Abstract
Collective cell migration plays a fundamental role in many biological phenomena such as immune response, embryogenesis and tumorigenesis. In the present work, we propose a reaction–diffusion finite element model of the lateral line primordium migration in zebrafish. The population is modelled as a continuum with embedded discrete motile cells, which are assumed to be viscoelastic and able to undergo large deformations. The Wnt/ß-catenin–FGF and cxcr4b–cxcr7b signalling pathways inside the cohort regulating the migration are described through coupled reaction–diffusion equations. The coupling between mechanics and the molecular scenario occurs in two ways. Firstly, the intensity of the protrusion–contraction movement of the cells depends on the cxcr4b concentration. Secondly, the intra-synchronization between the active deformations and the adhesion forces inside each cell is triggered by the cxcr4b–cxcr7b polarity. This influences the inter-synchronization between the cells and results in two main modes of migration: uncoordinated and coordinated. The main objectives of the work were (i) to validate our assumptions with respect to the experimental observations and (ii) to decipher the mechanical conditions leading to efficient migration of the primordium. To achieve the second goal, we will specifically focus on the role of the leader cells and their position inside the population.





Similar content being viewed by others
Notes
Wnt/ß-catenin is the canonical Wnt pathway.
FGF stands for fibroblast growth factor.
apc is a protein regulating Wnt/ß-catenin signalling and ensuring association with the microtubules, which is necessary for normal migration, via the C terminus.
SU5402 is a fibroblast growth factor receptor (FGFR)-specific tyrosine kinase inhibitor.
dkk1 is a diffusible inhibitor of the Wnt/ß-catenin pathway.
References
Allena R (2013) Cell migration with multiple pseudopodia: temporal and spatial sensing models. Bull Math Biol 75(2):288–316. doi:10.1007/s11538-012-9806-1
Allena R, Aubry D (2012) ‘Run-and-tumble’ or ‘look-and-run’? A mechanical model to explore the behavior of a migrating amoeboid cell. J Theor Biol 306:15–31. doi:10.1016/j.jtbi.2012.03.041
Allena R, Aubry D, Sharpe J (2013) On the mechanical interplay between intra- and inter-synchronization during collective cell migration: a numerical investigation. Bull Math Biol 75(12):2575–2599. doi:10.1007/s11538-013-9908-4
Allena R, Mouronval A-S, Aubry D (2010) Simulation of multiple morphogenetic movements in the Drosophila embryo by a single 3D finite element model. J Mech Behav Biomed Mater 3(4):313–323. doi:10.1016/j.jmbbm.2010.01.001
Aman A, Piotrowski T (2008) Wnt/beta-catenin and Fgf signaling control collective cell migration by restricting chemokine receptor expression. Dev cell 15(5):749–761. doi:10.1016/j.devcel.2008.10.002
Aman A, Piotrowski T (2009) Multiple signaling interactions coordinate collective cell migration of the posterior lateral line primordium. Cell Adhes Migr 3(4):365–368
Aman A, Piotrowski T (2011) Cell–cell signaling interactions coordinate multiple cell behaviors that drive morphogenesis of the lateral line. Cell Adhes Migr 5(6):499–508. doi:10.4161/cam.5.6.19113
Anand RJ, Leaphart CL, Mollen KP, Hackam DJ (2007) The role of the intestinal barrier in the pathogenesis of necrotizing enterocolitis. Shock (Augusta, Ga) 27(2):124–133. doi:10.1097/01.shk.0000239774.02904.65
Arciero JC, Mi Q, Branca MF, Hackam DJ, Swigon D (2011) Continuum model of collective cell migration in wound healing and colony expansion. Biophys J 100(3):535–543. doi:10.1016/j.bpj.2010.11.083
Bausch AR, Möller W, Sackmann E (1999) Measurement of local viscoelasticity and forces in living cells by magnetic tweezers. Biophys J 76(1 Pt 1):573–579
Couzin ID, Krause J, Franks NR, Levin SA (2005) Effective leadership and decision-making in animal groups on the move. Nature 433(7025):513–516. doi:10.1038/nature03236
Dambly-Chaudière C, Cubedo N, Ghysen A (2007) Control of cell migration in the development of the posterior lateral line: antagonistic interactions between the chemokine receptors CXCR4 and CXCR7/RDC1. BMC Dev Biol 7:23. doi:10.1186/1471-213X-7-23
David NB, Sapède D, Saint-Etienne L, Thisse C, Thisse B, Dambly-Chaudière C, Ghysen A (2002) Molecular basis of cell migration in the fish lateral line: role of the chemokine receptor CXCR4 and of its ligand, SDF1. Proc Natl Acad Sci 99(25):16297–16302. doi:10.1073/pnas.252339399
Di Costanzo E, Natalini R, Preziosi L (2014) A hybrid mathematical model for self-organizing cell migration in the zebrafish lateral line. J Math Biol. doi:10.1007/s00285-014-0812-9
Dong C, Slattery MJ, Rank BM, You J (2002) In vitro characterization and micromechanics of tumor cell chemotactic protrusion, locomotion, and extravasation. Ann Biomed Eng 30(3):344–355
Drury JL, Dembo M (2001) Aspiration of human neutrophils: effects of shear thinning and cortical dissipation. Biophys J 81(6):3166–3177
Friedl P, Gilmour D (2009) Collective cell migration in morphogenesis, regeneration and cancer. Nat Rev Mol Cell Biol 10(7):445–457. doi:10.1038/nrm2720
Fukui Y, Uyeda TQP, Kitayama C, Inoué S (2000) How well can an amoeba climb? Proc Natl Acad Sci 97(18):10020–10025. doi:10.1073/pnas.97.18.10020
Gamba L, Cubedo N, Ghysen A, Lutfalla G, Dambly-Chaudière C (2010) Estrogen receptor ESR1 controls cell migration by repressing chemokine receptor CXCR4 in the zebrafish posterior lateral line system. Proc Natl Acad Sci 107(14):6358–6363. doi:10.1073/pnas.0909998107
Gompel N, Cubedo N, Thisse C, Thisse B, Dambly-Chaudière C, Ghysen A (2001) Pattern formation in the lateral line of zebrafish. Mech Dev 105(1–2):69–77
Graner, Glazier (1992) Simulation of biological cell sorting using a two-dimensional extended Potts model. Phys Rev Lett 69(13):2013–2016
Haas P, Gilmour D (2006) Chemokine signaling mediates self-organizing tissue migration in the zebrafish lateral line. Dev Cell 10(5):673–680. doi:10.1016/j.devcel.2006.02.019
Holzapfel GA (2000) Nonlinear solid mechanics: a continuum approach for engineering, 1st edn. Wiley, Hoboken
Ilina O, Friedl P (2009) Mechanisms of collective cell migration at a glance. J Cell Sci 122(18):3203–3208. doi:10.1242/jcs.036525
Kabla AJ (2011) Collective cell migration: leadership, invasion and segregation. arXiv:1108.4286 [physics, q-bio]. Retrieved from http://arxiv.org/abs/1108.4286
Laurent VM, Kasas S, Yersin A, Schäffer TE, Catsicas S, Dietler G, Meister J-J et al (2005) Gradient of rigidity in the lamellipodia of migrating cells revealed by atomic force microscopy. Biophys J 89(1):667–675. doi:10.1529/biophysj.104.052316
Lecaudey V, Cakan-Akdogan G, Norton WHJ, Gilmour D (2008) Dynamic Fgf signaling couples morphogenesis and migration in the zebrafish lateral line primordium. Development (Cambridge, England) 135(16):2695–2705. doi:10.1242/dev.025981
López-Schier H (2010) Fly fishing for collective cell migration. Curr Opin Genet Dev 20(4):428–432. doi:10.1016/j.gde.2010.04.006
Lubarda V (2004) Constitutive theories based on the multiplicative decomposition of deformation gradient: thermoelasticity, elastoplasticity, and biomechanics. Appl Mech Rev 57(2):95–109
Nechiporuk A, Raible DW (2008) FGF-dependent mechanosensory organ patterning in zebrafish. Science (New York, NY) 320(5884):1774–1777. doi:10.1126/science.1156547
Rørth P (2007) Collective guidance of collective cell migration. Trends Cell Biol 17(12):575–579. doi:10.1016/j.tcb.2007.09.007
Serra-Picamal X, Conte V, Vincent R, Anon E, Tambe DT, Bazellieres E, Trepat X (2012) Mechanical waves during tissue expansion. Nat Phys 8(8):628–634
Sherratt JA, Murray JD (1990) Models of epidermal wound healing. Proc Biol Sci/R Soc 241(1300):29–36. doi:10.1098/rspb.1990.0061
Sherratt JA, Murray JD (1991) Mathematical analysis of a basic model for epidermal wound healing. J Math Biol 29(5):389–404
Streichan SJ, Valentin G, Gilmour D, Hufnagel L (2011) Collective cell migration guided by dynamically maintained gradients. Phys Biol 8(4):045004. doi:10.1088/1478-3975/8/4/045004
Taber LA (2004) Nonlinear theory of elasticity: applications in biomechanics. World Scientific, Singapore
Tambe DT, Hardin CC, Angelini TE, Rajendran K, Park CY, Serra-Picamal X, Trepat X (2011) Collective cell guidance by cooperative intercellular forces. Nat Mater 10(6):469–475. doi:10.1038/nmat3025
Trepat X, Wasserman MR, Angelini TE, Millet E, Weitz DA, Butler JP, Fredberg JJ (2009) Physical forces during collective cell migration. Nat Phys 5(6):426–430. doi:10.1038/nphys1269
Valentin G, Haas P, Gilmour D (2007) The chemokine SDF1a coordinates tissue migration through the spatially restricted activation of Cxcr7 and Cxcr4b. Curr Biol 17(12):1026–1031. doi:10.1016/j.cub.2007.05.020
Vedel S, Tay S, Johnston DM, Bruus H, Quake SR (2013) Migration of cells in a social context. Proc Natl Acad Sci 110(1):129–134. doi:10.1073/pnas.1204291110
Vitorino P, Meyer T (2008) Modular control of endothelial sheet migration. Genes Dev 22(23):3268–3281. doi:10.1101/gad.1725808
Yamao M, Naoki H, Ishii S (2011) Multi-cellular logistics of collective cell migration. PLoS One 6(12):e27950. doi:10.1371/journal.pone.0027950
Acknowledgments
This work was initiated and partially completed while Rachele Allena was a visitor to the Mathematical Institute, Oxford. The authors are grateful to Dr. Tatjana Piotrowski and Pr. Denis Aubry for useful discussions.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Appendix
Appendix
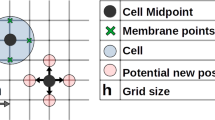
1.1 LLP Geometry
The cell network \(\Omega _n \)is defined by a characteristic function \(h_n \left( {{\varvec{p}}} \right) \) as follows:
with round being the classical integer function and \({{\varvec{p}}}=\left( {p_x ,p_y } \right) \) the initial position of any particle of the system .
The ECM domain \(\Omega _\mathrm{ECM}\) is identified by the characteristic function \(h_\mathrm{ECM} \left( {{\varvec{p}}} \right) \) which reads
Each cell inside the population is denoted by \(c(i,j)\) where the indices \(i\) and \(j\) vary as follows:
with \(N_\mathrm{c} =\frac{L}{r_\mathrm{c}}, i_\mathrm{max } =18\) and \(n_\mathrm{{c,\max }} =\frac{l}{r_\mathrm{c} }\) being the number of cells along the two axes of the ellipse (Fig. 2b, c).
The domain \(\Omega _{\mathrm{c}_{i,j} } \) occupied by each cell \(c(i,j)\) is defined through a characteristic function as follows
Each cell is equipped with a frontal \(\partial \Omega _{\mathrm{sf}_{i,j} }\) and a rear \(\partial \Omega _{\mathrm{sr}_{i,j}}\) adhesion region (Fig. 2d) described, respectively, by two characteristic functions
where \(\left( {{{\varvec{a}}},{{\varvec{b}}}} \right) \) defines the scalar product and \(l_\mathrm{f}\) and \(l_\mathrm{r}\) are the distances of \({{\varvec{c}}}_{i,j}\) from the frontal and rear adhesion surfaces, respectively.
The ellipse is divided into cell rows \(r(i)\) (Fig. 2b), which are numbered, similarly to the single cells, from the “stern” (left) to the “bow” (right) of the ellipse (\(1\le i\le N_\mathrm{c} =i_\mathrm{max } \)) (Fig. 2c) and are defined through a characteristic function as
1.2 Leading and Trailing Edge of the LLP
The Wnt/ß-catenin–FGF network is mainly based on the spatial polarization of the LLP. We define the leading, \(\Omega _\mathrm{front}\), and the trailing, \(\Omega _\mathrm{rear}\), edges of the LLP through the characteristic functions \(h_\mathrm{front}\) and \(h_\mathrm{rear}\), respectively, as follows:
where \(p_{x0}\) is the axial coordinate defining the boundary between the leading and the trailing edges.
1.3 Description of Mutants
In the following, we define the reaction–diffusion equations that have been used to describe the molecular and chemokine patterns specific to each mutant as mentioned in Sect. 2.2.
-
apc embryo
$$\begin{aligned} \frac{\partial \left[ W \right] }{\partial t}&= \underbrace{D_a \nabla ^{2}\left[ W \right] }_\mathrm{diffusion}+\underbrace{S_a \left[ W \right] \left( {1-\left[ W \right] } \right) }_{signalling}-\underbrace{R_\mathrm{a} \left[ W \right] \left[ F \right] }_\mathrm{reaction\, by\, dkk1}\end{aligned}$$(15)$$\begin{aligned} \frac{\partial \left[ F \right] }{\partial t}&= \underbrace{D_\mathrm{b} \nabla ^{2}\left[ F \right] }_\mathrm{diffusion}+\underbrace{P_\mathrm{b} \left[ W \right] \left( {1-\left[ F \right] } \right) }_\mathrm{production}-\underbrace{R_\mathrm{b} \left[ F \right] \left[ W \right] }_\mathrm{reaction\, by\, sef}\end{aligned}$$(16)$$\begin{aligned} \frac{\partial \left[ {c_4 } \right] }{\partial t}&= \underbrace{P_\mathrm{c} \left[ {c_4 } \right] \left( {1-\left[ {c_4 } \right] } \right) }_\mathrm{production}-\underbrace{R_\mathrm{c} \left[ {c_4 } \right] \left[ F \right] }_\mathrm{reaction\, by\, Fgf}\end{aligned}$$(17)$$\begin{aligned} \frac{\partial \left[ {c_7 } \right] }{\partial t}&= \underbrace{P_\mathrm{d} \left[ {c_7 } \right] \left( {1-\left[ {c_7 } \right] } \right) }_\mathrm{production}-\underbrace{R_\mathrm{d} \left[ {c_7 } \right] \left[ W \right] }_\mathrm{reaction\, by\, Wnt} \end{aligned}$$(18) -
SU5402 embryo
$$\begin{aligned} \frac{\partial \left[ W \right] }{\partial t}&= \underbrace{D_\mathrm{a} \nabla ^{2}\left[ W \right] }_\mathrm{diffusion}+\underbrace{S_\mathrm{a} \left[ W \right] \left( {1-\left[ W \right] } \right) h_\mathrm{front} }_\mathrm{signalling}-\underbrace{R_\mathrm{a} \left[ W \right] \left[ F \right] h_\mathrm{rear} }_\mathrm{reaction\, by\, dkk1}\end{aligned}$$(19)$$\begin{aligned} \frac{\partial \left[ F \right] }{\partial t}&= \underbrace{D_\mathrm{b} \nabla ^{2}\left[ F \right] }_\mathrm{diffusion}-\underbrace{R_\mathrm{b} \left[ F \right] \left[ W \right] h_\mathrm{front} }_\mathrm{reaction\, by\, sef}\end{aligned}$$(20)$$\begin{aligned} \frac{\partial \left[ {c_4 } \right] }{\partial t}&= \underbrace{P_\mathrm{c} \left[ {c_4 } \right] \left( {1-\left[ {c_4 } \right] } \right) }_\mathrm{production}-\underbrace{R_\mathrm{c} \left[ {c_4 } \right] \left[ F \right] }_{\mathrm{reaction}\, {by}\, Fgf}\end{aligned}$$(21)$$\begin{aligned} \frac{\partial \left[ {c_7 } \right] }{\partial t}&= \underbrace{P_\mathrm{d} \left[ {c_7 } \right] \left( {1-\left[ {c_7 } \right] } \right) }_\mathrm{production}-\underbrace{R_\mathrm{d} \left[ {c_7 } \right] \left[ W \right] }_{\mathrm{reaction\, by}\, Wnt} \end{aligned}$$(22) -
dkk1 embryo
$$\begin{aligned} \frac{\partial \left[ W \right] }{\partial t}&= \underbrace{D_\mathrm{a} \nabla ^{2}\left[ W \right] }_\mathrm{diffusion}+\underbrace{S_\mathrm{a} \left[ W \right] \left( {1-\left[ W \right] } \right) h_\mathrm{front} }_\mathrm{signalling}-\underbrace{R_\mathrm{a} \left[ W \right] \left[ F \right] }_{\mathrm{reaction}\, \mathrm{by}\, dkk1}\end{aligned}$$(23)$$\begin{aligned} \frac{\partial \left[ F \right] }{\partial t}&= \underbrace{D_\mathrm{b} \nabla ^{2}\left[ F \right] }_\mathrm{diffusion}+P_\mathrm{b} \left[ W \right] \left( {1-\left[ F \right] } \right) -\underbrace{R_\mathrm{b} \left[ F \right] \left[ W \right] h_\mathrm{front} }_{\mathrm{reaction\, by}\, sef}\end{aligned}$$(24)$$\begin{aligned} \frac{\partial \left[ {c_4 } \right] }{\partial t}&= \underbrace{P_\mathrm{c} \left[ {c_4 } \right] \left( {1-\left[ {c_4 } \right] } \right) }_\mathrm{production}-\underbrace{R_\mathrm{c} \left[ {c_4 } \right] \left[ F \right] }_{\mathrm{reaction\, by}\, Fgf}\end{aligned}$$(25)$$\begin{aligned} \frac{\partial \left[ {c_7 } \right] }{\partial t}&= \underbrace{P_\mathrm{d} \left[ {c_7 } \right] \left( {1-\left[ {c_7 } \right] } \right) }_\mathrm{production}-\underbrace{R_\mathrm{d} \left[ {c_7 } \right] \left[ W \right] }_{\mathrm{reaction\, by}\, Wnt} \end{aligned}$$(26)
1.4 Constitutive Model
As mentioned in Sect. 2.3, the behaviour of the cells is described through a generalized viscoelastic 2D Maxwell model (Allena 2013; Allena and Aubry 2012).
The Cauchy stress, \(\varvec{\sigma }\), is assumed to be the sum of the solid (\(\varvec{\sigma }_\mathrm{s}\)) and the fluid (\(\varvec{\sigma }_\mathrm{f}\)) Cauchy stresses, while the deformation gradient \({{\varvec{F}}}\) is equal to the solid (\({{\varvec{F}}}_\mathrm{s}\)) and the fluid (\({{\varvec{F}}}_\mathrm{f}\)) deformation gradients.
The decomposition of the deformation gradient (Allena et al. 2010; Lubarda 2004) is used to describe the solid deformation tensor, \({{\varvec{F}}}_\mathrm{s}\), which is then given by
where \({{\varvec{F}}}_\mathrm{se} \) is the elastic deformation tensor responsible for the stress generation and \({{\varvec{F}}}_\mathrm{sa}\) is the active deformation tensor responsible for the pulsating movement (protrusion–contraction) of each cell. Similarly, the fluid deformation tensor \({{\varvec{F}}}_\mathrm{f}\) is the multiplicative decomposition of the fluid elastic (\({{\varvec{F}}}_\mathrm{fe}\)) and the fluid viscoelastic (\({{\varvec{F}}}_\mathrm{fv}\)) gradients.
Both the solid \(\varvec{\sigma }_\mathrm{se}\) and the fluid elastic \(\varvec{\sigma }_\mathrm{fe}\) Cauchy stresses are given by isotropic hyperelastic models \(\bar{{\varvec{\sigma }}}_\mathrm{se}\) and \(\bar{{\varvec{\sigma }}}_\mathrm{fe}\), respectively, as
with \({{\varvec{e}}}_\mathrm{se}\) and \({{\varvec{e}}}_\mathrm{fe}\) the Euler–Almansi deformation tensors for the solid elastic and the fluid elastic phases, respectively. Additionally, \(\varvec{\sigma }_\mathrm{fe}\) has to be expressed in the actual configuration according to the multiplicative decomposition described above. Finally, the deformation rate \(\dot{{{\varvec{e}}}}_\mathrm{fv} \) is related to the deviator part of the fluid viscous stress \(\varvec{\sigma }_\mathrm{fv}^D\) as follows:
where \(\mu _\mathrm{fv} \) is the viscosity and the dot is the derivative with respect to time.
1.5 Coordinated and Uncoordinated Migration
The characteristic functions \(h_\mathrm{c}\) and \(h_\mathrm{uc}\) are expressed as follows
with \(\wedge \) being the Boolean operator AND and \(c_\mathrm{max}\) and \(c_\mathrm{min}\) being two thresholds fixed here to 0.9 and 0.2, respectively.
The terms \(e_{a,c}\) and \(e_{a,uc}\) describe the cyclic deformation of protrusion–contraction, and they read
where \(t\) is time.
For the coordinated migration, \(\alpha _c\) is set to 2 and \(T\) indicates the duration of a migration period which has been fixed here to 60 s (Allena and Aubry 2012; Dong et al. 2002). Additionally, a wave progressively covers the LLP from the “bow” to the “stern” to activate, one by one, the cell row \(r(i) \) with a velocity equal to \(\frac{2t}{T}\). The wave is expressed by the characteristic function \(h_{wave} \left( {{{\varvec{p}}},t} \right) \) as follows:
For the uncoordinated migration, \(\alpha _{uc_{ij}}\) and \(T_{uc_{ij}}\) may vary between 0 and 1 and between 60 and 120 s, respectively, for each cell \(c(i,j)\).
Rights and permissions
About this article
Cite this article
Allena, R., Maini, P.K. Reaction–Diffusion Finite Element Model of Lateral Line Primordium Migration to Explore Cell Leadership. Bull Math Biol 76, 3028–3050 (2014). https://doi.org/10.1007/s11538-014-0043-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11538-014-0043-7