Abstract
Background
There are few molecular markers driving treatment selection in later lines of treatment for advanced colorectal cancer patients. The vast majority of patients who progress after first- and second-line therapy undergo chemotherapy regardless of molecular data.
Objective
We aimed to assess the prognostic and predictive effects of specific RAS mutations on overall survival of patients receiving regorafenib (rego), trifluridine/tipiracil (TFD/TPI), or both.
Patients and methods
This was a retrospective observational study based on data from a previous study of our research network, involving nine Italian institutions over a 10-year timeframe (2012–2022). Extended RAS analysis, involving KRAS exon 2–4 and NRAS exon 2–4, and BRAF were the main criteria for inclusion in this retrospective evaluation. Patients with BRAF mutation were excluded. Patients were classified according to treatment (rego- or TFD/TPI-treated) and RAS mutational status (wild-type [WT], KRAS codon 12 mutations, KRAS codon 13 mutations, KRAS rare mutations and NRAS mutations, KRAS G12C mutation and KRAS G12D mutation).
Results
Overall, 582 patients were included in the present analysis. Overall survival did not significantly differ in rego-treated patients according to RAS extended analysis, although a trend toward a better median survival in patients carrying G12D mutation (12.0 months), Codon 13 mutation (8.0 months), and Codon 12 mutation (7.0 months) has been observed, when compared with WT patients (6.0 months). Overall survival did not significantly differ in TFD/TPI-treated patients according to RAS extended analysis, although a trend toward a better median survival in WT patients had been observed (9.0 months) in comparison with the entire population (7.0 months). Patients receiving both drugs displayed a longer survival when compared with the population of patients receiving rego alone (p = 0.005) as well as the population receiving TFD/TPI alone (p < 0.001), suggesting a group enriched for favorable prognostic factors. However, when each group was analyzed separately, the addition of TFD/TPI therapy to the rego-treated group improved survival only in all-RAS WT patients (p = 0.003). Differently, the addition of rego therapy to TFD/TPI-treated patients significantly improved OS in the Codon 12 group (p = 0.0004), G12D group (p = 0.003), and the rare mutations group (p = 0.02), in addition to all-RAS WT patients (p = 0.002). The rego-TFD/TPI sequence, compared with the reverse sequence, significantly improved OS only in the KRAS codon 12 group (p = 0.003).
Conclusions
Our data demonstrate that RAS mutations do not affect outcome in rego-treated patients as well as TFD/TPI-treated patients. Nevertheless, a trend toward a higher efficacy of rego in RAS-mutated (in particular codon 12, rare RAS mutations, and G12D) patients has been recorded. The rego-TFD/TPI sequence seems to be superior to the reverse sequence in patients carrying an RAS codon 12 mutation, although the impact of other factors as disease burden or performance status cannot be excluded.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
There are few molecular markers driving treatment selection in later lines of advanced colorectal cancer patients, despite a growing commitment toward molecular characterization aimed at treatment personalization. |
Regorafenib, trifluridine/tipiracil, fruquintinib, and the combination of trifluridine/tipiracil (TFD/TPI) with bevacizumab demonstrated effectiveness in third-/fourth-line of therapy in randomized, phase III studies, irrespective of molecular characterization. |
The vast majority of patients progressing after first- and second-line therapy undergo chemotherapy regardless of molecular data. |
We aimed to assess the prognostic and predictive effects of specific RAS mutations on overall survival of patients receiving regorafenib, trifluridine/tipiracil, or both. |
1 Introduction
Despite a growing commitment toward precision medicine, there are few molecular markers driving treatment selection in later lines of treatment for advanced colorectal cancer (CRC) patients [1]. A small percentage of patients (6–7%) who carry a clinically actionable BRAFV600E mutation are eligible for targeted therapy with a combination of encorafenib and cetuximab [2]. Patients with microsatellite instable tumors (about 5%) are eligible for immune checkpoint inhibitors [3, 4]. Moreover, small molecule inhibitors (sotorasib and adagrasib) showed activity in KRAS G12C-mutated CRCs (3–4%), in monotherapy or in combination with anti-epidermal growth factor receptor (EGFR) agents [5, 6]. Regorafenib (rego), trifluridine/tipiracil (TFD/TPI), fruquintinib, and the combination of TFD/TPI with bevacizumab demonstrated effectiveness in third-/fourth-line of therapy in randomized, phase III studies [7,8,9,10], irrespective of molecular characteristics. Therefore, the vast majority of patients with an advanced CRC progressing after first- and second-line therapy will be treated regardless of molecular data.
In Italy, as in many Western Countries, the combination of TFD/TPI and bevacizumab and fruquintinib are not yet available in clinical practice. For this reason, most patients receive rego or TFDD/TPI based on physician’s judgment. Many studies attempted to demonstrate the superiority of one drug over the other [11,12,13,14,15], but no significant differences have been documented in terms of efficacy. On the other hand, the two drugs differ in their adverse effects. Rego displays more symptomatic adverse events such as asthenia, hand-foot syndrome, and skin reactions, while TFD/TPI-related adverse effects are generally more asymptomatic (neutropenia, anemia) [16]. Therefore, TFD/TPI is more often prescribed in older patients or in patients with worse performance status [16, 17].
A previous publication from our research network [17] aimed to assess the outcomes in a large retrospective series of metastatic CRC (mCRC) patients treated with rego, TFD/TPI, or both, in late lines. Even in our study, overall survival (OS) displayed no significant differences between patients receiving rego or TFD/TPI. A subgroup analysis in patients treated with the rego-TFD/TPI sequence showed a significantly higher OS when compared with patients receiving the reverse sequence. At multivariate analysis, better performance status, male sex, lower disease burden (liver metastases only) and wild-type (WT) RAS status were the factors affecting OS.
Recently, KRAS G12 mutations have been reported to be associated with reduced OS in patients with mCRC treated with TFD/TPI [18]. This evidence was obtained in a discovery cohort and replicated in a large real-world cohort and in a retrospective analysis of RECOURSE patients [7].
Unfortunately, there are no studies properly aimed at assessing the efficacy of rego according to RAS mutational status. The RAS gene is the most commonly mutated oncogene in CRC, occurs in 45% of cases, and its mutations result in a constitutive activation of RAS protein [1, 8]. Rego is a multikinase inhibitor that blocks the activity of several protein kinases, including those involved in the downstream signaling cascade of EGFR.
Thus, the current analysis was mainly designed to assess the prognostic and predictive effects of specific RAS mutations on patients with refractory mCRC receiving rego, TFD/TPI, or both, hypothesizing that rego could work better in patients carrying RAS mutation(s). This kind of information could help physicians to select treatments in later lines, based on efficacy data in addition to the pattern of adverse effects.
2 Patients and Methods
The present analyses are based on data from a previous study of our research network, which was presented in a recent publication [17]. Nine Italian institutions contributed to this 10-year (2012–2022) retrospective observational study. The study was approved by the Ethics Committee (2022-no.1021/CE Lazio 1) and was carried out in conformity with the Declaration of Helsinki. To protect sensitive information, all data were anonymized and patients were identified only by their initials and a number. In accordance with the law, the lead investigator served as the data manager and had access to the complete database.
2.1 Patients
Criteria for study inclusion have been extensively described in the former publication [17]. Extended RAS analysis, involving KRAS exon 2–4 and NRAS exon 2–4, and BRAF was the main criteria for inclusion in this retrospective evaluation. Patients with BRAF mutation were excluded. If data about codon mutation were available, even in the absence of specific information concerning amino acid substitution, patients were considered eligible. Patients were considered WT if no mutation involving KRAS exon 2–4, NRAS exon 2–4, and BRAF was detected. Briefly, inclusion criteria were histologically confirmed stage IV adenocarcinoma of the colon or rectum with unresectable metastatic disease; age >18 years; Eastern Cooperative Oncology Group performance status (ECOG PS) 0–2; progression to at least two prior regimens of standard chemotherapy using fluoropyrimidine, irinotecan, oxaliplatin, anti-vascular endothelial growth factor (VEGF) antibodies (bevacizumab and aflibercept), or anti-EGFR antibodies (cetuximab or panitumumab); known RAS mutation status; adequate organ function at the start of treatment.
2.2 Treatment Details
Study details have been extensively described in the former publication [17]. Briefly, TFD/TPI was administered orally twice daily at a dose of 35 mg/m2 on days 1–5 and 8–12 of a 28-day cycle [7], and rego was administered at a standard dose of 160 mg once daily for 21 days of a 28-day cycle. The ReDos dose-escalation strategy of rego (starting dose 80 mg/day orally with weekly escalation, per 40 mg increment, to 160 mg/day, permitted if there were no significant drug-related adverse events) was used according to the physician decision [8, 19]. Eligible patients were deemed to be treated until disease progression or unacceptable toxicity.
2.3 Endpoints and Statistical Analysis
The objective of the study was to investigate the prognostic and predictive effect of specific RAS mutations on patients with refractory CRC receiving rego, TFD/TPI, or both. OS was defined as the interval time between the start of treatment (rego or TFD/TPI) and death from any cause, or last follow-up in the case of patients lost to follow-up. Time to progression (TTP) was defined as the interval time between the start of treatment and disease progression (clinical, radiological, or both) or death. Patients not experiencing an event were censored at the time of last follow-up. Analyses were based on aggregated retrospective data comparing outcome by RAS extended analysis (provided by the investigators). Patients were classified according to treatment in two groups (rego- or TFD/TPI-treated) and stratified in six groups according to RAS mutational status: (1) patients with tumor harboring no mutations involving KRAS exon 2–4 and NRAS exon 2–4 (WT); (2) patients with tumor harboring a mutation involving KRAS codon 12 (KRAS codon 12); (3) patients with tumor harboring a mutation involving codon 13 (KRAS codon 13); (4) patients with tumor harboring a rare KRAS mutation or an NRAS mutation (rare mutations); (5) patients with tumor harboring a KRAS G12C mutation (KRAS G12C); (6) patients with tumor harboring a KRAS G12D mutation (KRAS G12D). Patients carrying G12C or G12D mutations were considered distinctly, since these two were the only mutations for which a targeted treatment is available [5, 6, 20].
The pertinent data were compiled using descriptive statistics. Possible relationships were assessed using the Chi-square and Fisher’s exact tests. The Kaplan–Meier product limit approach was used to compute both progression-free survival (PFS) and OS, and the log-rank test was used to evaluate differences between subgroups. Significance was established at p = 0.05. All of the statistical analyses were performed using MedCalc Statistical software, version 14.8.1 (MedCalc Software, Ostend Belgium).
3 Results
3.1 Patients
Overall, 866 patients were included in the former study [17], of whom 10 (1.1%) carried a BRAF mutation and were excluded from further analysis. For 274 patients (31.6%), data about RAS extended analysis were not available and thus these patients were excluded from the present study. Overall, 582 patients were included in the present analysis (Online Resource Table 1), of whom 270 (46.6%) were WT, 212 (36.4%) carried a codon 12 KRAS mutation, 49 (8.4%) carried a codon 13 mutation, and 51 (8.7%) carried other KRAS rare mutations or NRAS mutations. Among codon 12 KRAS mutations, the G12D mutation was the most frequent. Details on detected mutations of our population are summarized in Table 1; patient characteristics are summarized in Table 2 and Table 3. In particular, when considering rego-treated patients (Table 2), characteristics were well-balanced between groups, with the exception of number of previous lines of therapy (>4 lines was less frequent in the G12C and G12D groups) and previous administered drugs, since anti-EGFRs were restricted to WT patients. About 50% of patients in each group received TFP/TPI in addition to rego (before or after, with a well-balanced distribution). Even for TFD/TPI-treated patients, characteristics were well-balanced between groups, with the exception of number of previous lines of therapy (>4 lines was less frequent in the G12C and G12D groups) and previously administered drugs, since anti-EGFRs were restricted to all WT patients. About 50% of patients in each group received rego in addition to FTD/TPI (before or after, with a well-balanced distribution).
3.2 Efficacy Outcome According to RAS Extended Analysis in Regorafenib-Treated Patients
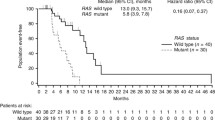
OS did not significantly differ in rego-treated patients according to RAS extended analysis (p = 0.69) (Fig. 1), although a trend toward a better median survival in patients carrying G12D mutation (12.0 months), Codon 13 mutation (8.0 months) and Codon 12 mutation (7.0 months) has been observed, when compared with WT patients (6.0 months). Accordingly, TTP did not differ significantly in rego-treated patients according to RAS extended analysis (p = 0.71) (Online Resource Fig. 1). However, a non-statistically significant benefit was observed in patients carrying a Codon 12 mutation (3.6 months), a rare mutation (3.9 months), or a G12C mutation (4.5 months), when compared with WT patients (3.0 months).
Patients receiving TFD/TPI (before or after, 184 patients) in addition to rego showed a statistically significant gain in OS compared with those not exposed to TFD/TPI (9 vs. 5 months; p = 0.005) (Online Resource Fig. 2a), suggesting an enrichment for favorable prognostic factors. When focusing on rego-treated patients who were exposed to TFD/TPI, a trend toward statistical significance was observed for WT patients (10 months), Codon 13 (12 months), and G12D (14 months; p = 0.05) (Online Resource Fig. 2b).
3.3 Efficacy Outcome According to RAS Extended Analysis in Trifluridine/Tipiracil-Treated Patients
OS did not significantly differ in TFD/TPI-treated patients according to RAS extended analysis (p = 0.09) (Fig. 2), although trend toward a better median survival was observed in WT patients (9 months) in comparison with the whole population (7.0 months). Accordingly, TTP did not significantly differ in TFD/TPI-treated patients according to RAS extended analysis (p = 0.86) (Online Resource Fig. 3). However, a non-statistically significant trend was observed in favor of WT patients (4.0 months) when compared with the whole population (3.7 months).
Patients receiving rego in addition to TFD/TPI (before or after, 188 patients) showed a statistically significant gain in OS when compared with those not exposed to rego (10 vs. 5 months; p < 0.001) (Online Resource Fig. 4a), suggesting an enrichment for favorable prognostic factors. According to RAS extended analysis, a survival gain was equally distributed among groups, without statistically significant differences (Online Resource Fig. 4b).
3.4 Efficacy Outcome According to Each Subgroup
Since no differences concerning efficacy of rego and TFD/TPI according to RAS extended analysis were documented, we analyzed each subgroup separately. These analyses are to be considered exploratory.
Patient characteristics were well-balanced in each population. Statistically significant differences in previously administered drugs for WT, Codon 12, and G12D patients were registered, with missing data being significantly more frequent for the rego-treated population, likely due to the issue that some patients in the rego group had been treated previously. Details of the comparison of patient characteristics in each group have been reported in Online Resource Table 2.
No differences in terms of the response rate were registered between the rego-treated population and the TFD/TPI-treated population in each RAS subgroup. Details about response rate have been reported in Online Resource Table 3. Moreover, concerning dose intensity, dose reductions were more frequent in rego-treated patients (compared with TFD/TPI patients), with statistically significant differences in WT, Codon 12 and Rare subgroups. Details on dose reductions have been reported in Online Resource Table 4, and details of comparison of patient characteristics according to the treatment sequence in each group have been reported in Online Resource Table 5.
Efficacy outcome in the WT group: In WT patients, median rego-related OS was significantly longer in patients receiving TFD/TPI (overall 79 patients [45.0%]; 46 before, 33 after) in addition to rego (p = 0.003) (Fig. 3a). At univariate analysis, including age, sex, number of previous lines, synchronous disease, PS, and sites of disease, no statistically significant differences in terms of OS were observed, with the exception of patients with PS <2 (p = 0.001) and patients with a synchronous disease (p = 0.002). Multivariate analysis was not performed because of the low sample size.
At the same time, median TFD/TPI-related OS was significantly longer in patients receiving rego (overall 80 patients [45.1%]; 32 before, 48 after) in addition to TFD/TPI (p = 0.002) (Fig. 3b). At univariate analysis, including age, sex, number of previous lines, synchronous disease, PS, and sites of disease, no statistically significant differences in terms of OS were observed.
When focusing on the treatment administration sequence (comparing patients who received rego followed by TFD/TPI or the reverse sequence), no statistically significant differences were documented (p = 0.13) (Online Resource Fig. 5).
Efficacy outcome in the Codon 12 group: In Codon 12-mutated patients, median rego-related OS showed no differences between patients receiving TFD/TPI (overall 47 patients [52.8%]; 26 before, 21 after) in addition to rego (p = 0.16) (Fig. 4a). Conversely, median TFD/TPI-related OS was significantly longer in patients receiving rego (overall 49 patients [54.4%]; 19 before, 30 after) in addition to TFD/TPI (p = 0.0004) (Fig. 4b). At univariate analysis, including age, sex, number of previous lines, synchronous disease, PS, and sites of disease, no statistically significant differences in terms of OS were observed.
When focusing on the treatment administration sequence, patients receiving rego before TFD/TPI displayed a significantly longer OS (p = 0.003) (Fig. 5). At univariate analysis, including age, sex, number of previous lines, synchronous disease, PS, and sites of disease, no statistically significant differences in terms of OS were observed, with the exception of patients with liver involvement alone (p = 0.0002). Multivariate analysis was not performed because of the low sample size.
Efficacy outcome in the Codon 13 group: In Codon 13-mutated patients, median rego-related OS showed no differences between patients receiving TFD/TPI (overall 17 patients [58.6%]; 8 before, 9 after) in addition to rego (p = 0.35) (Online Resource Fig. 6a). Accordingly, median TFD/TPI-related OS showed no differences between patients receiving rego (overall 17 patients [44.7%]; 9 before, 8 after) in addition to TFD/TPI (p = 0.11) (Online Resource Fig. 6b).
When focusing on the treatment administration sequence, no statistically significant differences were documented (p = 0.12) (Online Resource Fig. 7), although a trend toward a better performance in patients receiving rego followed by TFD/TPI was observed.
Efficacy outcome in the rare mutations group: In the Rare group, median rego-related OS showed no differences between patients receiving TFD/TPI (overall 16 patients [53.3%]; 10 before, 6 after) in addition to rego (p = 0.80) (Online Resource Fig. 8a). Conversely, median TFD/TPI-related OS was significantly longer in patients receiving rego (overall 16 patients [43.2%]; 5 before, 11 after) in addition to TFD/TPI (p = 0.02) (Online Resource Fig. 8b). At univariate analysis, including age, sex, number of previous lines, synchronous disease, PS, and sites of disease, no statistically significant differences in terms of OS were observed, with the exception of patients with liver involvement alone (p = 0.0002). Multivariate analysis was not performed because of the low sample size.
When focusing on the treatment administration sequence, a trend toward a better performance in patients receiving rego first was observed (p = 0.09) (Online Resource Fig. 9).
Efficacy outcome in the G12C group: In the G12C group, median rego-related OS showed no differences between patients receiving TFD/TPI (overall 6 patients [60.0%]; 4 before, 2 after) in addition to rego (p = 0.98) (Online Resource Fig. 10a). Accordingly, median TFD/TPI-related OS was not significantly longer in patients receiving rego (overall 7 patients [50.0%]; 2 before, 5 after) in addition to TFD/TPI (p = 0.65) (Online Resource Fig. 10b).
When focusing on the treatment administration sequence, no statistically significant differences were documented (p = 0.73) (Online Resource Fig. 11).
Efficacy outcome in the G12D group. In patients in the G12D group, median rego-related OS showed no differences between patients receiving TFD/TPI (overall 19 patients [61.2%]; 9 before, 10 after) in addition to rego (p = 0.42) (Online Resource Fig. 12a). Conversely, median TFD/TPI-related OS was significantly longer in patients receiving rego (overall 19 patients [36.5%]; 10 before, 9 after) in addition to TFD/TPI (p = 0.03) (Online Resource Fig. 12b). At univariate analysis, including age, sex, number of previous lines, synchronous disease, PS, and sites of disease, no statistically significant differences in terms of OS were observed.
When focusing on the treatment administration sequence, no statistically significant differences were documented (p = 0.73) (Online Resource Fig. 13).
4 Discussion
To our knowledge, this is the first report evaluating the efficacy of rego and TFD/TPI in later lines of real-life treatment of CRC patients, according to extended RAS analysis.
Our study suggests that extended RAS mutation status does not affect the outcome of heavily pretreated CRC patients, neither when receiving TFD/TPI nor when receiving rego. Nevertheless, a trend toward a higher efficacy of rego in RAS-mutated patients has been documented (in particular in patients carrying G12D and Codon 12 mutations), when compared with RAS WT patients. Our study also demonstrated that about 50% of patients are able to receive both drugs independently of molecular characteristics. Patients receiving both drugs surely represent a favorable prognostic factor-enriched population, since the median OS is significantly higher when compared with monotherapy, both in our study and in other papers [9,10,11,12,13,14,15]. Rego-related OS was significantly longer when considering patients receiving TFD/TPI in addition to rego, and, accordingly, TFD/TPI-related survival was significantly longer when considering patients receiving rego in addition to TFD/TPI. Interestingly enough, the scenario changed when each molecular subgroup was considered separately. While, in RAS WT patients, both rego-related OS and TFD/TPI-related OS were longer in patients receiving both drugs, in mutated groups (Codon 12, Codon 13, Rare, G12C and G12D), rego-related survival was not significantly improved in patients receiving TFD/TPI in addition to rego. Differently, TFD/TPI-related OS was significantly longer when considering patients receiving rego in addition to TFD/TPI, not only in RAS WT group but also in the Codon 12, Rare and G12D groups. In particular, these data were highly significant in Codon 12 (p = 0.0003). Accordingly, only in this group (Codon 12), the rego followed by TFD/TPI sequence was statistically superior to the reverse sequence. Interestingly enough, the G12C subgroup displayed a different clinical behavior, probably due to the worse prognosis of patients carrying this mutation [21].
A recent paper [18] analyzing two independent real-world datasets (960 patients overall) and an independent validation cohort based on the global phase III RECOURSE trial (800 patients) demonstrated that KRAS codon 12 mutations are predictive of reduced TFD/TPI efficacy. On the contrary, all other patients benefited from TFD/TPI therapy compared with placebo. Unfortunately, no data about patients receiving rego in addition to TFD/TPI were available for this population. Our data are consistent with the data reported above since rego-related OS of the Codon 12 group did not benefit from the addition of TFD/TPI.
A Japanese study [16] comparing rego and TFD/TPI efficacy in a large (7279 patients) nationwide database showed a significantly longer survival in patients receiving TFD/TPI first compared with those receiving rego first. However, 39% of patients in the rego-first group received subsequent TFD/TPI treatment, whereas only 25% of the patients in the TFD/TPI-first group received subsequent rego treatment. This imbalance likely affected the outcome, since patients receiving both drugs displayed a significantly higher survival. This evidence, reported in this study, is in line with our results as well as with other reports [9,10,11,12,13,14,15,16,17]. In this regard, the percentage of patients receiving both drugs (about 50% in our study, much higher than other reports) deserves further attention since, considering the high variability among reports [22], we suspect it could be attributed to factors other than patient-related or disease-related factors.
Last, but not least, our study provides a picture of the molecular landscape of patients reaching later lines of treatment in advanced CRC. The proportion of patients carrying a BRAF mutation is very low. The percentage of patients with no RAS mutation (WT) is 46.3% and is in line with literature data [21] and with patients treated in the first-line. G12D is the most common codon 12 mutation and G13D is the most common codon 13 mutation, in line with available evidence on CRC RAS mutation epidemiology [23].
The main limitation of our study is represented by its retrospective nature, which might have affected the results due to its intrinsic selection bias. Another important limitation is the long interval time over which patients have been included. Last, but not least, the sample size of the subgroups analysis is quite limited.
Although our study demonstrates that efficacy of rego and TFD/TPI in later lines of therapy of advanced CRC patients is not significantly affected by extended RAS mutational status, it identifies subgroups of patients in whom rego seems to work better. In patients carrying KRAS codon 12 mutations, rarer KRAS mutations and NRAS mutations and KRAS G12D mutation, the addition of rego (before or after) to TFD/TPI significantly improves TFD/TPI-related survival, but is not the same when adding TFD/TPI to rego. Moreover, only for patients with CRC harboring KRAS codon 12 mutations, the rego followed by TFD/TPI sequence seems significantly superior to the reverse sequence. Because of the small sample size of some subgroups, we cannot exclude that other factors such as disease burden or PS could play an important role in our results.
5 Conclusion
Although our results cannot be considered conclusive, they are in line with previous evidence [18] investigating the efficacy of TFD/TPI in relation to KRAS mutational status, and add another important piece to the puzzle of treatment personalization of advanced CRC patients. Bearing in mind that no more than 50% of patients will receive both drugs in the history of their disease, we think that available data could suggest to consider rego anticipation (in comparison with TFD/TPI) in patients carrying a codon 12 mutation.
References
Benson AIB, Venook AP, Al-Hawary MM, et al. NCCN clinical practice guidelines in oncology: colon cancer, version 2.2021. J Natl Compr Canc Netw. 2021;19:329–59.
Tabernero J, Grothey A, Van Cutsem E, et al. Encorafenib plus cetuximab as a new standard of care for previously treated BRAF V600E-mutant metastatic colorectal cancer: updated survival results and subgroup analyses from the BEACON study. J Clin Oncol. 2021;39:273–84.
Overman MJ, McDermott R, Leach JJ, et al. Nivolumab in patients with metastatic DNA mismatch repair-deficient or microsatellite instability-high colorectal cancer (CheckMate 142): an open-label, multicentre, phase 2 study. Lancet Oncol. 2017;18(9):1182–91.
André T, Shiu KK, Kim TW, et al. Pembrolizumab in microsatellite-instability–high advanced colorectal cancer. N Engl J Med. 2020;383(23):2207–18.
Fakih MG, Kopetz S, Kuboki Y, et al. Sotorasib for previously treated colorectal cancers with KRASG12C mutation (CodeBreaK100): a prespecified analysis of a single-arm, phase 2 trial. Lancet Oncol. 2022;23(1):115–24. https://doi.org/10.1016/S1470-2045(21)00605-7.
Yaeger R, Weiss J, Pelster MS, et al. Adagrasib with or without cetuximab in colorectal cancer with mutated KRAS G12C. N Engl J Med. 2023;388(1):44–54. https://doi.org/10.1056/NEJMoa2212419.
Mayer RJ, Van Cutsem E, Falcone A, et al. Randomized trial of TAS-102 for refractory metastatic colorectal cancer. N Engl J Med. 2015;372:1909–19.
Grothey A, Van Cutsem E, Sobrero A, et al. Regorafenib monotherapy for previously treated metastatic colorectal cancer (CORRECT): an international, multicentre, randomised, placebo-controlled, phase 3 trial. Lancet. 2013;381:303–12.
Dasari NA, Lonardi S, Garcia-Carbonero R, et al. LBA25 FRESCO-2: a global phase III multiregional clinical trial (MRCT) evaluating the efficacy and safety of fruquintinib in patients with refractory metastatic colorectal cancer [abstract]. Ann Oncol. 2022;33(Suppl 7):S1391–2.
Prager GW, Tajeb J, Fakih M, et al. Trifluridine-Tipiracil and bevacizumab in refractory metastatic colorectal cancer. N Engl J Med. 2023;388:1657–67. https://doi.org/10.1056/NEJMoa2214963.
Moriwaki T, Fukuoka S, Taniguchi H, et al. Propensity score analysis of regorafenib versus trifluridine/tipiracil in patients with metastatic colorectal cancer refractory to standard chemotherapy (REGOTAS): a Japanese Society for Cancer of the Colon and Rectum multicenter observational study. Oncologist. 2018;23:7–15.
Masuishi T, Taniguchi H, Hamauchi S, et al. Regorafenib versus trifluridine/tipiracil for refractory metastatic colorectal cancer: a retrospective comparison. Clin Colorectal Cancer. 2017;16:e15-22.
Tanaka A, Sadahiro S, Suzuki T, et al. Retrospective study of regorafenib and trifluridine/tipiracil efficacy as a third-line or later chemotherapy regimen for refractory metastatic colorectal cancer. Oncol Lett. 2018;16:6589–97.
Ogata M, Kotaka M, Ogata T, et al. Regorafenib vs trifluridine/tipiracil for metastatic colorectal cancer refractory to standard chemotherapies: a multicenter retrospective comparison study in Japan. PLoS One. 2020;15: e0234314.
Patel AK, Abhyankar R, Brais LK, et al. Trifluridine/tipiracil and regorafenib in patients with metastatic colorectal cancer: a retrospective study at a tertiary oncology center. Oncologist. 2021;26:e2161-2169.
Nakashima M, Takeuchi M, Kawakami K. Effectiveness and safety of regorafenib vs. Trifluridine/Tipiracil in unresectable colorectal cancer: a retrospective cohort study. Clin Colorectal Cancer. 2020;19(4):e208–25.
Signorelli C, Calegari MA, Basso M, et al. Treatment settings and outcomes with regorafenib and trifluridine/tipiracil at third-line and beyond in metastatic colorectal cancer: a real-world multicenter retrospective study. Curr Oncol. 2023;30(6):5456–69. https://doi.org/10.3390/curroncol30060413.
van de Haar J, Ma X, Ooft SN, et al. Codon-specific KRAS mutations predict survival benefit of trifluridine/tipiracil in metastatic colorectal cancer. Nat Med. 2023;29:605–14. https://doi.org/10.1038/s41591-023-02240-8.
Bekaii-Saab TS, Ou FS, Ahn DH, et al. Regorafenib dose-optimization in pts with refractory metastatic colorectal cancer (ReDOS): a randomized, multicentre, open-label, phase 2 study. Lancet Oncol. 2019;20(8):1070–82. https://doi.org/10.1016/S1470-2045(19)30272-4.
Hallin J, Bowcut V, Calinisan A, et al. Anti-tumor efficacy of a potent and selective non-covalent KRASG12D inhibitor. Nat Med. 2022;28(10):2171–82. https://doi.org/10.1038/s41591-022-02007-7.
Koulouridi P, Karagianni M, Messaritakis I, et al. Prognostic value of KRAS mutations in colorectal cancer patients. Cancers. 2022;14:3320. https://doi.org/10.3390/cancers14143320.
Nevala-Plagemann C, Sama S, Ying J, et al. A real-world comparison of regorafenib and trifluridine/tipiracil in refractory metastatic colorectal cancer in the United States. Natl Compr Canc Netw. 2023;21(3):257–64. https://doi.org/10.6004/jnccn.2022.7082.
Timar J, Kashofer K. Molecular epidemiology and diagnostics of KRAS mutations in human cancer. Cancer Metastasis Rev. 2020;39:1029–38. https://doi.org/10.1007/s10555-020-09915-5.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
Open access funding provided by Università Cattolica del Sacro Cuore within the CRUI-CARE Agreement.
Conflicts of Interest/Competing Interests
Carlo Signorelli reports consultant’s fee from Amgen and Bayer. Maria Alessandra Calegari reports consulting or advisory role for Merck, Servier and Pierre Fabre. Lisa Salvatore reports speakers’ and consultant’s fees from MSD, Astra-Zeneca, Servier, Bayer, Merck, Amgen, Pierre-Fabre, GSK, Takeda, and Incyte. Carmelo Pozzo reports consulting or advisory role for Amgen, Servier and Eli-Lilly. Giampaolo Tortora is supported by funds from the Ministero della Salute (Ricerca Corrente 2022). Michele Basso, Jessica Lucchetti, Ina Valeria Zurlo, Emanuela Dell’Aquila, Giulia Arrivi, Federica Zoratto, Fiorenza Santamaria, Rosa Saltarelli, Giovanni Trovato, Giulia Caira, Lorenzo Angotti, Marta Schirripa, Annunziato Anghelone, Francesco Schietroma, and Mario Giovanni Chilelli declare that they have no conflicts of interest that might be relevant to the contents of this manuscript.
Ethics Approval
The study was approved by the Ethics Committee (2022-no.1021/CE Lazio 1) and was carried out in conformity with the Declaration of Helsinki.
Consent to Participate
Informed consent was obtained from all patients prior to participating in this study.
Consent to Publish
Not applicable.
Availability of Data and Materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Code Availability
Not applicable.
Authors’ Contributions
The authors met the criteria for authorship as recommended by the International Committee of Medical Journal Editors.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Basso, M., Signorelli, C., Calegari, M.A. et al. Efficacy of Regorafenib and Trifluridine/Tipiracil According to Extended RAS Evaluation in Advanced Metastatic Colorectal Cancer Patients: A Multicenter Retrospective Analysis. Targ Oncol 19, 371–382 (2024). https://doi.org/10.1007/s11523-024-01050-3
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11523-024-01050-3