Abstract
The COVID-19 pandemic has had a considerable impact on global health and economics. The impact in African countries has not been investigated thoroughly via fitting epidemic models to the reported COVID-19 deaths. We downloaded the data for the 12 most-affected countries with the highest cumulative COVID-19 deaths to estimate the time-varying basic reproductive number (\({R}_{0}(t)\)) and infection attack rate. We develop a simple epidemic model and fitted it to reported COVID-19 deaths in 12 African countries using iterated filtering and allowing a flexible transmission rate. We observe high heterogeneity in the case-fatality rate across the countries, which may be due to different reporting or testing efforts. South Africa, Tunisia, and Libya were most affected, exhibiting a relatively higher \({R}_{0}(t)\) and infection attack rate. Thus, to effectively control the spread of COVID-19 epidemics in Africa, there is a need to consider other mitigation strategies (such as improvements in socioeconomic well-being, healthcare systems, the water supply, and awareness campaigns).
Similar content being viewed by others
1 Introduction
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), a novel coronavirus that has caused the current coronavirus disease pandemic (COVID-19), has strongly affected all regions, including Africa. On February 14, 2020, the disease hit Africa when the first case was reported in Cairo, Egypt (Gilbert et al. 2020; Musa et al. 2020b; World Health Organization 2021a, b). Since then, the disease has spread across Africa, epi-centered in South Africa, and after a year (i.e., by March 25, 2021), over 7 million people have been infected, including 155,675 thousand related deaths in African region by December 27, 2021 (World Health Organization 2021a, b; World Health Organization 2021c). The disease, caused by SARS-CoV-2, emerged in China at the end of 2019 and has affected more than 282.8 million individuals and killed over 5.4 million people globally by December 27, 2021 (World Health Organization 2021a). COVID-19 shows a faster spreading ability than other coronaviruses of the same family (i.e., SARS-CoV and MERS-CoV) (Chowell et al. 2004; Gilbert et al. 2020; Hu et al. 2020; Li et al. 2020; Lin et al. 2018); it has also destroyed overstretched healthcare systems and weakened the socioeconomic sector worldwide.
In the early phase of the epidemic, the 12 most-affected African countries with COVID-19 deaths were classified into three clusters/groups based on their similarity in exposure risk that originated from three Chinese provinces, namely Guangdong, Fujian, and Beijing, which represented three different levels of risks of importation (high, medium, and relatively low) (Gilbert et al. 2020; Musa et al. 2021a). Each group corresponded to different Chinese airports in the associated province as the main sources of entry risk. Egypt, Algeria, and South Africa were identified as the highest importation risk groups with moderate to high magnitudes of high epidemic response, followed by Nigeria, Ethiopia, Sudan, Morocco, and Kenya, which were classified as moderate importation risk groups with variable magnitudes of response to epidemics with high vulnerability. The last group includes Tunisia, Cameroon, and Zambia, classified as having relatively low importation risk with variable magnitude to respond to epidemics with high vulnerability (Gilbert et al. 2020; World Health Organization 2021b).
With vaccines being developed recently, many are still in clinical trials, and some are already commercially used against COVID-19 infection (World Health Organization 2020). Nonpharmaceutical interventions (NPIs) (which include social distancing, facemasks, total or partial lockdown, contact tracing, quarantine, and isolation) are currently the main strategies to mitigate/prevent the spread of COVID-19 and have helped significantly reduce the mortality rate across the globe (Ferguson et al. 2020; Iboi et al. 2020; World Health Organization 2021a).
The epidemic situation in most African countries has been similar since the beginning of the pandemic in the region. Some of the reasons for this include the following. Most of the NPIs measures are challenging to comply with within most African countries, likely due to socioeconomic behavioral response issues, even during the lockdown period. Also, there is a lack of governmental financial support, as is being provided for local communities and businesses in developed countries. Most African people find it challenging to comply with NPIs measures. For example, ignoring lockdown and quarantine policies, engaging in communal activities to earn incomes for their families, and even helping other most vulnerable populations such as migrants, stateless people, and forcibly displaced refugees. In addition, most African countries cannot afford to get a reasonable quantity of vaccines for their people due to the devastating economic situation caused by the COVID-19 pandemic (Gilbert et al. 2020; Mehtar et al. 2020; Musa et al. 2020b, 2021b; Van Zandvoort et al. 2020).
Epidemiological modeling studies have long been used to understand and shed light on the transmission and spread of infectious diseases, including COVID-19 (Chen et al. 2020; Gilbert et al. 2020; He et al. 2021; Li et al. 2020; Musa et al. 2020b). Further, several works (Gu et al. 2020; Musa et al. 2021b; Roser et al. 2020) suggested a reasonable proportion of un-reported/under-ascertainment in the number of COVID-19 cases and deaths in African counties, which has largely contributed to the fast-spreading of the disease. This was likely due to the low testing rate, especially during the early epidemic following a high positivity rate when the testing rate significantly improved. Some reports investigated the seroprevalence of SARS-CoV-2 among blood donors’ in African countries to explore the actual impact of the pandemic in the region. In particular, Uyoga et al. (2021) examined the seroprevalence of anti-SARS-CoV-2 immunoglobulin G antibodies among Kenyan blood donors to determine the extent to which COVID-19 spread in the community. They found that the crude seroprevalence (i.e., amount of SARS-CoV-2 pathogen in blood serum) was estimated at 5.6%, indicating that SARS-CoV-2 exposure is more extensive than case-based surveillance. Another work (in the preprint by the submission date of this manuscript) by Sykes et al. (2021) studied the seroprevalence of SARS-CoV-2 among blood donors in South Africa. The results showed that SARS-CoV-2 prevalence differs by race group and province, with seroprevalence among black donors higher than white donors and other main population subgroups. Their estimation of the seroprevalence (which crudely equals the infection attack rate if we ignore seroreversion) in each of the four cities of the study area was 62.5 in Eastern Cape (95% CI 58.9–66.1); 31.8 in Northern Cape (95% CI 25.3–38.3); 45.5 in Free State (95% CI 39.9–51.1); and 52.1 in KwaZulu Natal (95% CI 49.1–55.2). These results differed from the work of Uyoga et al. (2021), in which the given estimates of the seroprevalence in Kenya (provided by age group) ranged from 3.4 to 10.0 (95% CI 1.6–19.5). Thus, there is a need for more epidemiological studies to describe the pandemic of COVID-19 in African countries (Ndaye et al. 2021).
In this paper, we propose a simple epidemic model to study the transmission dynamics of SARS-CoV-2 in Africa. We also discuss some of the epidemiological characteristics and NPIs implementation and implications in Africa. In particular, we estimate the \(R_{0} \left( t \right)\) and IAR of COVID-19 for the 12 most-affected African countries that experienced high COVID-19-related deaths. The findings of this study are expected to provide valuable insights and suggest effective COVID-19 control measures in Africa. NPIs measures play a crucial role in preventing and controlling SARS-CoV-2 transmission worldwide. However, African countries have some difficulties, especially regarding compliance with NPIs measures. Thus, we report/highlight some essential factors that should be emphasized/improved to control the disease effectively. Simulations results of the proposed model reveal \(R_{0} \left( t \right)\) and IAR’s estimated values for the top 12 countries with COVID-19-related deaths in Africa. These parameters are essential epidemiological parameters to curtail the spread of COVID-19 in Africa and beyond.
2 Materials and Methods
2.1 COVID-19 Epidemic Data
A set of COVID-19 observations indexed and arranged in time (Fanoodi et al. 2019) for cases and deaths were extracted from the public domain of the World Health Organization (WHO) dashboard for disease surveillance reports of the 12 African countries with the most COVID-19 deaths. Those are Zambia, Cameroon, Nigeria, Libya, Sudan, Kenya, Ethiopia, Algeria, Tunisia, Morocco, Egypt, and South Africa (World Health Organization 2021a). The time series distribution for the daily confirmations of COVID-19 cases and deaths for the 12 countries with the highest number of deaths in Africa by February 12, 2021, are visualized in Fig. 1, showing the trends of the epidemics. The COVID-19 cases and fatalities are represented by black and red dotted curves, respectively. Based on the data scenarios, we observed that Libya exhibited the first wave of the epidemic. Other counties have shown at least two waves of the COVID-19 epidemic patterns, with South Africa hit worst for both the cases and deaths. The country’s population data were obtained from the Worldometer, available via https://www.worldometers.info/population/ (Worldometer 2020). We used COVID-19 deaths data primarily since the COVID-19 cases can be easily influenced by reporting policies and efforts. However, COVID-19 deaths are underreported. To better understand underreporting, we obtained information on excess deaths for South Africa from an online source (The Wall Street Journal 2021). For details of the IFR, see Supplementary Sect. 4.
2.2 The SEIHRD Epidemic Model
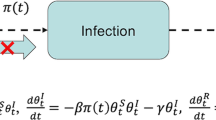
A susceptible–exposed–infectious–hospitalized–removed–dead (SEIHRD) model was proposed based on the classic SEIR-type (He et al. 2020; Musa et al. 2020a; Zhao et al. 2018) to obtain the fitting results with biologically reasonable parameter values. The model was fitted to the deaths data to visualize the COVID-19 scenario in the 12 most-affected countries with COVID-19 deaths in Africa to shed light on suitable NPIs measures that could appropriately and timely help curtail the outbreaks with very reduced socioeconomic consequences. In the classic SEIR-type model, the population is subdivided into four different compartments according to infection status, representing susceptible (S), exposed (E), infectious (I), and recovered (R).
Based on the general epidemiological features of SARS-CoV-2 (Chen et al. 2020; Li et al. 2020), we considered two additional compartments for infected individuals, which are ‘hospitalized infected’ and ‘dead’ compartments, represented by \(H\) and \(D\), respectively. Susceptible individuals can become exposed after coming into contact with infected people. Exposed individuals can progress to the infectious compartment after the latency period. Infectious individuals can move to the hospitalized compartment, which comes from either mild or severe cases. Infectious individuals can recover/be removed following effective treatment (or naturally) or die from the infection and enter the \(D\) compartment. The proposed model (Fig. 2), represented by the following nonlinear ordinary differential equations, is presented below.
Here, parameter \(\beta\) represents the flexible time-varying transmission rate, while parameter \(\sigma\) denotes the disease progression rate from the latent stage to infection status. Parameter \(\gamma\) measures the rate at which individuals move from \(I\) to \(H\) or \(R\), while parameter \(\xi\) denotes the SARS-CoV-2-induced death rate from the \(H\) compartment. We have lumped asymptomatically infected and symptomatically infected compartments since we are interested in exploring the overall transmission behavior in African counties, which shows similar epidemic patterns, especially during the early phase of the outbreaks. Although the current model’s structure is relatively simpler than many previous studies, it has a flexible transmission rate. Thus, it is not surprising that our model captures the dynamics well in these countries.
In this model, the parameter \(\rho\) represents both the proportion of hospitalization among infections and the share of deaths among hospitalizations. Note that hospitalization can be interpreted as symptomatic cases. Thus, the infection fatality ratio (IFR) is given by \(\rho^{2}\). We fitted the weekly integrated \(D\) to the reported mortality data for each of the 12 countries with the highest number of COVID-19-related deaths in Africa. Note that H could also be interpreted as reported cases. Since we only fit D to reported deaths rather than H to reported cases (or hospitalization), the exact definition of H is not crucial. We could define the proportions separately (the numbers of hospitalizations among infections, and the share of deaths among hospitalizations), but that would introduce one more parameter that would require more information or assumption with virtually the same fitting performance. Classic theory tells us that the dynamics of the model mainly depend on \(\beta\) and \(\gamma\) and less sensitive to other parameters.
The other assumptions include negative binominal measurement noise in reporting with an overdispersion parameter \(\phi\) and a time-varying transmission rate (\(\beta )\), which is an exponential cubic spline function with 7 nodes. The effective basic reproduction number \({R}_{0}\left(t\right)\) is given by\({R}_{0}\left(t\right)\approx \beta (t)/\gamma\). For more details regarding the model fitting process, see (He et al. 2020; Musa et al. 2020a; Zhao et al. 2018). Iterated filtering algorithms are used to fit the model with an additional death class to the reported COVID-19 deaths for the 12 countries with the most deaths in Africa to quantify epidemic scenarios in Africa using the estimate of reproduction number. Thus, the proposed model was fitted to the SARS-CoV-2 mortality data for each of the 12 African countries. We estimated the IFR (with some restriction, see supplementary Sect. 4) and the time-varying transmission rate of COVID-19 in African countries.
3 Results
In Figs. 3 and 4, we used the proposed SEIHRD model (depicted in Fig. 2) to simulate the COVID-19 mortality datasets (weekly) for the 12 African countries with the most COVID-19 deaths to explore the dynamic characteristics of SARS-CoV-2 in Africa. We estimated the time-varying basic reproductive number, \({R}_{0}\left(t\right)\), and the infection attack rate, IAR, for each of the 12 countries, as depicted in Figs. 3 and 4, respectively. Figure 3 shows the fitting results of nine of the 12 countries with the most COVID-19 deaths (Algeria, Cameroon, Egypt, Ethiopia, Kenya, Morocco, Libya, Nigeria, Sudan, Tunisia, and Zambia), which were hit milder or had a relatively lower reporting rate. Figure 4 displays the fitting results of three of the 12 countries with the most COVID-19 deaths (Libya, South Africa, and Tunisia), which hit harder or had a relatively higher reporting rate. The weekly confirmed deaths are represented by the red circle, with the black curve representing the medium simulation, and the gray shaded region denoting the 95% confidence interval (CI) based on 1000 simulations. By February 2021, Cameroon, Sudan, Algeria, and Tunisia were witnessing the third wave of the COVID-19 epidemic, whereas Zambia, Nigeria, Libya, Kenya, Ethiopia, Morocco, Egypt, and South Africa were observing the second wave of the SARS-CoV-2 epidemic. The increase in transmission rate in these countries was likely due to the partial or non-compliance of the NPIs measures, age factor (the share of the elderly population), seasonality, or behavioral changes (see, for instance, (Moges et al. 2014)). For South Africa, we fitted our model to excess deaths, as seen in Fig. 4d. With the assumption IFR \(\ge 0.\) 68%, we yielded a similar estimated IAR per using the reported COVID-19. The excess death rate is approximately 3.4-fold of the reported COVID-19 rate.
Time series fitting results of weekly confirmed COVID-19 deaths (in red circles) in nine of the 12 African countries with the most COVID-19 deaths, which were hit milder (with low reporting rates). Deaths are population standardized (i.e., deaths per 1 million people). The median of the simulation is represented by the black curve, and the time-varying basic reproductive number (\({R}_{0}(t)\)) is denoted by the blue dashed curve. The 95% confidence interval of the simulation is shown by the shaded (gray) region. The estimated IAR is displayed in the title of each panel
Time series fitting results of weekly confirmed COVID-19 deaths (in red circles) in three of the 12 African countries with most COVID-19 deaths, which were hit harder (or with a relatively higher reporting effort): a Libya, b South Africa, c Tunisia and d South Africa with excess deaths. Deaths are population standardized (i.e., deaths per 1 million people). The median of the simulation is represented by the black curve, and the time-varying basic reproductive number (\({R}_{0}(t)\)) is denoted by the blue dashed curve. The 95% confidence interval of the simulation is shown by the shaded (gray) region. The estimated IAR is portrayed in the title of each panel
In Table 1, we obtained estimated values of the SARS-CoV-2 infection attack rate (IAR, the ratio of mortality per case in a particular population) for the 12 countries with the most deaths in Africa by February 2021. South Africa was hit hardest with an IAR of 0.414, followed by Tunisia with an IAR of 0.213 and then Libya with an IAR of 0.155, while Cameroon and Algeria were hit the mildest, with IARs of 0.022 and 0.042, respectively. These results indicate the impacts of NPIs, and thus highlight the imperative need to implement other control strategies to mitigate epidemics in Africa and beyond. We presume that the reported COVID-19 deaths are relatively reliable.
In Supplementary Table S1, we report the COVID-19 index case(s) scenarios for the 12 countries with the most deaths in Africa. SARS-CoV-2 was first reported in Egypt on February 14, 2020, followed by exponentially growing cases that spread in all parts of Africa, with South Africa as the epicenter until now. Consequently, the estimated seroprevalences of SARS-COV-2 (i.e., the estimated proportion of individuals in a population with positive test for COVID-19) are reported in Supplementary Table S2, which indicates that South Africa has an estimated seroprevalence of approximately 50% from blood donors (Shaw et al. 2021; Sykes et al. 2021). This result highlights that South Africa has been hit harder, which may be a consequence of the high proportion of mild or asymptomatic cases, comprising up to 80% of total infections (Eckerle and Meyer 2020).
Supplementary Table S4 presents the results of the model’s estimated parameters by employing the log-likelihood estimation technique. South Africa, Egypt, and Tunisia have log-likelihood values of − 301.112, − 263.928, and − 232.474, respectively. These outcomes signal that South Africa has the highest transmission potential compared to other countries. In Supplementary Table S4, we present a summary of the estimated results of the time-varying transmission rate using a fixed number of nodes (\({n}_{m}\)), 7. Different values of the time-varying transmission rate were obtained based on the different simulation runs. The state values of the model were assigned, with initial values given in Supplementary Table S4.
4 Discussion and Conclusions
It has been more than a year since COVID-19 emerged in China and spread rapidly to over 200 countries and territories. African countries have slightly strengthened their preparedness plans against unexpected disease outbreaks such as the COVID-19 pandemic based on the lessons learned. Those include timely responses; airport surveillance and temperature screening at ports of entry; improvement in diagnostic centers for testing/mass testing; strengthened collaboration with other countries in other regions like China, the US, and the UK (for instance, the deployment of medical personnel from the UK, donations of testing kits and PPEs by the Jack Ma Foundation, etc.) (Gilbert et al. 2020; World Health Organization 2021b). Africa was fortunate to report its first case of COVID-19 at least a month after many countries on other continents had already reported thousands of cases and deaths (Li et al. 2020; World Health Organization 2021a; Zhao et al. 2020); making the continent advantageous over others for timely preparations and responses to the pandemic in a variety of ways based on the lessons learned.
To visualize the actual scenario of the SARS-CoV-2 in 12 African countries with the most deaths, we simulated the model using weekly mortality data for each of the 12 countries to guide policymaking for effectively controlling the COVID-19 pandemic in Africa. To achieve reasonable fittings and estimates, we made some assumptions; in particular, we divided these countries into two groups. Group 1) consists of South Africa, Libya, Tunisia, with a value of \(\rho\) above 0.045 (i.e., IFR > 0.2%). These countries reported more deaths per capita, which suggests a severe impact and/or efficient reporting of deaths, and their IARs were 41.4%, 15.5%, and 21.3%, respectively. Group 2) consists of nine other countries (with a value of \(\rho\) between 0.01 and 0.04, i.e., 0.01% < IFR < 0.16%), which shows mild impact and/or underreporting of deaths. Their IARs varies between 2.2 and 20.2%, with a median of 6.2%. In Fig. 3, we depicted the time series fitting results of weekly reported COVID-19 deaths in 9 of the 12 countries (Algeria, Cameroon, Ethiopia, Kenya, Morocco, Nigeria, Sudan, and Zambia) that were hit milder or had a low reporting rate, and Fig. 4 reveals the time series fitting results of weekly reported COVID-19 deaths in 3 of the 12 countries (Libya, South Africa, and Tunisia) that were hit harder or had a relatively higher reporting rate.
The excess death has been one of the main quantities representing a more reliable metric for comparing and understanding the impacts of the pandemic among countries. Excess death is used to estimate the additional number of mortalities before and during the pandemic period more frequently than the presumed number of fatalities. It has become one of the crucial factors in identifying the relative effectiveness of prevention and control measures between countries. Although inconsistencies in reporting and testing between countries could affect the estimation of epidemiological parameters, excess mortality could help overcome this inherent inequality; however, its role in helping us understand why mortality varies among countries is still inadequate. Thus, we used excess deaths as a proxy for deaths due to COVID-19 to have a clear picture of the current situation in African counties, even though it may not represent the actual scenario (Beaney et al. 2020; Roser et al. 2020). Furthermore, according to Li et al. (2021), excess deaths = true COVID-19 deaths—avoided death due to lockdown effect + added deaths due to lockdown effect. Thus, it is clear that excess deaths will be a good proxy only when the last two terms on the right hand of the equation cancel each other. Suppose the second term is larger than the third term. Excess deaths could still be an underestimate of the actual COVID-19 deaths. Nevertheless, it addressed the underreporting in the reported COVID-19 deaths to some extent. All the above assumes that all causes of deaths should be at the average level of the previous five years, i.e., without a large-scale, long-term trend. See Supplementary Figures S2 and S3 for the comparison of excess deaths and COVID-19 reported death and a log-likelihood profile for IFR for South Africa, respectively.
Due to the asymptomatic feature of COVID-19, which could be up to 84% (Gu et al. 2020), and non-compliance of NPIs by the general public (which includes non-compliance for community testing surveillance, quarantine/isolation policy, lockdown, facemask use, and social distancing), it is highly likely that COVID-19 cases were underreported or under-ascertained in African countries (Gu et al. 2020; Musa et al. 2021b). Consequently, South Africa, as the epicenter of the pandemic in Africa, has recorded the highest morbidity and mortality cases of CVID-19 cases, which has eventually been one of the significant reasons for the occurrence of underreporting or under-ascertainment issues since the healthcare system was overwhelmed. Besides, the government cannot follow up with all the contacts for effective contact tracing, which results in a sizeable unobserved number of cases and deaths. Recent report (Abdool Karim 2020; National Institute for Communicable Diseases 2020) suggested that only about one in every four mild cases would be ascertained in South Africa.
Africa’s COVID-19 responses and control strategies were largely not context-specific (Iwuoha and Aniche 2020; Uyoga et al. 2021). The responses and mitigation strategies via NPIs employed in Africa were described as replication or simply the COVID-19 copy-and-paste policies that were implemented in countries other than Africa, such as China, United Kingdom, Germany and United States, despite the variability in epidemiological characteristics, differences in weather and climate, and different socioeconomic growth levels, which probably impact the transmission of the disease in Africa. Moreover, the copy-and-paste approach (also known as the ‘one-size-fits-all’ policy) is hugely adopted and implemented in African countries. Although, some of those (stringent) measures were needless and turned out to be useless, causing more socioeconomic hardship to vulnerable populations. For instance, many African countries impulsively adopted the same lockdown policy without the requisite knowledge of the COVID-19 clinical and epidemiological features in the region, which could have serious negative consequences to the communities (World Health Organization 2021b).
Furthermore, social distancing policies and restrictions on social gatherings (such as banning weddings, religious activities, and funeral ceremonies) have been imposed to curtail the spread of the virus, even though it is almost practically impossible for many African countries to comply. Mandatory wearing of facemasks in public places and the use of hand sanitizers were also pervasive COVID-19 control measures in Africa, even though compliance is very difficult and problematic due to ignorance, poverty, resource constraints, and a lack of awareness (Iwuoha and Aniche 2020; World Health Organization 2021b). Although some successes have been achieved concerning these response strategies, critics have described them as one-size-fits-all or copy-and-paste approaches because of inappropriateness and unfavorability to local settings in Africa (Dzisi and Dei 2020; Nyarko et al. 2020; Roser et al. 2020). In addition, one more important control measure is school closure, which has been massively criticized in Africa since many countries on the continent spontaneously closed down schools with the absence of exhaustive e-learning systems and inadequate infrastructure, as has been practiced in many counties of continents other than Africa (Iwuoha and Aniche 2020).
Some of the main challenges regarding the fight against the COVID-19 pandemic in Africa include the imposition of the hand-hygiene policy when the majority of Africans do not have access to an adequate and potable water supply, temperature screening at airports for travelers without considering asymptomatic carriers (asymptomatically infected people), the impossibility of work from home policies, and an insufficient quantity of PPEs, especially for frontline health workers, insufficient data sharing, and illegal crossing of borders at non-official points of entry (Arakpogun et al. 2020; Ataguba 2020; Irlacher and Koch 2021; World Health Organization 2021b). During lockdowns, food prices have skyrocketed significantly in many countries on the continent due to panic buying and interruptions in the food supply chain within the community and between countries (Vos et al. 2020). Although lockdowns have helped control the spread of the disease, they plunged millions of people into extreme poverty (Iwuoha and Aniche 2020; Nyarko et al. 2020; World Health Organization 2021b). And likely has greater consequences and can kill more people than COVID-19 due to its negative impacts that have exhausted countless individuals (Iwuoha and Aniche 2020). Aside from the extreme poverty caused (likely) due to the effects of lockdowns, there were issues of human rights abuses by government officials or security agencies that added to the hardships already emanating from the lockdown policy in Africa (Amadasun 2020).
Another unexpected repercussion of NPIs in Africa was the effect of already fragile healthcare systems. Neglecting public health needs in the wake of COVID-19 has been reported with an unprecedented reduction in health service usage, causing more threats concerning other infectious illnesses such as malaria, HIV/AIDS, and meningitis (Abdool Karim 2020; Jewell et al. 2020; Sherrard-Smith et al. 2020). These implications may even cause severe consequences during the post-COVID-19 pandemic because of their socioeconomic destruction. We also observed that most of the top 12 African countries with the most COVID-19 deaths reported their first COVID-19 case(s) between February and March 2020 (see Supplementary Table S1), which indicates that most of the stringency index policies are similar in most countries in Africa. Future work plans to extend the current study by incorporating possible reinfection or vertical transmission in the model to explore its impact on the overall transmission dynamics in African countries (Tang et al. 2021; Musa et al. 2021c).
Overall, Africa has done remarkably well in awareness and enlightenment campaigns by the media and civil society in disseminating health education and communication on COVID-19 prevention and control, which helps in curtailing the spread of SARS-CoV-2 in the African region (World Health Organization 2021b). Even though a more favorable/suitable policy needs to be considered, which can be implemented and easily complied with by the general public to effectively control the spread of COVID-19, with significantly reduced economic hardships and negative consequences, especially during a post-COVID-19 pandemic. With the assumed range of IFR and flexible transmission rate, we model a simple unified model of the reported deaths in 12 African countries. The group choice for countries was justified by the tests per capita performed across countries and the raw case-fatality rate across counties (Supplementary Table S3). For instance, South Africa performed more tests per capita than other countries, which implies a relatively higher reporting effort. The raw case-fatality rate is high in South Africa. Combining these two aspects, we decide to allocate South Africa to the high \(\rho\) group. Our fitting result reveals that South Africa, Libya, Tunisia, Ethiopia, and Morocco were hit harder with the SAR-CoV-2 epidemic in Africa. Thus, we suggest an imperative need to improve the socioeconomic situation, quality water supply, and healthcare system (including the provision of adequate health workers, PPEs, and test kits) in Africa to effectively and timely control SARS-CoV-2 outbreaks.
In conclusion, we proposed a simple unified model to report deaths scenarios in 12 African countries and excess deaths in South Africa. We gained a qualitative idea of the severity in African countries. In the next step, we plan to associate the reconstructed \({R}_{0}\left(t\right)\) with government control measures and possible voluntary social distancing in each country. In summary, we conducted large-scale data analysis and modeling work for the 12 African countries with the most deaths cases and shed light on the possible IFR and infection attack rate. Due to different economic levels, control measures, and testing and reporting efforts, we found considerable variation across countries. Our estimated infection attack rates, which are based on the assumed range of IFR, are largely in line with population-level serological studies. Our work is essential since it is the first large-scale population-level modeling work for these African countries. Along with population-level serological studies, our work provides insight into the possible severity in these countries.
References
Abdool Karim SS (2020) The South African response to the pandemic. New Engl J Med 382:e95
Amadasun S (2020) COVID-19 palaver: ending rights violations of vulnerable groups in Africa. World Dev 134:105054
Arakpogun E, El Sahn Z, Prime KS et al. (2020) Africa’s resilience in the face of COVID-19 pandemic: let’s talk about it!. Available at SSRN 3640311
Ataguba JE (2020) COVID-19 pandemic, a war to be won: understanding its economic implications for Africa. Appl Health Econ Health Policy 18(3):325–328
Beaney T, Clarke JM, Jain V et al (2020) Excess mortality: the gold standard in measuring the impact of COVID-19 worldwide? J R Soc Med 113:329–334
Chen N, Zhou M, Dong X et al (2020) Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet 395:507–513
Chowell G, Castillo-Chavez C, Fenimore PW et al (2004) Model parameters and outbreak control for SARS. Emerg Infect Dis 10:1258
Dzisi EKJ, Dei OA (2020) Adherence to social distancing and wearing of masks within public transportation during the COVID 19 pandemic. Transp Res Interdiscip Perspect 7:100191
Eckerle I, Meyer B (2020) SARS-CoV-2 seroprevalence in COVID-19 hotspots. Lancet 396:514–515
Fanoodi B, Malmir B, Jahantigh FF (2019) Reducing demand uncertainty in the platelet supply chain through artificial neural networks and ARIMA models. Comput Biol Med 113:103415
Ferguson N, Laydon D, Nedjati Gilani G et al (2020) Report 9: Impact of non-pharmaceutical interventions (NPIs) to reduce COVID19 mortality and healthcare demand. Imp Coll Lond. https://doi.org/10.25561/77482
Gilbert M, Pullano G, Pinotti F et al (2020) Preparedness and vulnerability of African countries against importations of COVID-19: a modelling study. Lancet 395:871–877
Gu X, Mukherjee B, Das S et al (2020) COVID-19 prediction in South Africa: estimating the unascertained cases-the hidden part of the epidemiological iceberg. medRxiv. https://doi.org/10.1101/2020.12.10.20247361
He D, Zhao S, Lin Q et al (2020) New estimates of the Zika virus epidemic attack rate in Northeastern Brazil from 2015 to 2016: A modelling analysis based on Guillain-Barré Syndrome (GBS) surveillance data. PLoS Negl Trop Dis 14:e0007502
He D, Artzy-Randrup Y, Musa SS et al (2021) The unexpected dynamics of COVID-19 in Manaus, Brazil: herd immunity versus interventions. medRxiv. https://doi.org/10.1101/2021.02.18.21251809
Hu B, Guo H, Zhou P et al (2020) Characteristics of SARS-CoV-2 and COVID-19. Nat Rev Microbiol 19(3):141–154
Iboi EA, Sharomi OO, Ngonghala CN et al (2020) Mathematical modeling and analysis of COVID-19 pandemic in Nigeria. Math Biosci Eng 17(6):7192–7220
Irlacher M, Koch M (2021) Working from home, wages, and regional inequality in the light of Covid-19. Jahrbücher für Nationalökonomie und Statistik 241(3):373–404
Iwuoha VC, Aniche ET (2020) Covid-19 lockdown and physical distancing policies are elitist: towards an indigenous (Afro-centred) approach to containing the pandemic in sub-urban slums in Nigeria. Local Environ 25:631–640
Jewell BL, Mudimu E, Stover J et al (2020) Potential effects of disruption to HIV programmes in sub-Saharan Africa caused by COVID-19: results from multiple mathematical models. Lancet HIV 7:e629–e640
Li Q, Guan X, Wu P et al (2020) Early transmission dynamics in Wuhan, China, of novel coronavirus–infected pneumonia. N Engl J Med 382:1199–1207
Li C, Zhang X, Zhu Y et al (2021) Excess pneumonia and influenza death as herald wave of COVID-19 in England and Wales, United Kingdom. J Infect 82(2):282–327
Lin Q, Chiu AP, Zhao S et al (2018) Modeling the spread of middle east respiratory syndrome coronavirus in Saudi Arabia. Stat Methods Med Res 27:1968–1978
Mehtar S, Preiser W, Lakhe NA et al (2020) Limiting the spread of COVID-19 in Africa: one size mitigation strategies do not fit all countries. Lancet Glob Health 8:e881–e883
Moges AG, Tamiya N, Yamamoto H (2014) Emerging population ageing challenges in Africa: a case of Ethiopia Kokusai Hoken Iryo. J Int Health 29:11–15
Musa SS, Zhao S, Gao D et al (2020a) Mechanistic modelling of the large-scale Lassa fever epidemics in Nigeria from 2016 to 2019. J Theor Biol 493:110209
Musa SS, Zhao S, Wang MH et al (2020b) Estimation of exponential growth rate and basic reproduction number of the coronavirus disease 2019 (COVID-19) in Africa. Infect Dis Poverty 9:1–6
Musa SS, Qureshi S, Zhao S et al (2021a) Mathematical modeling of COVID-19 epidemic with effect of awareness programs. Infect Dis Model 6:448–460
Musa SS, Zhao S, Hussaini N et al (2021b) Estimation of COVID-19 under-ascertainment in Kano, Nigeria during the Early Phase of the Epidemics. Alex Eng J 60:4547–4554
Musa SS, Bello UM, Zhao S et al (2021c) Vertical transmission of SARS-CoV-2: a systematic review of systematic reviews. Viruses 13(9):1877
National Institute for Communicable Diseases (2020) South African COVID-19 Modelling Consortium (Projections), https://www.nicd.ac.za/diseases-a-z-index/disease-index-covid-19/surveillance-reports/modelling-consortium-projections/. Accessed from 10 Oct 2021
Ndaye AN, Hoxha A, Madinga J et al (2021) Challenges in interpreting SARS-CoV-2 serological results in African countries. Lancet Glob Health 9(5):e588–e589
Nyarko R, Boateng E, Kahwa I et al (2020) The impact on public health and economy using lockdown as a tool against COVID-19 pandemic in Africa: a perspective. J Epidemiol Public Health Rev. https://doi.org/10.16966/2471-8211.188
Roser M, Ritchie H, Ortiz-Ospina E et al. (2020) Coronavirus pandemic (COVID-19). Our world in data. https://ourworldindata.org/coronavirus?te=1&nl=nicholas-kristof&emc=edit_nk_20200622
Shaw JA, Meiring M, Cummins T et al (2021) Higher SARS-CoV-2 seroprevalence in workers with lower socioeconomic status in Cape Town, South Africa. PLoS ONE 16:e0247852
Sherrard-Smith E, Hogan AB, Hamlet A et al (2020) The potential public health consequences of COVID-19 on malaria in Africa. Nat Med 26:1411–1416
Sykes W, Mhlanga L, Swanevelder R et al. (2021) Prevalence of anti-SARS-CoV-2 antibodies among blood donors in Northern Cape, KwaZulu-Natal, Eastern Cape, and Free State provinces of South Africa in January 2021 Research Square https://doi.org/10.21203/2Frs.3.rs-233375%2Fv1
Tang X, Musa SS, Zhao S et al (2021) Reinfection or reactivation of severe acute respiratory syndrome coronavirus 2: a systematic review front. Public Health 9:663045. https://doi.org/10.3389/fpubh.2021.663045
The Wall Street Journal (2021) Coronavirus. South Africa's Drop in COVID-19 Cases Adds Questions About Waves of Infections. 2021. Retrieved from https://www.wsj.com/articles/south-africas-drop-in-covid-19-cases-adds-to-questions-about-waves-of-infections-11615734003. Accessed from 1 Apr 2021
Uyoga S, Adetifa IM, Karanja HK et al (2021) Seroprevalence of anti–SARS-CoV-2 IgG antibodies in kenyan blood donors. Science 371:79–82
Van Zandvoort K, Jarvis CI, Pearson CA et al (2020) Response strategies for COVID-19 epidemics in African settings: a mathematical modelling study. BMC Med 18:1–19
Vos R, Martin W, Laborde D (2020) How much will global poverty increase because of COVID-19 Intl Food Policy Research Institute 1-28, https://www.ifpri.org/blog/how-much-will-global-poverty-increase-because-covid-19. Accessed 6 May 2021
World Health Organization (2020) Coronavirus disease (COVID-19), COVID-19 Vaccines, https://www.who.int/emergencies/diseases/novel-coronavirus-2019/covid-19-vaccines. Accessed 10 Oct 2021
World Health Organization (2021b) Regional Office for Africa. COVID-19 Strategic Response Plan in the WHO African Region, https://www.afro.who.int/fr/node/12891. Accessed from 4 Mar 2021
World Health Organization (2021a) Coronavirus disease (COVID-19) Dashboard, https://covid19.who.int/. Accessed from 10 Oct 2021
World Health Organization (2021c) Coronavirus disease (COVID-19) Weekly Epidemiological Update and Weekly Operational Update, https://www.who.int/emergencies/diseases/novel-coronavirus-2019/situation-reports/. Accessed from 29 Mar 2021
Worldometer (2020) COVID-19 Coronavirus Pandemic, https://www.worldometers.info/coronavirus/#countries. Accessed From 7 Apr 2021
Zhao S, Stone L, Gao D et al (2018) Modelling the large-scale yellow fever outbreak in Luanda, Angola, and the impact of vaccination. PLoS Negl Trop Dis 12:e0006158
Zhao S, Lin Q, Ran J et al (2020) Preliminary estimation of the basic reproduction number of novel coronavirus (2019-nCoV) in China, from 2019 to 2020: a data-driven analysis in the early phase of the outbreak. Int J Infect Dis 92:214–217
Acknowledgements
The authors are thankful to the handling editor and anonymous reviewers for their useful comments and suggestions, which were used to improve the paper from its initial version.
Funding
The work described in this paper was partially supported by a grant from the Research Grants Council of the Hong Kong Special Administrative Region, China (HKU C7123-20G). SL was supported by a grant from the Guangdong Recruitment Program of Oversea Experts (2020A1414010081). WM Wang was supported by the National Natural Science Foundation of China (Grant Nos. 12171192 and 12071173).
Author information
Authors and Affiliations
Contributions
SSM and DH contributed to conceptualization; SSM, SZ, XW, NH, WW, and DH contributed to formal analysis and writing—original draft; SL and DH contributed to writing—review and editing.
Corresponding author
Ethics declarations
Conflict of interests
We declare no competing interests.
Data Availability
All data used were obtained from the public domain, available from https://covid19.who.int/.
Ethics Approval
Ethics approval was not required since only publicly available data were used.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Musa, S.S., Wang, X., Zhao, S. et al. The Heterogeneous Severity of COVID-19 in African Countries: A Modeling Approach. Bull Math Biol 84, 32 (2022). https://doi.org/10.1007/s11538-022-00992-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11538-022-00992-x