Abstract
Background
The efficacy of systemic treatment for primary central nervous system lymphoma (PCNSL) is limited because of the blood–brain barrier (BBB) and the ineffectiveness of chemotherapy. The dual PI3K/HDAC inhibitor BEBT-908 has exhibited favorable in vivo distribution and activity in various cancers.
Objectives
The aims of this study were to assess the efficacy of BEBT-908 in brain orthotopic mouse models of hematological malignancies, to investigate its pharmacologic properties, and to elucidate the underlying mechanism of action.
Methods
We evaluated the anticancer activity of BEBT-908 in various hematological malignancies through cell viability assays. The impact of BEBT-908 on c-Myc expression and ferroptosis signaling pathways was assessed using Western blotting, qPCR, ROS detection, GSH/GSSG detection, and IHC. Pharmacokinetic and pharmacodynamic profiles were assessed through LC–MS/MS and Western blotting. The effects of BEBT-908 in vivo were examined using xenografts and brain orthotopic mouse models.
Results
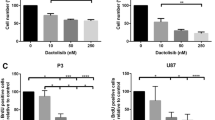
Our findings demonstrate that BEBT-908 exhibits promising anti-tumor activity in vitro and in vivo across multiple subtypes of hematological malignancies. Furthermore, BEBT-908 exhibits excellent BBB penetration and inhibits tumor growth in a brain orthotopic lymphoma model with prolonged survival of host mice. Mechanistically, BEBT-908 downregulated c-Myc expression, which contributed to ferroptosis, ultimately leading to tumor shrinkage.
Conclusion
Our study provides robust evidence for the dual PI3K/HDAC inhibitor BEBT-908 as an effective anti-cancer agent for PCNSL.






Similar content being viewed by others
References
Deckert M, Engert A, Brück W, et al. Modern concepts in the biology, diagnosis, differential diagnosis and treatment of primary central nervous system lymphoma. Leukemia. 2011;25(12):1797–807. https://doi.org/10.1038/leu.2011.169.
Houillier C, Soussain C, Ghesquières H, et al. Management and outcome of primary CNS lymphoma in the modern era: an LOC network study. Neurology. 2020;94(10):e1027–39. https://doi.org/10.1212/WNL.0000000000008900.
Shao L, Xu C, Wu H, et al. Recent progress on primary central nervous system lymphoma-from bench to bedside. Front Oncol. 2021;11: 689843. https://doi.org/10.3389/fonc.2021.689843.
Grommes C, DeAngelis LM. Primary CNS lymphoma. J Clin Oncol. 2017;35(21):2410–8. https://doi.org/10.1200/JCO.2017.72.7602.
Schabet M. Epidemiology of primary CNS lymphoma. J Neurooncol. 1999;43(3):199–201. https://doi.org/10.1023/a:1006290032052.
Schultz C, Scott C, Sherman W, et al. Preirradiation chemotherapy with cyclophosphamide, doxorubicin, vincristine, and dexamethasone for primary CNS lymphomas: initial report of radiation therapy oncology group protocol 88-06. J Clin Oncol. 1996;14(2):556–64. https://doi.org/10.1200/JCO.1996.14.2.556.
Eyre TA, Kirkwood AA, Wolf J, et al. Stand-alone intrathecal central nervous system (CNS) prophylaxis provide unclear benefit in reducing CNS relapse risk in elderly DLBCL patients treated with R-CHOP and is associated increased infection-related toxicity. Br J Haematol. 2019;187(2):185–94. https://doi.org/10.1111/bjh.16070.
Gleeson M, Counsell N, Cunningham D, et al. Central nervous system relapse of diffuse large B-cell lymphoma in the rituximab era: results of the UK NCRI R-CHOP-14 versus 21 trial. Ann Oncol. 2017;28(10):2511–6. https://doi.org/10.1093/annonc/mdx353.
Ferreri AJ, Cwynarski K, Pulczynski E, et al. Chemoimmunotherapy with methotrexate, cytarabine, thiotepa, and rituximab (MATRix regimen) in patients with primary CNS lymphoma: results of the first randomisation of the International Extranodal Lymphoma Study Group-32 (IELSG32) phase 2 trial. Lancet Haematol. 2016;3(5):e217–27. https://doi.org/10.1016/S2352-3026(16)00036-3.
Chen T, Liu Y, Wang Y, et al. Evidence-based expert consensus on the management of primary central nervous system lymphoma in China. J Hematol Oncol. 2022;15(1):136. https://doi.org/10.1186/s13045-022-01356-7.
Wilson MR, Eyre TA, Kirkwood AA, et al. Timing of high-dose methotrexate CNS prophylaxis in DLBCL: a multicenter international analysis of 1384 patients. Blood. 2022;139(16):2499–511. https://doi.org/10.1182/blood.2021014506.
von Baumgarten L, Illerhaus G, Korfel A, Schlegel U, Deckert M, Dreyling M. The diagnosis and treatment of primary CNS lymphoma. Dtsch Arztebl Int. 2018;115(25):419–26. https://doi.org/10.3238/arztebl.2018.0419.
Mendez JS, Grommes C. Treatment of primary central nervous system lymphoma: from chemotherapy to small molecules. Am Soc Clin Oncol Educ Book. 2018;38:604–15. https://doi.org/10.1200/EDBK_200829.
Langner-Lemercier S, Houillier C, Soussain C, et al. Primary CNS lymphoma at first relapse/progression: characteristics, management, and outcome of 256 patients from the French LOC network. Neuro Oncol. 2016;18(9):1297–303. https://doi.org/10.1093/neuonc/now033.
Yu H, Kong H, Li C, et al. Bruton’s tyrosine kinase inhibitors in primary central nervous system lymphoma-evaluation of anti-tumor efficacy and brain distribution. Transl Cancer Res. 2021;10(5):1975–83. https://doi.org/10.21037/tcr-21-50.
Korfel A, Schlegel U, Herrlinger U, et al. Phase II trial of temsirolimus for relapsed/refractory primary CNS lymphoma. J Clin Oncol. 2016;34(15):1757–63. https://doi.org/10.1200/JCO.2015.64.9897.
Ghesquieres H, Chevrier M, Laadhari M, et al. Lenalidomide in combination with intravenous rituximab (REVRI) in relapsed/refractory primary CNS lymphoma or primary intraocular lymphoma: a multicenter prospective “proof of concept” phase II study of the French Oculo-Cerebral lymphoma (LOC) Network and the Lymphoma Study Association (LYSA)†. Ann Oncol. 2019;30(4):621–8. https://doi.org/10.1093/annonc/mdz032.
Roberts AW, Davids MS, Pagel JM, et al. Targeting BCL2 with venetoclax in relapsed chronic lymphocytic leukemia. N Engl J Med. 2016;374(4):311–22. https://doi.org/10.1056/NEJMoa1513257.
Zhang X, Wu Y, Sun X, et al. The PI3K/AKT/mTOR signaling pathway is aberrantly activated in primary central nervous system lymphoma and correlated with a poor prognosis. BMC Cancer. 2022;22(1):190. https://doi.org/10.1186/s12885-022-09275-z.
Tarantelli C, Gaudio E, Hillmann P, et al. The novel TORC1/2 kinase inhibitor PQR620 has anti-tumor activity in lymphomas as a single agent and in combination with venetoclax. Cancers (Basel). 2019;11(6):775. https://doi.org/10.3390/cancers11060775.
Jain N, Singh S, Laliotis G, et al. Targeting phosphatidylinositol 3 kinase-β and -δ for Bruton tyrosine kinase resistance in diffuse large B-cell lymphoma. Blood Adv. 2020;4(18):4382–92. https://doi.org/10.1182/bloodadvances.2020001685.
Zhang X, Liu Y. Targeting the PI3K/AKT/mTOR signaling pathway in primary central nervous system lymphoma: current status and future prospects. CNS Neurol Disord Drug Targets. 2020;19(3):165–73. https://doi.org/10.2174/1871527319666200517112252.
Grommes C, Gavrilovic I, Miller AM, et al. Phase Ib of copanlisib in combination with ibrutinib in recurrent/refractory primary CNS lymphoma (PCNSL). Blood. 2019;134(Suppl 1):1598. https://doi.org/10.1182/blood-2019-126214.
Rahmani M, Aust MM, Benson EC, Wallace L, Friedberg J, Grant S. PI3K/mTOR inhibition markedly potentiates HDAC inhibitor activity in NHL cells through BIM- and MCL-1-dependent mechanisms in vitro and in vivo. Clin Cancer Res. 2014;20(18):4849–60. https://doi.org/10.1158/1078-0432.CCR-14-0034.
Fan F, Liu P, Bao R, et al. A dual PI3K/HDAC inhibitor induces immunogenic ferroptosis to potentiate cancer immune checkpoint therapy. Cancer Res. 2021;81(24):6233–45. https://doi.org/10.1158/0008-5472.CAN-21-1547.
Kapadia B, Nanaji NM, Bhalla K, et al. Fatty acid synthase induced S6Kinase facilitates USP11-eIF4B complex formation for sustained oncogenic translation in DLBCL. Nat Commun. 2018;9(1):829. https://doi.org/10.1038/s41467-018-03028-y.
Wu W, Bi C, Credille KM, et al. Inhibition of tumor growth and metastasis in non-small cell lung cancer by LY2801653, an inhibitor of several oncokinases, including MET. Clin Cancer Res. 2013;19(20):5699–710. https://doi.org/10.1158/1078-0432.CCR-13-1758.
Dong Y, Tu R, Liu H, Qing G. Regulation of cancer cell metabolism: oncogenic MYC in the driver’s seat. Signal Transduct Target Ther. 2020;5(1):124. https://doi.org/10.1038/s41392-020-00235-2.
Kurland JF, Tansey WP. Myc-mediated transcriptional repression by recruitment of histone deacetylase. Cancer Res. 2008;68(10):3624–9. https://doi.org/10.1158/0008-5472.CAN-07-6552.
Alborzinia H, Flórez AF, Kreth S, et al. MYCN mediates cysteine addiction and sensitizes neuroblastoma to ferroptosis. Nat Cancer. 2022;3(4):471–85. https://doi.org/10.1038/s43018-022-00355-4.
Anderson GR, Wardell SE, Cakir M, et al. PIK3CA mutations enable targeting of a breast tumor dependency through mTOR-mediated MCL-1 translation. Sci Transl Med. 2016;8(369): 369ra175. https://doi.org/10.1126/scitranslmed.aae0348.
Martz CA, Ottina KA, Singleton KR, et al. Systematic identification of signaling pathways with potential to confer anticancer drug resistance. Sci Signal. 2014;7(357):ra121. https://doi.org/10.1126/scisignal.aaa1877.
Li L, Li Y, Que X, et al. Prognostic significances of overexpression MYC and/or BCL2 in R-CHOP-treated diffuse large B-cell lymphoma: a systematic review and meta-analysis. Sci Rep. 2018;8(1): 6267. https://doi.org/10.1038/s41598-018-24631-5.
Fujimoto K, Shinojima N, Hayashi M, Nakano T, Ichimura K, Mukasa A. Histone deacetylase inhibition enhances the therapeutic effects of methotrexate on primary central nervous system lymphoma. Neurooncol Adv. 2020;2(1): vdaa084. https://doi.org/10.1093/noajnl/vdaa084.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Funding
This study is funded by National Science and Technology Major Project of China (2016ZX09101002), Guangdong Pearl River Talents Plan (2014ZT05Y232), Guangzhou Municipal Science and Technology Project (201909020004).
Conflict of interest
C. Qian and X. Cai are inventors of BEBT-908 and founders of Guangzhou BeBetter Medicine Technology Co., Ltd. N.Wang, Z.Mo, L.Pan, M.Zhou, X.Ye, X.Liu, F.Chen, Y. Xiong, F.Fan, W.Li declare that they have no conflicts of interest that might be relevant to the contents of this article.
Institutional Review Board Statement
The animal study was approved by Curis Inc and Ruiye Animal Model Inc Animal Use and Care Committee.
Consent to Participate and Publish
Not applicable due to the study being preclinical in nature.
Data Availability
The data generated in the present study are included in the article and the Online Supplementary Materials.
Code Availability
Not applicable.
Author Contributions
Conceptualization: WL, FF, XL, CQ; Methodology: NW, ZM, LP, MZ, XY, FC; Validation: WL, FF, NW; Formal Analysis: FF, NW; Investigation: WL, FF; Resources: WL, CQ, XC, YX; Data curation: FF, NW; Writing—original draft preparation: NW, FF; Writing—review and editing: FF, XL; Supervision: FF, XL; Project administration: WL, FF; Funding acquisition: CQ.
Additional information
Xiong Cai and Changgeng Qian are former employees of Curis, Inc.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wang, N., Mo, Z., Pan, L. et al. Dual PI3K/HDAC Inhibitor BEBT-908 Exhibits Potent Efficacy as Monotherapy for Primary Central Nervous System Lymphoma. Targ Oncol 18, 941–952 (2023). https://doi.org/10.1007/s11523-023-01006-z
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11523-023-01006-z