Abstract
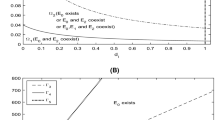
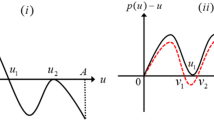
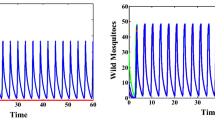
By studying an infection-age structured model, we consider the effects of releasing sterile males and the fertility of infected mosquitoes on the mosquito-borne diseases transmission including the extinction of mosquitoes, the elimination and persistence of diseases. Firstly, equivalent integral equations are established to prove the well-posedness of solutions. Then, the main results of disease dynamics are given. By taking chikungunya as a numerical simulation example, an optimal releasing threshold is given according to our presupposed control standard. When the fertility disturbance of infected mosquitoes is small, the high releasing amount plays a main role on the control of the disease; however, when the fertility disturbance is large, the initial distributions and the fertility of infected mosquitoes are the key factors to control the disease. Mathematically, the fertility of infected mosquitoes makes the system have complex dynamics with multiple positive equilibria and bistability.










Similar content being viewed by others
References
Criteria for control and elimination of malaria, Ministry of Health of the People’s Republic of China Standardization Administration of China, GB 26345-2010, http://www.nhc.gov.cn/wjw/s9499/201106/de37ee7388634b458b3eb7d923ce01ba.shtml
Technical guide for chikungunya fever Prevention and Control. https://wenku.baidu.com/view/1221d33a6394dd88d0d233d4b14e852459fb3992.html
Alphey L (2014) Genetic control of mosquitoes. Annu Rev Entomol 59:205–224
Anguelov R, Dumont Y, Lubuma J (2012) Mathematical modeling of sterile insect technology for control of anopheles mosquito. Comput Math Appl 64:374–389
Atkinson M, Su Z, Alphey N, Alphey L, Wein L (2007) Analyzing the control of mosquito-borne diseases by a dominant lethal genetic system. Proc Natl Acad Sci USA 104:9540–9545
Boete C, Agusto F, Reeves R (2014) Impact of mating behaviour on the success of malaria control through a single inundative release of transgenic mosquitoes. J Theoret Biol 347:33–43
Bowman C, Gumel A, van den Driessche P, Wu J, Zhu H (2005) A mathematical model for assessing control strategies against West Nile virus. Bull Math Biol 67:1107–1133
Buckner E, Alto B, Lounibos L (2013) Vertical transmission of key west dengue-1 virus by aedes aegypti and aedes albopictus (diptera: Culicidae) mosquitoes from florida. J Med Entomol 50:1291–1297
Cai L, Ai S, Li J (2014) Dynamics of mosquitoes populations with different strategies for releasing sterile mosquitoes. SIAM J Appl Math 74:1786–1809
Charlwood J, Smith T, Billingsley P, Takken W, Lyimo E, Meuwissen J (1997) Survival and infection probabilities of anthropophagic anophelines from an area of high prevalence of plasmodium falciparum in humans. Bull Entomol Res 87:453–455
Chen Y, Zhou S, Yang J (2016) Global analysis of an SIR epidemic model with infection age and saturated incidence. Nonlinear Anal-Real World Appl 30:16–31
Chitnis N, Hyman J, Cushing J (2008) Determining important parameters in the spread of malaria through the sensitivity analysis of a mathematical model. Bull Math Biol 70:1272–1296
Dufourd C, Dumont Y (2013) Impact of environmental factors on mosquito dispersal in the prospect of sterile insect technique control. Comput Math Appl 66:1695–1715
Farkas J, Hinow P (2010) Structured and unstructured continuous models for Wolbachia infections. Bull Math Biol 72:2067–2088
Ferguson H, Rivero A, Read A (2003) The influence of malaria parasite genetic diversity and anaemia on mosquito feeding and fecundity. Parasitology 127:9–19
Fister K, McCarthy M (2013) Optimal control of insects through sterile insect release and habitat modification. Math Biosci 244:201–212
Fu G, Lees R, Nimmo D, Aw D, Jin L, Gray P, Berendonk T, White-Cooper H, Scaife S, Kim Phuc H, Marinotti O, Jasinskiene N, James A, Alphey L (2010) Female-specific flightless phenotype for mosquito control. Proc Natl Acad Sci USA 107:4550–4554
Gubler D (1998) Resurgent vector-borne diseases as a global health problem. Emerg Infect Dis 4:442–450
Harrus S, Baneth G (2005) Drivers for the emergence and re-emergence of vector-borne protozoal and bacterial diseases. Int J Parasit 35:1309–1318
Hu L, Yang C, Hui Y, Yu J (2021) Mosquito control based on pesticides and endosymbiotic bacterium wolbachia. Bull Math Biol 83:1–24
Huang M, Luo J, Hu L, Zheng B, Yu J (2017) Assessing the efficiency of Wolbachia driven Aedes mosquito suppression by delay differential equations. J Theor Biol 440:1–11
Huang J, Ruan S, Yu P, Zhang Y (2019) Bifurcation analysis of a mosquito population model with a saturated release rate of sterile mosquitoes. SIAM J Appl Dyn Syst 18:939–972
Hughes H, Britton N (2013) Modelling the use of wolbachia to control dengue fever transmission. Bull Math Biol 75:796–818
Killeen G, McKenzie F, Foy B, Schieffelin C, Billingsley P, Beier J (2000) A simplified model for predicting malaria entomologic inoculation rates based on entomologic and parasitologic parameters relevant to control. Am J Trop Med Hyg 62:535–544
Lees R, Gilles J, Hendrichs J, Vreysen M, Bourtzis K (2015) Back to the future: the sterile insect technique against mosquito disease vectors. Curr Opin Insect Sci 10:156–162
Lewis M, van den Driessche P (1993) Waves of extinction from sterile insect release. Math Biosci 116:221–247
Li J (2004) Simple mathematical models for interacting wild and transgenic mosquito populations. Math Biosci 189:39–59
Li J, Cai L, Li Y (2017) Stage-structured wild and sterile mosquito population models and their dynamics. J Biol Dynam 11:79–101
Liu H, Yu J, Zhu G (2006) Analysis of a vector-host malaria model with impulsive effect and infection-age. Adv Complex Syst 9:237–248
Lou Y, Zhao X (2011) A reaction-diffusion malaria model with incubation period in the vector population. J Math Biol 62:543–568
Manore C, Hickmann K, Xu S, Wearing H, Hyman J (2014) Comparing dengue and chikungunya emergence and endemic transmission in A. aegypti and A. albopictus. J Math Biol 356:174–191
Rock K, Wood D, Keeling M (2015) Age-and bite-structured models for vector-borne diseases. Epidemics 12:20–29
Sachs J, Malaney P (2002) The economic and social burden of malaria. Nature 415:680–685
Sutherst R (2004) Global change and human vulnerability to vector-borne diseases. Clin Microbiol Rev 17:136–173
Taghikhani R, Sharomi O, Gumel A (2020) Dynamics of a two-sex model for the population ecology of dengue mosquitoes in the presence of Wolbachia. Math Biosci 328:108426
Thomas D, Donnelly C, Wood R, Alphey L (2000) Insect population control using a dominant, repressible lethal, genetic system. Science 287:2474–2476
Waltz E (2016) US reviews plan to infect mosquitoes with bacteria to stop disease. Nature 533:450–453
Werren J (1997) Biology of wolbachia. Annurev Ento 42:587–609
White S, Rohani P, Sait S (2010) Modelling pulsed releases for sterile insect techniques: fitness costs of sterile and transgenic males and the effects on mosquito dynamics. J Appl Ecol 47:1329–1339
Xi Z, Khoo C, Dobson S (2005) Wolbachia establishment and invasion in an Aedes aegypti laboratory population. Science 310:326–328
Xue L, Cao X, Wan H (2021) Releasing wolbachia-infected mosquitos to mitigate the transmission of Zika virus. J Math Anal Appl 496:1–27
Yu J (2018) Modeling mosquito population suppression based on delay differential equations. SIAM J Appl Math 78:3168–3187
Yu J, Li J (2020) Global asymptotic stability in an interactive wild and sterile mosquito model. J Differ Equ 269:6193–6215
Zheng X, Zhang D (2019) Incompatible and sterile insect techniques combined eliminate mosquitoes. Nature 572:56–61
Acknowledgements
We are extremely grateful to the critical comments and invaluable suggestions made by anonymous honorable reviewers. We sincerely thank Professor Jinzhi Lei and Xiyin Liang for helpful discussions on mathematical modeling and numerical computation. This work is supported by the National Natural Science Foundation of China (Nos. 11871371,11871179,11971023).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Sun, X., Liu, S., Lv, Y. et al. Effects of Sterile Males and Fertility of Infected Mosquitoes on Mosquito-Borne Disease Dynamics. Bull Math Biol 84, 31 (2022). https://doi.org/10.1007/s11538-022-00991-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11538-022-00991-y