevalence and control of vancomycin-resistant enterococci. J Infect Dis 193(10):1473–1474. https://doi.org/10.1086/503439
Niewiadomska A, Jayabalasingham B, Seidman JC et al (2019) Population-level mathematical modeling of antimicrobial resistance: a systematic review. BMC Med 81:17. https://doi.org/10.1186/s12916-019-1314-9
Okuma K, Iwakawa K, Turnidge JD et al (2002) Dissemination of new methicillin-resistant Staphylococcus aureus clones in the community. J Clin Microbiol 40(11):4289–94. https://doi.org/10.1128/JCM.40.11.4289-4294.2002
Otter J, French G (2006) Nosocomial transmission of community-associated methicillin-resistant Staphylococcus aureus: an emerging threat. Lancet Infect Dis 6(12):753–755. https://doi.org/10.1016/S1473-3099(06)70636-3
Peng H, Liu D, Ma Y et al (2018) Comparison of community- and healthcare-associated methicillin-resistant Staphylococcus aureus isolates at a chinese tertiary hospital, 2012–2017. Sci Rep 8(17):916. https://doi.org/10.1038/s41598-018-36206-5
Popovich KJ, Weinstein RA, Hota B (2008) Are community-associated methicillin-resistant staphylococcus aureus (mrsa) strains replacing traditional nosocomial mrsa strains? Clin Infect Dis 46:787–794. https://doi.org/10.1086/52871
Robotham JV, Scarff CA, Jenkins DR et al (2006) Methicillin-resistant Staphylococcus aureus (MRSA) in hospitals and the community: model predictions based on the UK situation. J Hosp Infect 65(S2):93–99. https://doi.org/10.1016/S0195-6701(07)60023-1
Rosenthal VD, Maki DG, Jamulitrat S et al (2010) International nosocomial infection control consortium (INICC) report, data summary for 2003–2008, issued June 2009. J Hosp Infect 38(2):95–104. https://doi.org/10.1016/j.ajic.2009.12.004
Saiman L, Keefe MO, Graham PLI, et al (2003) Hospital transmission of community-acquired methicillin-resistant Staphylococcus aureus among postpartum women. J Hosp Infect 37(10, 15):1313–1319. https://doi.org/10.1086/379022
Scott EA, Bloomfield SF (1990) The survival and transfer of microbial contamination via cloths, hands and utensils. J Appl Bacteriol 68(3):271–278. https://doi.org/10.1111/j.1365-2672.1990.tb02574.x
Stuart AM, Humphries AR (1998) Dynamical systems and numerical analysis. Cambridge University Press, Cambridge
Terefe Y, Kassa SM (2020) A sex-structured model for the transmission of trichomoniasis with possible reinfection. Int J Biomath 13(7):2050,062. https://doi.org/10.1142/S179352452050062X
Terefe YA (2020) A sex-structured model for the transmission of trichomoniasis with possible reinfection. Math Popul Stud 48(2):81–103. https://doi.org/10.1080/08898480.2020.1767416
Tsuji BT, Rybak MJ, Cheung CM et al (2007) Community-and health care-associated methicillin-resistant staphylococcus aureus: a comparison of molecular epidemiology and antimicrobial activities of various agents. Diagn Micr Infec Dis 58(1):41–47. https://doi.org/10.1016/j.diagmicrobio.2006.10.021
Tsuzuki S, Matsunaga N, Yahara K et al (2021) Disease burden of bloodstream infections caused by antimicrobial-resistant bacteria: a population-level study, Japan, 2015–2018. Int J Infect Dis 108:119–124. https://doi.org/10.1016/j.ijid.2021.05.018
Turner J, Begon M, Bowers RG et al (2003a) A model appropriate to the transmission of a human food-borne pathogen in a multi-group managed herd. Int J Infect Dis 57(4):175–198. https://doi.org/10.1016/s0167-5877(03)00006-0
Turner J, Bowers RG, Begon M et al (2003b) A semi-stochastic model of the transmission of E. coli O157 in a typical UK dairy herd: dynamics, sensitivity analysis and intervention/prevention strategies. J Theor Biol 241(4):806–822. https://doi.org/10.1016/j.jtbi.2006.01.020
Turner J, Bowers RG, Clancy D, et al (2003c) A network model of E.coli O157 transmission within a typical UK dairy herd: the effect of heterogeneity and clustering on the prevalence of infection. J Theor Biol 254(1):45–54. https://doi.org/10.1016/j.jtbi.2008.05.007
van den Driesche P, Watmough J (2002) Reproduction numbers and sub-threshold endemic equilibria for the compartmental models of disease transmission. Math Biosci 180(1–2):29–48. https://doi.org/10.1016/S0025-5564(02)00108-6
Wang L (2012) Modelling environmental contamination of antibiotic-resistant bacteria in hospitals. PhD thesis, University of Miami, Coral Gables, Florida
Wang S, Ruan S (2017) Modelling nosocomial infections of methicillin-resistant Staphylococcus aureus with environment contamination. Sci Rep 580:7. https://doi.org/10.1038/s41598-017-00261-1
Wang X, Xiao Y, Wang J et al (2011) A mathematical model of effects of environmental contamination and presence of volunteers on hospital infections in China. J Theoret Biol 293:161–173. https://doi.org/10.1016/j.jtbi.2011.10.009
Wang X, Gautam R, Pinedo PJ et al (2013) A stochastic model for transmission, extinction and outbreak of E. coli O157:H7 in cattle as affected by ambient temperature and cleaning practices. J Math Biol 69(2):161–173. https://doi.org/10.1007/s00285-013-0707-1
WHO (2021) Policy guidance on integrated antimicrobial stewardship activities. https://www.who.int/publications-detail-redirect/9789240025530
Wood JC, McKendrick IJ, Gettinby G (2007) A simulation model to assess herd-level intervention strategies against E. coli O157. Epidemiol Infect 135(5):749–764. https://doi.org/10.1017/S0950268806007436
Woodford N, Ward ME, Kaufmann ME et al (2004) Community and hospital spread of Escherichia coli producing CTX-M extended- spectrum \(\beta \)-lactamases in the UK. J Antimicrob Chemother 54(4):735–743. https://doi.org/10.1093/jac/dkh424
Zetola N, Francis JS, Nuermberger EL et al (2005) Community-acquired meticillin-resistant Staphylococcus aureus: an emerging threat. Lancet Infect Dis 5(5):275–286. https://doi.org/10.1016/S1473-3099(05)70112-2
Zhang XS, Chase-Topping ME, McKendrick IJ et al (2010) Spread of E. coli O157:H7 infection among Scottish cattle farms: stochastic models and model selection. Epidemics 2(1):11–20. https://doi.org/10.1016/j.epidem.2010.02.001
Acknowledgements
The authors are grateful to Dr Haileyesus Getahun from the WHO for his personal advice and valuable discussion to prepare this manuscript. The authors are also grateful to the anonymous reviewers and the Editor-in-Chief for their constructive suggestions and comments to improve the manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Appendices
Appendix A: Biologically Feasible Region
Theorem A.1
Model (10) is a dynamical system on the biologically feasible region
where \(M=\frac{1}{\nu }\left( (\xi _1+\xi _2)\frac{\Lambda _1}{\gamma _P}+\xi _3\frac{\Lambda _2}{\mu }\right) \).
Proof
We want to show that for non-negative initial data (4), system (10) possesses a unique non-negative solution in the region \(\Omega \) for all time \(t\ge 0\). The proof will follow two steps.
Following the approach in Busenberg and Cooke (1993), Terefe (2020) and Kassa et al. (2021), it can be shown that any solution of (10) corresponding to non-negative initial conditions is non-negative.
In a second step, we show that any solution of model system (10) satisfies some a priori estimates. By adding the first five equations of (10), we obtain
where
By applying Gronwall inequality on (A2), we obtain
Hence, for \(0 \le N_{P0} \le \frac{\Lambda _1}{\mu _m}\), we get
for all \(t\ge 0\).
If \(N_P(t)> \frac{\Lambda _1}{\mu _m}\), then from (A3), we get
Thus
Likewise, by adding the last two equations of (10) related to the healthcare workers, we obtain
which leads into
Then, for \(0\le N_{H0}\le \frac{\Lambda _2}{\mu }\),
Finally, from the last equation of (10) which is related to the contaminated environment, we obtain
By applying Gronwall inequality, we have
Hence, from (A5), when \(0\le E_0\le \frac{M}{\nu }\), we infer that
Combining the above results and using Theorem 2.1.5 in Stuart and Humphries (1998), we conclude that (10) defines a dynamical system on \(\Omega \). \(\square \)
Appendix B: Computation of the Reproduction Number
By using the theory of next generation operator in Diekmann and Heesterbeek (2000); van den Driesche and Watmough (2002) and the notation in the references, the matrix F (for the new infection terms) with \(X=(P_{CCA},P_{ICA},P_{CHA},P_{IHA},H_{CW},E)\), is given by
The matrix for transition terms linearized at the disease-free equilibrium is given by
The inverse of matrix V can be obtained to be
where \(\Phi _{CIHA} = \frac{\tau _{CHA}\phi _{IHA}}{k_4k_5}\), \(\Phi _{CICA} = \frac{\tau _{CCA}\phi _{ICA}}{k_1k_2}\), \(\Phi _M = (1-\Phi _{CIHA}-\Phi _{CICA}+\Phi _{CIHA}\times \Phi _{CICA})\).
Then the reproduction number \(\mathcal {R}_0\) of the model is given as the spectral radius of the matrix \(FV^{-1}\).
Appendix C: Global Stability of the Disease-Free Equilibrium
Theorem C.1
The disease-free equilibrium \(E_0\) of model (10) given at (12) is globally asymptotically stable if \(\mathcal {R}_0 <1\) .
Proof
To prove the global asymptotic stability of \(E_0\) for \(\mathcal {R}_0<1\), we apply the method investigated in Kamgang and Sallet (2008). Let \(X=(X_1,X_2)\) with
denote the number of uninfected and infected individuals in system (10), respectively. Then the system in (10) can be written as
where \(X_1^*=\left( \frac{\Lambda _1}{\gamma _P},\frac{\Lambda _2}{\mu }\right) \),
\(A_1(X)=\left( \begin{array}{cc} -\gamma _P&{} 0 \\ 0 &{} -\mu \\ \end{array} \right) \),
and
The five sufficient conditions of Kamgang–Sallet theorem are checked as follows:
-
1.
System (10) is a dynamical system on \(\Omega \). This is proved in Theorem A.1.
-
2.
The equilibrium \(X_1^*\) is GAS for the subsystem \(\dot{X}_1=A_1(X_1,0)(X_1-X_1^*)\). More precisely, we obtain
$$\begin{aligned} \dot{X}_1= & {} \left( \begin{array}{c} \Lambda _1-\gamma _p P\\ \Lambda _2-\mu H_{UW}\\ \end{array} \right) , \text{ then }\; X_1 = \left( \begin{array}{c} \frac{\Lambda _1}{\gamma _P}+\left( P_0-\frac{\Lambda _1}{\gamma _P}\right) e^{(-\gamma _p t)}\\ \frac{\Lambda _2}{\mu }+\left( H_{UW0}-\frac{\Lambda _2}{\mu }\right) e^{(-\mu t)}\\ \end{array} \right) \rightarrow \left( \begin{array}{c} \frac{\Lambda _1}{\gamma _P}\\ \frac{\Lambda _2}{\mu }\\ \end{array} \right) \\= & {} X^*_1\;~\text{ as }\;~t\rightarrow \infty . \end{aligned}$$Hence, \(X^*_1\) is globally asymptotically stable.
-
3.
The matrix \(A_2(X)\) is Metzler (i.e., all the off-diagonal elements are non-negative) and irreducible for any given \(X\in \Omega \).
-
4.
There exists an upper-bound matrix \(\bar{A}_2\) for the set
$$\begin{aligned} \mathcal {M}=\left\{ A_2(X):X\in \Omega \right\} . \end{aligned}$$Indeed, the upper bound of \(\mathcal {M}\) is given by \(\bar{A}_2=\left( \begin{array}{cccccc} \beta _{CA} \sigma _1 -k_1&{} \beta _{CA} +\phi _{ICA}&{}0&{}0&{} \frac{\beta _{CA}\epsilon _1 \Lambda _1}{\gamma _P}&{}\frac{\beta _e \Lambda _1}{\gamma _P K}\\ \tau _{CCA}&{}-k_2 &{} 0&{}0&{}0&{}0 \\ 0&{}0&{} \beta _{HA} \sigma _2 -k_3&{} \beta _{HA} +\phi _{ICA}&{} \frac{\beta _{HA}\epsilon _2 \Lambda _1}{\gamma _P}&{}\frac{\beta _e \Lambda _1}{\gamma _P K}\\ 0&{}0&{} \tau _{CHA}&{}-k_4&{} 0&{}0 \\ \frac{\beta _{CA}\sigma _1 \Lambda _2}{\mu }&{} \frac{\beta _{CA}\Lambda _2}{\mu }&{} \frac{\beta _{HA}\sigma _2 \Lambda _2}{\mu }&{} \frac{\beta _{HA}\Lambda _2}{\mu }&{}\frac{\beta _H \Lambda _2}{\mu }-k_5&{}\frac{\beta _e \Lambda _2}{\mu K} \\ 0&{}\xi _1&{}0&{}\xi _2&{}\xi _3&{}-\nu \\ \end{array} \right) .\)
-
5.
For \(\mathcal {R}_0<1\) in (14)
$$\begin{aligned} \alpha (\bar{A}_2)=\max \left\{ Re(\lambda ):\lambda \;~eigenvalue \;~of \bar{A}_2\right\} \le 0. \end{aligned}$$
Hence, by the Kamgang–Sallet global stability theorem in Kamgang and Sallet (2008), the disease-free equilibrium is globally asymptotically stable for \(\mathcal {R}_0<1\). \(\square \)
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Terefe, Y.A., Kassa, S.M. & Njagarah, J.B.H. Impact of the WHO Integrated Stewardship Policy on the Control of Methicillin-Resistant Staphyloccus aureus and Third-Generation Cephalosporin-Resistant Escherichia coli: Using a Mathematical Modeling Approach. Bull Math Biol 84, 97 (2022). https://doi.org/10.1007/s11538-022-01051-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11538-022-01051-1