Abstract
Background
Oxaliplatin-containing adjuvant regimens (folinic acid, fluorouracil, and oxaliplatin/capecitabine and oxaliplatin [FOLFOX/CAPOX]) are used after curative resection of colorectal cancer (CRC). However, real-world evidence regarding treatment sequences and outcomes in patients with early recurrence CRC after adjuvant chemotherapy is limited.
Objective
We aimed to describe the patient characteristics, treatment sequence, and overall duration of second-line (2L) therapy in patients with early recurrence CRC who received adjuvant chemotherapy (FOLFOX/CAPOX) followed by folinic acid, fluorouracil, and irinotecan (FOLFIRI) + anti-angiogenesis drugs (AA) or FOLFIRI + anti-epidermal growth factor receptor (EGFR) antibodies.
Methods
This retrospective study analyzed Japanese administrative data from November 2014 to March 2023 of adult patients who underwent CRC resection surgery, started FOLFOX/CAPOX ≤3 months (mo) after surgery, and had early CRC recurrence. Early recurrence was defined as initiation of FOLFIRI+AA or FOLFIRI+anti-EGFR antibodies as 2L therapy, ≤12 mo of discontinuing adjuvant chemotherapy. Patient characteristics, treatment sequence, median time to treatment discontinuation (mTTD), i.e., duration between the start and end dates of 2L therapy (Kaplan–Meier method), and factors associated with 2L time to treatment discontinuation constituted the study outcomes (Cox regression model). Subgroup analyses were performed for timing of early CRC recurrence (≤6 mo and 6–12 mo) and tumor sidedness.
Results
Among the 832 selected patients (median age [minimum–maximum] 67 (24–86) years, 56.4% male), CAPOX (71.3%) was more commonly used than FOLFOX (28.7%) as adjuvant therapy. FOLFIRI+AA (72.5%) was used more commonly than FOLFIRI+anti-EGFR antibodies (27.5%) in 2L. AA and anti-EGFR antibodies groups had similar mTTD: 6.2 mo (95% confidence interval 5.8, 6.9) and 6.1 mo (95% confidence interval 5.2, 7.4). Age ≥70 years showed significant association with shorter 2L treatment duration (hazard ratio 1.2, 95% confidence interval 1.0, 1.4; p = 0.03). The AA cohort’s mTTD was numerically shorter in the ≤6 mo recurrence subgroup compared with the 6–12 mo recurrence subgroup (6.1 mo vs 8.1 mo); the anti-EGFR antibodies cohort had similar mTTD (5.8 mo vs 6.2 mo). The AA and anti-EGFR antibodies cohorts also had similar mTTD in the left-sided CRC subgroup (6.5 mo vs 6.2 mo), but not in the right-sided subgroup (5.6 mo vs 3.9 mo).
Conclusions
This is the first administrative data-based real-world evidence on treatment sequence and outcomes for patients with early recurrence CRC treated with FOLFIRI+AAs or FOLFIRI+ anti-EGFR antibodies after adjuvant FOLFOX/CAPOX therapy in Japan. Both regimens had similar TTD, but relapse timing and tumor sidedness may influence their efficacy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
This real-world study is the first administrative data-based evidence on treatment sequences and outcomes for patients with early colorectal cancer recurrence treated with folinic acid, fluorouracil, and irinotecan (FOLFIRI) + anti-angiogenesis inhibitors (AAs) or FOLFIRI + anti-epidermal growth factor receptor antibodies after adjuvant folinic acid, fluorouracil, and oxaliplatin (FOLFOX)/capecitabine and oxaliplatin (CAPOX) therapy in Japan. |
This study shows that real-world treatment in Japan is consistent with Japanese and international guidelines. Both FOLFIRI + AA and FOLFIRI + anti-epidermal growth factor receptor antibody regimens had similar median time to treatment discontinuation, but the timing of relapse (≤6 months or 6–12 months) or tumor sidedness (left or right) may influence treatment efficacy. |
These real-world data will be useful to healthcare professionals in clinical practice. |
1 Introduction
Colorectal cancer (CRC) is one of the most common cancers globally, with over 1.9 million new CRC cases and 935,173 deaths reported in 2020 [1]. In Japan, CRC was the most common cancer with 148,505 new cases [2] and the second most common cause of mortality with 59,912 deaths in 2020 [2, 3]. Additionally, morbidity and mortality due to CRC has been projected to increase [3] despite availability of treatments for CRC [4]. In Japan, the recurrence rates after curative resection of CRC are 5.7%, 15.0%, and 31.8% for stage I, II, and III, respectively [5].
The National Comprehensive Cancer Network (NCCN) guideline recommends folinic acid, fluorouracil, and oxaliplatin (FOLFOX)/capecitabine and oxaliplatin (CAPOX) as the preferred adjuvant chemotherapy regimen, followed by capecitabine or fluorouracil/leucovorin [6]. Additional recommendations from the Japanese Society for Cancer of the Colon and Rectum (JSCCR) guidelines include use of uracil-tegafur/leucovorin or tegafur/gimeracil/oteracil (S-1) therapy (weak recommendation) [4]. After previous adjuvant FOLFOX/CAPOX within the past 12 months, the NCCN guidelines recommend folinic acid, fluorouracil, and irinotecan (FOLFIRI) with or without anti-angiogenesis drugs (AAs) [bevacizumab (BEV), ramucirumab (RAM), or aflibercept beta (AFL)] or anti-epidermal growth factor receptor (EGFR) antibodies (cetuximab [CET] and panitumumab [PANI]), as second-line (2L) therapy for recurrent CRC [6]. However, there is limited evidence to support these recommendations [7,8,9,10,11,12,13,14].
Additionally, no study describes the real-world utilization pattern of these treatments as 2L therapy in patients with early recurrence CRC. Considering the data gaps, it is essential to understand the current treatment patterns to provide optimal treatment options to patients with early recurrence CRC. Therefore, we conducted this study to describe the patient characteristics, treatment sequence, and overall duration of 2L therapy in patients with early recurrence CRC who received adjuvant chemotherapy (FOLFOX/CAPOX) followed by FOLFIRI + AAs or FOLFIRI + anti-EGFR antibodies. We also explored the factors associated with 2L therapy duration and analyzed sub-groups by timing of CRC recurrence and tumor sidedness.
2 Methods
2.1 Data Source
This retrospective cohort study analyzed data from the Medical Data Vision Co. Ltd. (MDV) database from April 2008 to March 2023. The MDV database comprises de-identified inpatient and outpatient administrative claims and Diagnosis Procedure Combination (DPC) data from acute care Japanese hospitals, i.e., hospitals treating severe illnesses for brief periods. The DPC is a case-mix classification system and is linked to a flat-fee payment system. As of March 2023, the MDV database included approximately 43.2 million patients from 475 acute-phase DPC hospitals (i.e., approximately 27% of DPC hospitals in Japan) [15]. Among these 475 hospitals, 236 were designated cancer care hospitals. These hospitals (classified by prefecture, community, or locality) are designated by the Japan Ministry of Health, Labour and Welfare. They provide specialized treatments and care to patients with cancer, co-ordinate and co-operate with each other, and also act as information centers for the patients [16].
This study was conducted in accordance with ethical principles originating from the Declaration of Helsinki and that were consistent with Good Pharmacoepidemiology Practices and the Japanese Ethical Guidelines for Medical and Health Research Involving Human Subjects [17]. Because of the retrospective nature of the study and use of de-identified data, ethical review by an institutional review board and informed consent from patients were not required.
2.2 Patient Population and Study Design
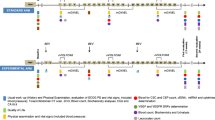
The study comprised patients aged ≥18 years at the index date with a confirmed CRC diagnosis (International Classification of Diseases, Tenth Revision codes C18–20); presence of CRC resection surgery and adjuvant therapy (FOLFOX/CAPOX, and other minor regimens recommended in the JSCCR guidelines [4] [Appendix 1 of the Electronic Supplementary Material (ESM)]) within 3 months after surgery during the study period (1 November, 2014 to 31 March, 2023 [Fig. 1]); and early CRC recurrence ≤12 months after the end of adjuvant chemotherapy. Early recurrence was defined as initiation of FOLFIRI + AAs or FOLFIRI + anti-EGFR antibodies for CRC as 2L therapy. November 2014 was selected as the start of the study period after considering the appropriate look-back and surgery time, before the approval of RAM for CRC in May 2016. Patients who initiated BEV, RAM, AFL, CET, or PANI within 3 months after CRC surgery or participated in a clinical trial after the index date were excluded from the analysis.
In this study, the index date was defined as the initiation date of the 2L therapy (FOLFIRI + AAs or anti-EGFR antibodies) within 12 months from the end of adjuvant therapy. Therapy started after the end of 2L was defined as third line (3L). The duration from 60 days prior to initiation of 2L (index date) to 1 day before the index date was defined as the baseline period.
2.3 Variables and Outcomes
The treatment sequence with CRC drugs as per JSCCR guidelines [4] (Appendix 1 of the ESM) for each patient was defined. Patients who received adjuvant therapies including FOLFOX/CAPOX and other minor regimens recommended in JSCCR guidelines were identified first. However, as FOLFOX/CAPOX are the major adjuvant therapies in Japan and/or globally, we defined patients who received FOLFOX/CAPOX as our study population and performed all detailed analyses on these patients hereafter.
All patient characteristics and hospital characteristics were analyzed at baseline. Anti-EGFR rechallenge, defined as re-introduction of anti-EGFR antibodies in patients who were initially responsive to 2L FOLFIRI + anti-EGFR antibodies, but developed progressive disease, and then switched to a non-anti-EGFR antibody regimen until disease progression [18], was also evaluated.
The adjuvant therapy regimen was defined as FOLFOX/CAPOX starting within 3 months after CRC resection. The start of 2L was defined as the initiation of FOLFIRI + AAs or anti-EGFR antibodies within 12 months from the end of adjuvant therapy. The 2L regimen included FOLFIRI + AAs or anti-EGFR antibodies prescribed within 28 days from the start of 2L. Regimens prescribed after the end of 2L and within 28 days from the start of 3L were defined as the 3L regimen. A line of therapy (LoT) for adjuvant therapy, 2L, and 3L was considered to have ended when the patient either terminated all the CRC drugs (Appendix 1 of the ESM) in the regimen for at least 180 days, or started a new CRC drug that was not included in the regimen, whichever occurred first. The end date of the LoT was the date of the last prescription + number of days of supply − 1 day, or 1 day before the next line starts, if such a line exists.
Time to treatment discontinuation (TTD) for 2L therapy was defined as the duration between the start and end dates of 2L. Patients were censored at the end date of the LoT if they had a <90-day interval between the end date of the LoT and the end of hospital data, without evidence of 3L. This was because such patients were still likely to be on treatment on the last visit.
2.4 Statistical Analysis
Treatment sequences were reported using Sankey charts. Characteristic data were reported descriptively, with continuous variables presented as mean, standard deviation, median, and range; and categorical variables presented as counts and percentages. Time to treatment discontinuation for 2L therapy was analyzed using the Kaplan–Meier method and factors associated with it were analyzed using a multivariable adjusted Cox proportional hazard model.
Subgroup analyses for 2L TTD were performed based on the timing of early CRC recurrence (recurrence ≤6 months and recurrence 6–12 months) and CRC sidedness at baseline (left or right). Sample selection and creation of analytic variables were performed using the Instant Health Data platform (Panalgo, Boston, MA, USA). All statistical analyses were conducted with R version 3.2.1 (R Foundation for Statistical Computing, Vienna, Austria) [19].
3 Results
3.1 Treatment Sequences
Of the 251,810 patients with a confirmed CRC diagnosis and CRC resection surgery in the database, 929 patients had CRC recurrence within 12 months after completion of adjuvant therapy and received 2L FOLFIRI + AAs or anti-EGFR antibodies (Fig. 2). Among them, 832 received FOLFOX/CAPOX as adjuvant therapy and 97 received other regimens in the adjuvant setting (Appendix 2 of the ESM). In the 832 patients, CAPOX (n = 593; 71.3%) was the more common adjuvant regimen than FOLFOX (n = 239; 28.7%) (Fig. 3). Among patients who received adjuvant FOLFOX/CAPOX, the most common 2L regimens were FOLFIRI + BEV (n = 483; 58.1%), FOLFIRI + PANI (n = 178; 21.4%), and FOLFIRI + RAM (n = 95; 11.4%) (Fig. 3). Among the 832 patients who received 2L, 519 (62.4%) received 3L therapy.
Treatment sequence in patients who received folinic acid, fluorouracil, and oxaliplatin/capecitabine and oxaliplatin (FOLFOX/CAPOX) adjuvant regimens. In third-line therapy (3L), all the regimens with five or fewer patients are combined as ‘Others’. AFL aflibercept beta; BEV bevacizumab; CAPE capecitabine; CET cetuximab; FOLFIRI folinic acid, fluorouracil, and irinotecan; FTD/TPI trifluridine/tipiracil; FU fluorouracil; Iri irinotecan; Oxali oxaliplatin; PANI panitumumab; RAM ramucirumab, Reg regorafenib; S1 tegafur/gimeracil/oteracil; UFT uracil-tegafur
3.2 Patient Characteristics at Baseline
Median (minimum–maximum) patient age at 2L initiation was 67 (24–86) years and 56.4% were male (Table 1). Most patients had left-sided CRC (65.5%) and liver or lung metastasis (54.8%). The most common comorbidities in the patients were hypertension (33.1%), diabetes mellitus (22.0%), liver diseases (20.4%), and neuropathy (18.0%). Most patients (76.3%) were treated in the designated cancer hospitals (76.3%), particularly in the surgery department (81.7%). Patient subgroups of recurrence within 6 months (n = 604) and in 6–12 months (n = 228) showed similar patient characteristics as the overall population. Patient characteristics by individual drugs are present in Appendix 3 of the ESM.
3.3 TTD of 2L
3.3.1 Overall Population
The median TTD (mTTD) for FOLFIRI + AAs and FOLFIRI + anti-EGFR antibodies treatment was 6.2 months (95% confidence interval [CI] 5.8, 6.9) and 6.1 months (95% CI 5.2, 7.4), respectively (Fig. 4). Individual AAs or anti-EGFR antibodies had similar mTTD (Appendix 4a and 4b of the ESM). In the study population, age ≥70 years was significantly associated with shorter 2L TTD (hazard ratio 1.2, 95% CI 1.0, 1.4; p = 0.03) whereas other factors (i.e., timing of CRC recurrence and CRC sidedness) were not significantly related with TTD (Appendix 5 of the ESM).
3.3.2 Timing of CRC Recurrence Subgroup
The mTTD for patients with early CRC recurrence in the ≤6 months subgroup was 6.1 months for FOLFIRI + AAs (95% CI 5.5, 6.5) and 5.8 months for FOLFIRI + anti-EGFR antibodies (95% CI 5.1, 7.8) (Fig. 5a). Among patients with early CRC recurrence in the 6–12 months subgroup, the mTTD was 8.1 months for FOLFIRI + AAs (95% CI 6.1, 9.2) and 6.2 months for FOLFIRI + anti-EGFR antibodies (95% CI 4.6, 8.0), respectively (Fig. 5b); thus showing a longer trend than the ≤6 months subgroup.
Time to treatment discontinuation of second-line therapy (2L) by timing of early recurrence. (a) and (b) show the time to treatment discontinuation of second-line therapy (2L) in patients with early recurrence <6 months and 6–12 months, respectively. AA anti-angiogenesis drugs; CI confidence interval; EGFR epidermal growth factor receptor
3.3.3 CRC Sidedness Subgroup
In patients with left-sided CRC, the mTTD was similar for FOLFIRI + AAs (6.5 months [95% CI 6.1, 7.6]) and FOLFIRI + anti-EGFR antibodies (6.2 months [95% CI 5.3, 7.8]) [Appendix 6a of the ESM]. In patients with right-sided CRC, the mTTD was numerically longer for FOLFIRI + AAs (5.6 months [95% CI 4.8, 6.7];) compared with FOLFIRI + anti-EGFR antibodies (3.9 months [95% CI 2.1, 7.4]) [Appendix 6b of the ESM].
4 Discussion
Although existing guidelines recommend FOLFOX/CAPOX as the preferred adjuvant regimen [4, 6], sufficient evidence on the optimal treatment sequences and outcomes in patients with early recurrence CRC after adjuvant chemotherapy is unavailable. The current study is the first real-world evidence of treatment patterns for patients with early recurrence CRC after FOLFOX/CAPOX adjuvant therapy and treated with FOLFIRI + AAs or FOLFIRI + anti-EGFR antibodies, and also the first real-world study in the patient population with no prior molecular targeted therapies. This real-world evidence would be useful to healthcare professionals and has important reference value in clinical practice. In line with NCCN guidelines [6], the current study defined early CRC recurrence as recurrence ≤12 months after adjuvant FOLFOX/CAPOX. We observed that real-world treatment of patients with early recurrence CRC in Japan is consistent with Japanese [4] and international [6] guidelines. FOLFOX/CAPOX were the most common regimens in the adjuvant setting and FOLFIRI + AAs or FOLFIRI + anti-EGFR antibodies were commonly used in 2L after oxaliplatin-containing adjuvant regimens to treat early recurrence CRC.
As a direct comparison of different studies may cause bias, and the definition for TTD and progression-free survival (PFS) varies, TTD can only be used as a reference for PFS. Although the population is different, the mTTD for FOLFIRI + AA reported in our study was similar to the 2L median PFS (mPFS) reported in global phase III studies with AAs, including the RAISE trial (RAM + FOLFIRI) [9], ML18147 trial (BEV + chemotherapy) [7], or VELOUR trial (AFL + FOLFIRI) [14]. However, it is important to note that unlike these phase III trials, patients did not receive prior molecular targeted therapies in our study. Additionally, in the phase II RAINCLOUD [20] trial that evaluated the efficacy of RAM + FOLFIRI in patients with early recurrence CRC who were pre-treated with FOLFOX/CAPOX without AAs, the mPFS was 6.2 months, which is also similar to the mTTD in our study.
We expect anti-EGFR antibodies were mainly used for patients with RAS/BRAF wild-type CRC in the current study. However, we cannot compare our results with other studies as no clinical trials specifically evaluated the clinical outcomes of FOLFIRI + anti-EGFR antibody use in early recurrence CRC. Nevertheless, the mTTD in the current study was similar to the mPFS observed in clinical trials evaluating 2L CET [21, 22] or PANI [23]. It is also important to note that patients with early recurrence, i.e., within a year of adjuvant therapy, usually had not received targeted treatment in the past. Limited 2L data exist for such patients but the mPFS of such patients from trials evaluating AFL + FOLFIRI [14] and irinotecan + S-1 [24] is similar to the mTTD of the current study.
Both NCCN [6] and JSCCR [4] guidelines recommend anti-EGFR antibody usage for patients with RAS/BRAF wild-type and left-sided tumors only. In the current study, among 229 patients treated with FOLFIRI + anti-EGFR antibodies, the majority had left-sided CRC (n = 196; 85.6%), which is in line with the NCCN and JSCCR guidelines. In the current study, 2L mTTD was similar in AA-treated or anti-EGFR antibody-treated patients with left-sided CRC (6.5 months and 6.2 months), but anti-EGFR antibody-treated patients with right-sided CRC had numerically shorter mTTD than those treated with AAs (3.9 months vs 5.6 months). Similar patterns were also observed in the PARADIGM study that evaluated panitumumab versus bevacizumab, known to be the standard first-line chemotherapy among patients with RAS wild-type metastatic CRC. The mPFS were similar for panitumumab versus bevacizumab (13.1 vs 11.9 months, hazard ratio 1.00, 95% CI 0.83, 1.20) for those with left-sided tumors. However, the mPFS was shorter in the panitumumab group (7.2 months) compared with the bevacizumab group (9.4 months) for right-sided tumors (hazard ratio 1.43, 95% CI 1.03–1.97) [25]. In line with guidelines [4, 6], our study results also suggest that FOLFIRI + AA may be more suitable than FOLFIRI + anti-EGFR antibodies for patients with right-sided CRC; however, further studies are needed, especially for early recurrence CRC.
The mTTD of FOLFIRI + AA and FOLFIRI + anti-EGFR antibodies were 6.1 months and 5.8 months in patients with CRC recurrence in ≤6 months, and 8.1 months and 6.2 months in patients with CRC recurrence in 6–12 months. These results suggest that the timing of relapse may be associated with the efficacy of AAs and anti-EGFR antibodies, but no statistically significant difference was observed in the Cox regression analysis.
Consistent with previous research [26], age ≥70 years was significantly associated with shorter 2L TTD in our study. This could be attributed to the higher proportion of right-sided tumors (age ≥70 years: 36.6% vs age <70 years: 28.0%) and comorbidities at baseline (hypertension [age ≥70 years: 43.3% vs age <70 years: 26.8%] and diabetes [age ≥70 years: 25.8% vs age <70 years: 19.7%]), thus making treatment risky [27]. Hence, treatment of early CRC recurrence in elderly patients requires a personalized treatment approach by taking into consideration their physical fitness, comorbidities, tolerability to side effects, tumor sidedness, and patient preferences [27, 28].
In this study, transition rate from 2L to 3L was 62.4%, which is similar to that of an early recurrence CRC population subset from Japan (63.7%) [26]. However, it is higher than the transition rate (from 2L to 3L) reported among patients with metastatic CRC in previous real-world studies from Canada, China, Japan, and the USA (31.5–55.5%) [29,30,31,32], probably owing to the difference in study populations, i.e., only patients with early recurrence CRC in the current study versus all patients with metastatic CRC in the previous studies. Nevertheless, our results suggest that many patients do not undergo post-progression therapy, thus implying the existence of unmet patient needs, as post-progression therapy may improve survival [33].
4.1 Strengths and Limitations
A major strength of this study is its novelty and use of the MDV database, which helped acquire real-world treatment practice data from many patients with CRC in Japan. This is especially relevant for patients who are under-represented in clinical trials, such as patients with early recurrence CRC after adjuvant chemotherapy. Nevertheless, this study has a few limitations. Although the MDV database is extensive, patients who received treatment in small medical centers were not included because of the nature of the database. Additionally, patients who underwent treatment at multiple hospitals may have been counted multiple times as the MDV database cannot track patients between hospitals. The database does not cover all clinical information, biomarker data, and treatment outcomes. Therefore, we could not investigate treatment patterns associated with CRC stage and biomarkers (e.g., RAS status) and analyze PFS or survival outcomes in detail. The study population included patients who received AAs or anti-EGFR antibodies in 2L, thus patients who may have received systemic therapies other than biologics were excluded. Hence, the results of this study may not be generalizable to all patients with CRC in Japan.
5 Conclusions
This is the first administrative, data-based, real-world evidence on treatment sequence and outcomes for patients with early recurrence CRC treated with FOLFIRI + AAs or FOLFIRI + anti-EGFR antibodies after adjuvant FOLFOX/CAPOX therapy in Japan. The treatment outcomes observed for patients with early recurrence CRC were similar to those from previous clinical trials in 2L advanced CRC. Both FOLFIRI + AAs and FOLFIRI + anti-EGFR antibodies for 2L regimens had similar TTD; however, the timing of relapse and tumor sidedness may influence the efficacy of these regimens.
References
International Agency for Research on Cancer. Colorectal cancer. World Health Organization; 2021. https://gco.iarc.fr/today/data/factsheets/cancers/10_8_9-Colorectum-fact-sheet.pdf. Accessed 16 Apr 2024.
International Agency for Research on Cancer. Japan fact sheet. The Global Cancer Observatory; 2021. https://gco.iarc.fr/today/data/factsheets/populations/392-japan-fact-sheets.pdf. Accessed 16 Apr 2024.
Xi Y, Xu P. Global colorectal cancer burden in 2020 and projections to 2040. Transl Oncol. 2021;14: 101174.
Hashiguchi Y, Muro K, Saito Y, Ito Y, Ajioka Y, Hamaguchi T, et al. Japanese Society for Cancer of the Colon and Rectum (JSCCR) guidelines 2019 for the treatment of colorectal cancer. Int J Clin Oncol. 2020;25:1–42.
Ahiko Y, Shida D, Kudose Y, Nakamura Y, Moritani K, Yamauchi S, et al. Recurrence hazard of rectal cancer compared with colon cancer by adjuvant chemotherapy status: a nationwide study in Japan. J Gastroenterol. 2021;56:371–81.
NCCN clinical practice guidelines in oncology (NCCN Guidelines®). Colon cancer. National Comprehensive Cancer Network; 2023. https://www.nccn.org/professionals/physician_gls/pdf/colon.pdf. Accessed 16 Apr 2024.
Bennouna J, Sastre J, Arnold D, Osterlund P, Greil R, Van Cutsem E, et al. Continuation of bevacizumab after first progression in metastatic colorectal cancer (ML18147): a randomised phase 3 trial. Lancet Oncol. 2013;14:29–37.
Iwamoto S, Takahashi T, Tamagawa H, Nakamura M, Munemoto Y, Kato T, et al. FOLFIRI plus bevacizumab as second-line therapy in patients with metastatic colorectal cancer after first-line bevacizumab plus oxaliplatin-based therapy: the randomized phase III EAGLE study. Ann Oncol. 2015;26:1427–33.
Tabernero J, Yoshino T, Cohn AL, Obermannova R, Bodoky G, Garcia-Carbonero R, et al. Ramucirumab versus placebo in combination with second-line FOLFIRI in patients with metastatic colorectal carcinoma that progressed during or after first-line therapy with bevacizumab, oxaliplatin, and a fluoropyrimidine (RAISE): a randomised, double-blind, multicentre, phase 3 study. Lancet Oncol. 2015;16:499–508.
Van Cutsem E, Tabernero J, Lakomy R, Prenen H, Prausova J, Macarulla T, et al. Addition of aflibercept to fluorouracil, leucovorin, and irinotecan improves survival in a phase III randomized trial in patients with metastatic colorectal cancer previously treated with an oxaliplatin-based regimen. J Clin Oncol. 2012;30:3499–506.
Peeters M, Price TJ, Cervantes A, Sobrero AF, Ducreux M, Hotko Y, et al. Randomized phase III study of panitumumab with fluorouracil, leucovorin, and irinotecan (FOLFIRI) compared with FOLFIRI alone as second-line treatment in patients with metastatic colorectal cancer. J Clin Oncol. 2010;28:4706–13.
Shitara K, Yonesaka K, Denda T, Yamazaki K, Moriwaki T, Tsuda M, et al. Randomized study of FOLFIRI plus either panitumumab or bevacizumab for wild-type KRAS colorectal cancer-WJOG 6210G. Cancer Sci. 2016;107:1843–50.
Giantonio BJ, Catalano PJ, Meropol NJ, O’Dwyer PJ, Mitchell EP, Alberts SR, et al. Bevacizumab in combination with oxaliplatin, fluorouracil, and leucovorin (FOLFOX4) for previously treated metastatic colorectal cancer: results from the Eastern Cooperative Oncology Group Study E3200. J Clin Oncol. 2007;25:1539–44.
Tabernero J, Van Cutsem E, Lakomy R, Prausova J, Ruff P, van Hazel GA, et al. Aflibercept versus placebo in combination with fluorouracil, leucovorin and irinotecan in the treatment of previously treated metastatic colorectal cancer: prespecified subgroup analyses from the VELOUR trial. Eur J Cancer. 2014;50:320–31.
Medical database overview (as of the end of March 2023). Number of actual patients: 43.22 million [Japanese]. Medical Data Vision; 2023. https://www.mdv.co.jp/press/2023/detail_1979.html. Accessed 16 Apr 2024.
Cancer Statistics in Japan: 2023. Foundation for promotion of cancer research; 2023. https://ganjoho.jp/public/qa_links/report/statistics/pdf/cancer_statistics_2023.pdf. Accessed 16 Apr 2024.
Ethical guidelines for medical and health research involving human subjects. Tokyo: Ministry of Education, Culture, Sports, Science and Technology, Ministry of Health, Labour and Welfare; 2018. https://www.lifescience.mext.go.jp/files/pdf/n2181_01.pdf. Accessed 16 Apr 2024.
Cremolini C, Montagut C, Ronga P, Venturini F, Yamaguchi K, Stintzing S, et al. Rechallenge with anti-EGFR therapy to extend the continuum of care in patients with metastatic colorectal cancer. Front Oncol. 2022;12: 946850.
R Core Team. R: a language and environment for statistical computing, 3.2.1 edn. Vienna: R Foundation for Statistical Computing; 2021.
Sugimoto N, Nakata K, Miyo M, Yoshioka S, Kagawa Y, Naito A, et al. P-76 Phase II study of FOLFIRI plus ramucirumab with recurrent colorectal cancer refractory to adjuvant chemotherapy with oxaliplatin/fluoropyrimidine (RAINCLOUD). Ann Oncol. 2022;33:S274.
Iwamoto S, Hazama S, Kato T, Miyake Y, Fukunaga M, Matsuda C, et al. Multicenter phase II study of second-line cetuximab plus folinic acid/5-fluorouracil/irinotecan (FOLFIRI) in KRAS wild-type metastatic colorectal cancer: the FLIER study. Anticancer Res. 2014;34:1967–73.
Passardi A, Scarpi E, Gelsomino F, Palladino MA, Casadei Gardini A, Turci D, et al. Impact of second-line cetuximab-containing therapy in patients with KRAS wild-type metastatic colorectal cancer: results from the ITACa randomized clinical trial. Sci Rep. 2017;7:10426.
Peeters M, Price TJ, Cervantes A, Sobrero AF, Ducreux M, Hotko Y, et al. Final results from a randomized phase 3 study of FOLFIRI ± panitumumab for second-line treatment of metastatic colorectal cancer. Ann Oncol. 2014;25:107–16.
Muro K, Boku N, Shimada Y, Tsuji A, Sameshima S, Baba H, et al. Irinotecan plus S-1 (IRIS) versus fluorouracil and folinic acid plus irinotecan (FOLFIRI) as second-line chemotherapy for metastatic colorectal cancer: a randomised phase 2/3 non-inferiority study (FIRIS study). Lancet Oncol. 2010;11:853–60.
Watanabe J, Muro K, Shitara K, Yamazaki K, Shiozawa M, Ohori H, et al. Panitumumab vs bevacizumab added to standard first-line chemotherapy and overall survival among patients with RAS wild-type, left-sided metastatic colorectal cancer: a randomized clinical trial. JAMA. 2023;329:1271–82.
Shinozaki E, Makiyama A, Kagawa Y, Satake H, Tanizawa Y, Cai Z, et al. Treatment sequences of patients with advanced colorectal cancer and use of second-line FOLFIRI with antiangiogenic drugs in Japan: a retrospective observational study using an administrative database. PLoS ONE. 2021;16: e0246160.
Itatani Y, Kawada K, Sakai Y. Treatment of elderly patients with colorectal cancer. Biomed Res Int. 2018;2018:2176056.
Millan M, Merino S, Caro A, Feliu F, Escuder J, Francesch T. Treatment of colorectal cancer in the elderly. World J Gastrointest Oncol. 2015;7:204–20.
McLean J, Rho YS, Kuruba G, Mamo A, Gilabert M, Kavan T, et al. Clinical practice patterns in chemotherapeutic treatment regimens for metastatic colorectal cancer. Clin Colorectal Cancer. 2016;15:135–40.
Xu R, Wang W, Zhu B, Lin X, Ma D, Zhu L, et al. Disease characteristics and treatment patterns of Chinese patients with metastatic colorectal cancer: a retrospective study using medical records from China. BMC Cancer. 2020;20:131.
Satake H, Kagawa Y, Shinozaki E, Tanizawa Y, Jin L, Cai Z, et al. Real-world data analysis of second-line antiangiogenic targeted treatments following anti-epidermal growth factor receptor monoclonal antibodies and first-line FOLFOX for patients with metastatic colorectal cancer. Adv Ther. 2022;39:2596–613.
Parikh RC, Du XL, Morgan RO, Lairson DR. Patterns of treatment sequences in chemotherapy and targeted biologics for metastatic colorectal cancer: findings from a large community-based cohort of elderly patients. Drugs Real World Outcomes. 2016;3:69–82.
Petrelli F, Barni S. Correlation of progression-free and post-progression survival with overall survival in advanced colorectal cancer. Ann Oncol. 2013;24:186–92.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This study was funded by Eli Lilly Japan K.K. Leo J. Philip Tharappel of Eli Lilly Services India Private Limited, Bengaluru, India provided medical writing and editorial support, which was funded by Eli Lilly Japan K.K.
Conflicts of Interest
Yoshinori Kagawa received honoraria from Bayer, Chugai, Eli Lilly Japan K.K., Merck, MSD, Ono, Sanofi, Taiho, Takeda, and Yakult. Chaochen Wang, Yongzhe Piao, Long Jin, Yoshinori Tanizawa, and Zhihong Cai are employees of Eli Lilly Japan K.K. and may hold stock and/or stock options in the company. Yu Sunakawa or his institution received grants or contracts from Chugai, Eli Lilly Japan K.K., Ohtsuka Pharmaceutical, Sanofi, Taiho, and Takeda. He received payments or honoraria from Bayer, Bristol-Myers Squibb, Chugai, Daiichi-Sankyo, Eli Lilly Japan K.K., Guardant Health, Merck Biopharma, MSD, Ono, Sysmex, Taiho, and Takeda. He also participated on an advisory board for Guardant Health, Merck Biopharma, and Ono.
Ethics Approval
This study was conducted in accordance with ethical principles originating from the Declaration of Helsinki and that were consistent with Good Pharmacoepidemiology Practices. This study used retrospective de-identified data so an ethical review and informed consent were not required, consistent with the Japanese Ethical Guidelines for Medical and Health Research Involving Human Subjects.
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Availability of Data and Material
The datasets generated and/or analyzed during the current study are available at Eli Lilly Japan K.K on reasonable request.
Code Availability
Not applicable.
Authors’ Contributions
Conception and design: CW, LJ, YK, YS. Collection and assembly of data: data analysis and/or interpretation: YK, CW, YP, LJ, YT, ZC, YS. Manuscript writing: YK, YS. Final approval of manuscript: YK, CW, YP, LJ, YT, ZC, YS. Accountable for all aspects of the work: YK, CW, YP, LJ, YT, ZC, YS.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Kagawa, Y., Wang, C., Piao, Y. et al. Real-World Evidence of FOLFIRI Combined with Anti-Angiogenesis Inhibitors or Anti-EGFR Antibodies for Patients with Early Recurrence Colorectal Cancer After Adjuvant FOLFOX/CAPOX Therapy: A Japanese Claims Database Study. Targ Oncol (2024). https://doi.org/10.1007/s11523-024-01063-y
Accepted:
Published:
DOI: https://doi.org/10.1007/s11523-024-01063-y