Abstract
Cyclin-dependent kinase 4 and 6 inhibitors (CDK4/6i) are the preferred regimen for patients with hormone receptor-positive and human epidermal growth factor receptor 2-negative (HR+/HER2−) advanced or metastatic breast cancer. However, the optimal treatment sequencing for CDK4/6i with other available therapeutic options is unclear. We conducted a targeted literature review to identify the current evidence on CDK4/6i treatment patterns in patients with breast cancer. The search was initially conducted in October 2021 and subsequently updated in October 2022. Biomedical databases and gray literature were searched, and bibliographies of included reviews were screened for relevant studies. The search identified ten reviews published since 2021 and 87 clinical trials or observational studies published since 2015. The included reviews discussed CDK4/6i usage with or without endocrine therapy (ET) in first-line and second-line treatment for patients with HR+/HER2− advanced or metastatic breast cancer, followed by ET, chemotherapy, or targeted therapy with ET. Clinical studies reported similar treatment sequences consisting of ET, chemotherapy, or targeted therapy with ET prior to CDK4/6i with ET, followed by ET monotherapy, chemotherapy, targeted therapy with ET, or continued CDK4/6i with ET. Current evidence suggests CDK4/6i are effective for HR+/HER2− advanced or metastatic breast cancer in earlier lines of therapy. Efficacy of CDK4/6i as measured by progression-free survival and overall survival was similar within a line of therapy regardless of the type of prior therapy. Survival on different post-CDK4/6i treatments was also similar within the same line of therapy. Additional research is needed to investigate the optimal place in therapy of CDK4/6i and the sequencing of treatments following progression on CDK4/6i.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Based on a targeted literature review on the place in therapy of cyclin-dependent kinase 4 and 6 inhibitors (CDK4/6i) in breast cancer published since 1 January, 2015, patients with hormone receptor-positive and human epidermal growth factor receptor 2-negative (HR+/HER2−) advanced or metastatic breast cancer commonly receive CDK4/6i in first-line or second-line treatment in combination with endocrine therapy, chemotherapy, or everolimus. |
The evidence suggests that CDK4/6i are generally more effective in earlier lines of therapy for the treatment of HR+/HER2− advanced or metastatic breast cancer, regardless of prior treatment received. |
CDK4/6i in combination with endocrine therapy is the preferred first-line treatment option for patients with HR+/HER2− advanced or metastatic breast cancer, and the preferred second-line option in patients without prior exposure to CDK4/6i in the metastatic setting. |
More research is needed to investigate optimal treatment sequencing of CDK4/6i rechallenging and treatment patterns following progression on CDK4/6i. |
1 Introduction
Breast cancer is the most commonly diagnosed cancer worldwide and is the leading cause of cancer-related mortality in women [1]. In 2020, there were 2.3 million newly diagnosed breast cancer cases and 685,996 deaths from breast cancer globally [1]. Breast cancer is a heterogeneous disease classified by disease stage and by four major subtypes based on molecular markers for hormone receptors (HR; i.e., estrogen receptor [ER] and progesterone receptor [PR]) and human epidermal growth factor receptor 2 (HER2) [2,3,4,5]. The most prevalent molecular subtype is HR positive and HER2 negative (HR+/HER2−), accounting for approximately 70% of cases in the USA and Canada [2,3,4]. Other subtypes include HR+/HER2+, HR-/HER2+, and HR−/HER2− (triple negative). Therapeutic options for breast cancer (e.g., surgery, chemotherapy, endocrine therapy [ET], and targeted therapy) vary depending on patient and disease-specific characteristics, including molecular subtype and disease stage [6].
The emergence of the cyclin-dependent kinase 4/6 inhibitors (CDK4/6i) has transformed the treatment landscape for patients with HR+/HER2− advanced or metastatic breast cancer (aBC/mBC). Since the US Food and Drug Administration (FDA) approved the first-in-class CDK4/6i, palbociclib, in 2015 [7], each of the currently approved CDK4/6i (palbociclib, ribociclib, and abemaciclib) has demonstrated clinical effectiveness and a well-tolerated safety profile when combined with ET to treat HR+/HER2− aBC/mBC in first-line (1L) and second-line (2L) settings [8,9,10,11,12,13,14,15]. As a result, CDK4/6i are a preferred 1L option for unresectable, HR+/HER2− aBC/mBC, and a preferred 2L option in patients without prior exposure to CDK4/6i in the metastatic setting [6, 16, 17]. Additionally, after demonstrating efficacy in treating HR+/HER2− early breast cancer (eBC) [18, 19], abemaciclib was the first CDK4/6i to receive FDA approval (October 2021) for adjuvant treatment of HR+/HER2− eBC [20]. Alternative treatments for endocrine-resistant breast cancer include other targeted therapies, such as the mammalian target of rapamycin inhibitor (mTORi) everolimus, and regimens using targeted therapy in combination with chemotherapy [6, 16, 17]. Overall, treatment selection is based on patient characteristics, guideline-recommended therapy, as well as physician and patient preferences [6, 16, 17]. Given the availability of various targeted treatments for breast cancer, ongoing research on genomic biomarkers aims to support the development of a personalized approach to treatment selection [17, 21].
Although CDK4/6i have established a clear clinical benefit when used to treat HR+/HER2− mBC, the optimal treatment sequencing for CDK4/6i and other available therapeutic options, particularly post-CDK4/6i treatment, is unclear. Following progression on CDK4/6i, treatment options include chemotherapy, ET monotherapy, alternative targeted therapies (e.g., mTORi, phosphoinositide 3-kinase inhibitor, poly (ADP-ribose) polymerase inhibitor [PARPi]), or continued CDK4/6 inhibition [16]. Establishing optimal treatment sequencing is critical for maximizing clinical benefit and improving patient outcomes.
In this study, we summarized the key current treatment guidelines and recommendations for CDK4/6i treatment for aBC/mBC and conducted a targeted literature review (TLR) to examine the current evidence on CDK4/6i treatment patterns in breast cancer across various disease stages and molecular subtypes. A TLR was conducted to provide a formal and focused review of the literature based on a structured search using selected key terms and phrases. We focused on the place in therapy of CDK4/6i for breast cancer, the clinical effectiveness of CDK4/6i across various treatment patterns, and of therapeutic options following CDK4/6i progression. Additionally, we summarized the treatment characteristics of patients who received CDK4/6i across various treatment modalities, as well as ongoing clinical trials investigating treatment options post-CDK4/6i progression.
2 Methods
The TLR was conducted on 15 October, 2021 to identify systematic reviews, narrative reviews, and clinical studies (i.e., clinical trials and observational studies) relevant to CDK4/6i treatment patterns. In comparison to a systematic literature review, this study provides a focused review of the most current literature while being less time intensive. The MEDLINE® biomedical databases including Epub ahead of print, in-process, other non-indexed citations, and daily status records were searched using the Ovid® interface, from the year 1946 up to 14 October, 2021. The database search strategy was developed and executed by an experienced information specialist, who provided comprehensive and thorough documentation of the search strategy and results. In addition to biomedical databases, gray literature sources were searched manually (13 and 14 October, 2021), including clinical trial registries (ClinicalTrials.gov, World Health Organization registry, European Union Drug Regulating Authorities Clinical Trials Database) and Google Scholar. Additionally, the bibliographies of included reviews were searched for references relevant to CDK4/6i treatment patterns. An updated search was conducted on 19 October, 2022 using the same search terms and strategy to include newly published evidence since the initial search date (i.e., 1946 up to 18 October, 2022). The complete search strategies for both the original and updated search are detailed in the Electronic Supplementary Material (ESM).
Study selection was based on pre-specified Population, Intervention, Comparison, Outcomes and Study design (PICOS) criteria (Table 1). Importantly, the PICOS criteria used to screen identified studies were set a priori. Clinical studies published since 1 January, 2015 were included. Systematic and narrative reviews published since 1 January, 2018 were initially included for screening. Given that over 30 relevant reviews were identified up to the search date (15 October, 2021), we further limited the search to only include reviews published since 1 January, 2021 to focus on the most recent reviews on the topics of interest.
Screening was conducted by a single reviewer at two levels. First, the titles and abstracts of records were reviewed for relevance based on the PICOS criteria. Then, the full text of records included after the first level of screening were reviewed in detail for formal inclusion in the review and data extraction.
Key details about study design and characteristics, baseline patient characteristics, clinical outcomes, treatment sequences investigated, post-CDK4/6i treatments and reported outcomes, and any quality-of-life outcomes captured in included studies were extracted by a single reviewer into a data extraction form in Microsoft Excel. To visually compare clinical outcomes across studies, bubble charts were created in Microsoft Excel using the extracted median overall survival (mOS) and median progression-free survival (mPFS) data, organized by study and line of therapy. In each bubble chart, study cohorts were presented along the X-axis with a unique reference number assigned to each cohort. The size of each bubble was used to represent the sample size in each cohort, and the bubbles were color coded by the treatment class received.
Clinical guidelines for the treatment of aBC/mBC published by the National Comprehensive Cancer Network® (NCCN®), American Society of Clinical Oncology (ASCO 2021), European Society for Medical Oncology (ESMO 2021), and the Arbeitsgemeinschaft Gynäkologische Onkologie (AGO) German Gynecological Oncology Group were also reviewed and summarized [6, 16, 17, 22].
3 Results
3.1 Review of Clinical Guidelines
Multiple breast cancer treatment guidelines are available for clinicians who treat breast cancer. Here, we summarize the most recent and relevant recommendations for the use of CDK4/6i as presented in key guidelines by the NCCN® in 2022, ASCO in 2021, ESMO in 2021, and the AGO in 2022 [6, 16, 17, 22].
3.1.1 Advanced or Metastatic Breast Cancer
All aforementioned guidelines recommend CDK4/6i for the treatment of patients with HR+/HER2− aBC/mBC [6, 16, 17, 22]. Notably, none of the included treatment guidelines name specific CDK4/6i treatments, but recommend the class broadly, as there have been no head-to-head clinical trials to date comparing the three approved CDK4/6i, and the efficacy of each appear to be similar [6, 16, 17, 22]. In patients with HR+/HER2− recurrent or stage IV disease who are postmenopausal (or premenopausal receiving ovarian ablation or suppression), the NCCN Clinical Practice Guidelines in Oncology recommend systemic therapy with CDK4/6i in combination with an aromatase inhibitor (AI) or fulvestrant as a preferred regimen in 1L, 2L, and subsequent-line therapy [5]. The NCCN Clinical Practice Guidelines in Oncology also note that data supporting treatment with continued CDK4/6i regimens upon progression on CDK4/6i are limited; therefore, CDK4/6i are recommended for patients without prior exposure to CDK4/6i treatment. Recent ASCO and ESMO guidelines provide more specific recommendations for CDK4/6i use: CDK4/6i combined with a non-steroidal AI is recommended for treatment-naïve patients in the mBC setting (i.e., in 1L) with HR+ mBC who are postmenopausal or premenopausal with chemical ovarian function suppression, and for male patients who are receiving a gonadotropin-releasing hormone analog [16, 17]. Additionally, CDK4/6i combined with fulvestrant is recommended either in 1L or after one prior line of chemotherapy in the metastatic setting for patients who experience progression on an AI or recurrence within 1 year of receiving an AI, and with no prior exposure to CDK4/6i in the metastatic setting [16, 17]. The ESMO guidelines also suggest CDK4/6i treatment could be continued after progression on prior CDK4/6i after a treatment-free interval of at least 12 months [16]. CDK4/6i combined with ET is also acceptable as a subsequent therapy for patients who have not received CDK4/6i in 1L and have progressive disease [16]. Additionally, ESMO guidelines recommend that ET alone in the first-line setting should be reserved for the small group of patients with comorbidities or a performance status that prevents the use of CDK4/6i.
3.1.2 Targeted Therapies for Genetic Subtypes
The NCCN, ASCO, ESMO, and AGO treatment guidelines all include similar treatment recommendations for patients with aBC/mBC with specific genetic mutations. Alpelisib with fulvestrant is recommended as a targeted therapy for patients with PIK3CA-mutated tumors, and PARPi (i.e., olaparib or talazoparib) is recommended for patients with germline BRCA1/BRCA2 (gBRCA1/2)-mutated tumors [6, 16, 17, 22]. Similarly, subsequent treatment options following progression on CDK4/6i in the ESMO guidelines include alpelisib with fulvestrant for PIK3CA-mutated tumors, PARPi for gBRCA1/2-mutated tumors, everolimus-based regimens, ET, and chemotherapies; however, the guidelines note that the optimal treatment sequence is uncertain post-CDK4/6i and depends on several factors, such as patient response to prior therapies, product availability, and patient preference [16]. Additionally, ASCO and AGO guidelines note there is evidence to suggest ESR1 mutations result in resistance to or reduced efficacy of AIs and tamoxifen; therefore, fulvestrant may be a more beneficial treatment in this population [17, 22].
3.2 Targeted Literature Review Results
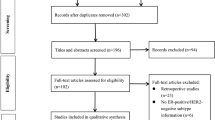
Our initial TLR identified 1223 records from the biomedical database searches, and 383 records from the gray literature searches. The updated search identified an additional 330 records from the biomedical database searches, and additional 370 records from the gray literature sources. The study selection process, including the numbers of records included and excluded at each step of the screening process, is summarized in the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flow diagram in Fig. 1. The complete list of studies included based on a full-text review is presented in the ESM. In summary, a total of 97 publication records, representing 92 unique relevant studies, were identified and extracted. These studies include nine narrative reviews, one systematic literature review, and 87 clinical studies (71 full-text publications, 13 abstracts, and 3 clinical trial records).

Source: adapted from The PRISMA 2020 statement [101]
Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) study selection flow diagram. EudraCT European Union Drug Regulating Authorities Clinical Trials Database, WHO World Health Organization. MA meta-analysis, NMA network meta-analysis, SLR systematic literature review.
3.3 Summary of Reviews
In total, ten reviews (nine narrative and one systematic review) were identified for inclusion. The list of reviews identified and the study and patient characteristics of each review are presented in the ESM. Consistent with treatment guidelines, the reviews confirmed CDK4/6i combined with ET as the preferred regimen for HR+/HER2− aBC/mBC (typically in 1L but could apply across all lines of therapy) [ESM]. While different treatment sequences were discussed across the reviews, there was consensus on treatment with CDK4/6i combined with ET in 1L; following progression on CDK4/6i, a targeted therapy combined with ET, or ET or chemotherapy alone, was the most common treatment sequence received by patients in the aBC/mBC setting based on response to prior therapy and patient preference (ESM). Additionally, a 2021 review by Loibl et al. summarized the phase III clinical trials that, at the time, were investigating the use of CDK4/6i combined with ET as a treatment for patients with eBC (PENELOPE-B, PALLAS, monarchE, and NATALEE) [23,24,25,26,27]. Results from these ongoing trials will shed further light on the efficacy and optimal sequencing of CDK4/6i in eBC in addition to aBC/mBC.
Subsequent treatment recommendation and selection following progression on CDK4/6i depends on a variety of factors, such as prior treatments, response to those therapies, and patient preference. Targeted treatment regimens were recommended for use in specific patient populations based on the mutation or biomarker status, such as alpelisib combined with ET for patients with aBC/mBC with phosphoinositide 3-kinase inhibitor-mutated tumors, or olaparib and talazoparib for patients with aBC/mBC with a germline BRCA1/2 mutation. Many ongoing clinical studies are investigating therapeutic options for the treatment of HR+/HER2− aBC/mBC post-CDK4/6i progression, with nearly 50 ongoing clinical trials and observational studies of post-CDK4/6i therapies identified in our review (ESM). Overall, the reviews suggested that optimal treatment sequencing with CDK4/6i is in combination with ET in 1L or for the treatment of patients with no prior history of CDK4/6i treatment. Based on the currently available clinical data, optimal treatment selection following progression on CDK4/6i-based therapies remains uncertain.
3.4 Summary of Clinical Studies
A total of 87 clinical studies (i.e., clinical trials and observational studies) were identified for inclusion. An overview of the characteristics of these studies is provided in Table 2. Of 87 studies, 71 studies were presented in full-text journal articles; 13 were presented in abstracts only, and three were completed trials indexed on the trial registry, ClinicalTrials.gov. Most studies (66 out of 87) were retrospective observational studies, six were prospective or prospective-retrospective observational studies, and 12 were clinical trials. Nearly all (80 out of 87) studies were based on patient data collected between 2017 and 2022, according to the end of the data collection period or follow-up reported in each study. The geographical scope of the studies included Europe, the Americas, and Asia, with most of the studies conducted in Europe (14 out of 87) and North America (36 out of 87). Nearly all studies investigated treatment patterns in patients with HR+/HER2− aBC/mBC, in alignment with the FDA-approved indications for CDK4/6i up to the search date [7, 20, 28], with the exception of one clinical trial that investigated a novel CDK4/6i dalpiciclib combined with pyrotinib and letrozole in the HR+ HER2+ population [29]. Additionally, the studies evaluated CDK4/6i in lines of therapy that ranged from 1L to third line (3L) and beyond, with 73 out of 87 studies reporting outcomes in multiple lines of therapy. Of the 73 studies that investigated CDK4/6i in multiple lines of therapy, 62 studies focused on 1L and later, 11 studies focused on 2L and later, while no studies exclusively investigated CDK4/6i in 3L and later. Many of the studies investigated or reported on post-CDK4/6i treatments, including chemotherapy monotherapy in 24 studies (28%), ET monotherapy in 26 studies (30%), and targeted therapies in 29 studies (33%; CDK4/6i in 16 studies, mTORi in 12 studies, and CDK4/6i combined with mTORi in 1 study).
3.5 CDK4/6i Treatment Patterns and Efficacy
3.5.1 Treatment Patterns
CDK4/6i treatment usage by line of therapy was reported by several studies conducted in Europe, the USA, and Japan [30,31,32,33,34]. A large observational study by Davie et al. in 2021 reported CDK4/6i treatment usage in over 2000 patients with HR+/HER2− aBC from France, Germany, Spain, Italy, the UK, and the USA using data collected by the Adelphi Real World Disease Specific Programme™ from March to June 2017. This study reported that CDK4/6i plus ET was received by 10% of patients in 1L, and 8% of patients in 2L; the majority of patients received ET only (54% in 1L and 39% in 2L), followed by chemotherapy only (21% in 1L and 23% in 2L) [30]. The study by Meegdes et al. in 2021 reported on CDK4/6i treatment usage in Dutch patients with HR+/HER2− aBC based on data from the Southeast Netherlands Advanced Breast cancer (SONABRE) Registry from 2009 to 2018. This study found that since August 2017 when CDK4/6i were reimbursed in the Netherlands, CDK4/6i combined with ET was received by 31% of 214 patients in 1L, and by 44% of 71 patients in 2L with no prior exposure to CDK4/6i [31]. Additionally, CDK4/6i combined with ET usage gradually increased over time from 2014 onward in all lines reported (i.e., 1L–3L), while the use of chemotherapy, ET, and mTORi decreased [31]. An observational study by Cui et al. in 2021 investigated CDK4/6i treatment usage in nearly 4000 women diagnosed with HR+/HER2− mBC in the USA between 1 January, 2013 and 31 January, 2019 based on data from the Flatiron Health database. In this study, in the 1L and 2L settings, 42.1% and 40.4% of patients received CDK4/6i-based regimens, respectively [32]. A retrospective study in Germany of data from the real-world registry PRAEGNANT reported a dramatic increase in CDK4/6i usage over time in the 1L setting for mBC, from 14.1% in 2016 when the first CDK4/6i was approved there in November, to 72.2% in 2022 [34]. A study of Japanese patients with aBC who received palbociclib based on claims data found that palbociclib was initially prescribed more commonly in 2L and later in 2017, and became more common as a 1L treatment steadily over time from 22.7% from December 2017 to June 2018, to 42.6% from July to December 2020 [33]. Overall, these data suggest a temporal trend of increased CDK4/6i use globally since their approval, particularly as an earlier line treatment for aBC/mBC. This is also consistent with the guidelines’ recommendations discussed above as CDK4/6i is becoming the standard option at 1L.
Of the 87 clinical studies, only two reported a detailed breakdown of the proportion of patients receiving different CDK4/6i treatment sequences across two lines of therapy for the treatment of mBC [35, 36] (ESM). The study by Goldschmidt et al. in 2018 reported a detailed breakdown of treatment sequences received by 147 patients with HR+/HER2− mBC treated with at least two lines of therapy based on data from 64 community oncologists in the USA collected between February and June 2017. Overall, a CDK4/6i-based regimen was received by 52.4% of patients in 1L and by 42.9% of patients in 2L [35]. The most common sequence was AI in 1L followed by CDK4/6i combined with fulvestrant in 2L, which 13.6% of patients received. Other commonly used sequences included chemotherapy followed by CDK4/6i combined with AIs (10.3%), CDK4/6i combined with AIs followed by chemotherapy (8.8%), and CDK4/6 combined with AIs followed by everolimus combined with AIs (8.8%) [35]. The study by Basile et al. in 2021 also investigated treatment patterns in 1L and 2L in 717 women with HR+/HER2− mBC who were treated between 2008 and 2020 in two Italian oncology departments. In this patient population, CDK4/6i combined with ET was received by 20% of patients in 1L and 8% of patients in 2L [36]. In the 1L setting, 27% of patients received chemotherapy followed by ET or chemotherapy in 2L, and 35% received ET in 1L followed by ET or chemotherapy in 2L. Only 3% of the cohort received CDK4/6i combined with ET in 1L followed by chemotherapy in 2L, 3% of patients received CDK4/6i combined with ET in 1L followed by ET in 2L, and 6% of patients received ET in 1L followed by CDK4/6i combined with ET in 2L [36].
Two studies compared the clinical effectiveness of different CDK4/6i treatment sequences (ESM) [36, 37]. Basile et al. reported that patients receiving CDK4/6i combined with chemotherapy in 1L followed by chemotherapy in 2L had significantly worse OS than those receiving CDK4/6i combined with ET in 1L followed by ET in 2L (hazard ratio: 6.95, p = 0.011) [36]. Another study by Jeong et al. in 2021 of a cohort of 88 patients with HR+/HER2− mBC in South Korea showed that patients who received CDK4/6i combined with ET in 1L followed by everolimus plus exemestane in 2L had longer mOS than patients who received the inverse sequence (46.8 vs 38.9 months; p = 0.151) [37].
3.5.2 Clinical Effectiveness
Forty clinical studies reported mPFS and 23 studies reported mOS of patients receiving CDK4/6i by line of therapy. Survival outcomes were reported from 1L to fourth line and beyond, and several studies reported outcomes for multiple lines of therapy. Median PFS and mOS outcomes reported in each study are summarized in Tables 3 and 4, respectively, along with the CDK4/6i treatment regimen and prior treatment received. To investigate the potential link between treatment sequence and survival, we grouped the data by the treatment class received prior to CDK4/6i, which included chemotherapy, ET, targeted therapy, and a mixture of therapies. Overall, clinical studies showed that the use of CDK4/6i-based regimens for aBC/mBC in 1L (Fig. 2) or 2L (Fig. 3) was associated with longer mPFS than in later lines of therapy beyond 2L (Fig. 4 for 2L+ to 3L and Fig. 5 for 3L+). Median PFS by line of therapy ranged from a maximum of 36.7 months in 1L to a minimum of 3.4 months in 3L or later (Table 3). Some studies reported mPFS and mOS grouped across multiple lines of therapy in which CDK4/6i was used; therefore, these were categorized separately (e.g., both 1L and 2L). Results from these studies followed the same trend of worse mPFS with later lines of therapy. Additionally, the type of therapy received in the line prior to CDK4/6i in the aBC/mBC setting did not appear to impact the mPFS within a line of therapy (Figs. 3, 4, 5). Overall, mOS by line of therapy generally showed a trend consistent with mPFS (Figs. 6, 7, 8). Median OS by line of therapy ranged from a maximum of 61.7 months in 1L to a minimum of 13 months in 3L or later (Table 4). However, mOS was reported in fewer studies and was not reached in many studies, thus the mOS trend was less conclusive.
Median progression-free survival (PFS, months) for cyclin-dependent kinase 4/6 inhibitor treatment are presented in the first-line setting. Each included patient population is presented along the X-axis with a unique reference number. The size of each bubble represents the sample size. A complete list of included studies and reported PFS is presented in Table 3. Ref # reference number
Median progression-free survival (PFS, months) for cyclin-dependent kinase 4/6 inhibitor treatments are presented in the first-line (1L) to second-line (2L) setting. Each included patient population is presented along the X-axis with a unique reference number. The size of each bubble represents the sample size. Data were further grouped and color coded by the treatment received prior to cyclin-dependent kinase 4/6 inhibitor treatment. A complete list of included studies and reported PFS is presented in Table 3. #L number of lines of therapy, CT chemotherapy, ET endocrine therapy, NR not reported, Ref # reference number
Median progression-free survival (PFS, months) for cyclin-dependent kinase 4/6 inhibitor treatment are presented in the second-line plus (2L+) to third-line (3L) setting. Each included patient population is presented along the X-axis with a unique reference number. The size of each bubble represents the sample size. Data were further grouped and color coded by the treatment received prior to cyclin-dependent kinase 4/6 inhibitor treatment. A complete list of included studies and reported PFS is presented in Table 3. #L number of lines of therapy, CT chemotherapy, NR not reported, Ref # reference number
Median progression-free survival (PFS) for cyclin-dependent kinase 4/6 inhibitor treatment are presented in the third-line plus (3L+) setting. Each included patient population is presented along the X-axis with a unique reference number. The size of each bubble represents the sample size. Data were further grouped and color coded by the treatment received prior to cyclin-dependent kinase 4/6 inhibitor treatment. A complete list of included studies and reported PFS are presented in Table 3. #L number of lines of therapy, 4L fourth line, 6L sixth line, CT chemotherapy, ET endocrine therapy, NR not reported, Ref # reference number
Median overall survival (OS, months) for cyclin-dependent kinase 4/6 inhibitor treatment is presented in the first-line setting. Each included patient population is presented along the X-axis with a unique reference number. The size of each bubble represents the sample size. A complete list of included studies and reported OS is presented in Table 4. #L number of lines of therapy, Ref # reference number
Median overall survival (OS, months) for cyclin-dependent kinase 4/6 inhibitor treatment is presented in the first-line plus (1L+) to second-line (2L) setting. Each included patient population is presented along the X-axis with a unique reference number. The size of each bubble represents the sample size. Data were further grouped and color coded by the treatment received prior to cyclin-dependent kinase 4/6 inhibitor treatment. A complete list of included studies and reported OS is presented in Table 4. #L number of lines of therapy, 3L third line, ET endocrine therapy, Ref # reference number
Median overall survival (OS, months) for cyclin-dependent kinase 4/6 inhibitor (CDK4/6i) treatment is presented in the second line plus (2L+) setting. Each included patient population is presented along the X-axis with a unique reference number. The size of each bubble represents the sample size. Data were further grouped and color coded by the treatment received prior to CDK4/6i treatment. A complete list of included studies and reported OS is presented in Table 4. #L number of lines of therapy, 1L first line, 3L third line, 4L fourth line, CT chemotherapy, ET endocrine therapy, Ref # reference number
In addition, 19 studies reported an association of specific clinical characteristics with clinical outcomes for patients receiving CDK4/6i (ESM). Common factors that are more likely to be associated with better efficacy include lower Eastern Cooperative Oncology Group performance status (vs higher), bone-only metastasis (vs visceral), receipt of fewer prior therapy lines (vs more), no prior chemotherapy (vs yes), no prior ET (vs yes), and treatment with CDK4/6i in earlier lines of therapy (vs later). Princic et al. was the only study that reported predictors of subsequent systemic therapy type following CDK4/6i progression. For example, patients who were rechallenged on a subsequent CDK4/6i-based regimen, compared with those who received subsequent chemotherapy, were likely to be older, have bone-only metastasis (vs visceral), have an AI as the prior CDK4/6i treatment partner (vs fulvestrant), have received any prior chemotherapy, and have lower breast cancer-related costs. Additionally, patients who were rechallenged on a subsequent CDK4/6i-based regimen were less likely to have received prior CDK4/6i for longer than 6 months compared with patients who received subsequent chemotherapy. Patients with recurrent (vs de novo) disease who received ET prior to metastasis were also less likely to receive subsequent ET than chemotherapy [38]. In support of the trend of PFS data by line of therapy shown in Figs. 2, 3, 4 and 5, Zhong et al. reported that a greater survival benefit was shown in patients who received palbociclib as 1L or 2L treatment for aBC based on univariate analyses; additionally, no prior chemotherapy for aBC, ≤ 1 line of prior ET, no primary resistance to ET, a fewer number of visceral metastasis sites, and no liver metastasis were significantly associated with a greater survival benefit [39]. Interestingly, Whitaker et al. reported an association between receiving CDK4/6i in 1L and OS in real-world populations by ethnicity. Patients who received CDK4/6i as part of 1L and 2L treatment had a similar mOS across ethnic groups; however, the non-Hispanic Black patient subgroup who received CDK4/6i only at 2L but not at 1L had worse OS after 2L initiation compared with White patients [40].
3.6 Post-CDK4/6i Treatment Patterns and Efficacy
Treatments following progression on CDK4/6i were investigated or reported across various treatment lines in 41 clinical studies (ESM). Studies that investigated or reported the first subsequent therapy received after CDK4/6i treatment primarily evaluated therapies used in 2L or 3L settings but ranged up to fourth line and beyond. Endocrine therapy was the most frequently investigated or reported post-CDK4/6i therapy, where it was used as monotherapy in 21 studies, in combination with chemotherapy in two studies, and in combination with targeted therapies in 11 studies. Chemotherapy was the second most investigated or reported post-CDK4/6i treatment, where it was used as a monotherapy in 20 studies. Use of targeted therapies post-CDK4/6i was investigated or reported in 22 studies, of which ten investigated or reported the use of everolimus-based combinations, including the TRINITI-1 clinical trial that investigated everolimus in combination with ribociclib and exemestane [41]. Rechallenging with a subsequent CDK4/6i-based regimen following CDK4/6i was investigated or reported in ten studies. Specifically, abemaciclib following initial palbociclib was investigated in two studies [42,43,44]. A study by Eziokwu et al. investigated the use of palbociclib combined with ET or abemaciclib combined with ET following progression on treatment with palbociclib combined with ET [45]. Two studies reported the use of investigational drugs as subsequent therapy following CDK4/6i; however, the specific treatment(s) used were not reported [48, 49].
Median PFS for post-CDK4/6i treatments was reported in 24 studies and mOS was reported in 14 studies, as presented in Tables 5 and 6. Efficacy of subsequent chemotherapy, ET, targeted therapies such as mTORi or alternate CDK4/6i treatment, or a mixture of these treatments following progression on CDK4/6i is shown in Fig. 9a for mPFS and Fig. 9b for mOS. Median PFS for 2L post-CDK4/6i treatments ranged from 3.25 months on fulvestrant to 17.7 months on either ET or ET combined with targeted agents [50, 51]. In 2L and later lines of therapy, most studies had a similar mPFS with a range from 1.8 months on standard-of-care therapies to 9.1 months on everolimus [52, 53]. One study conducted in a cohort of 30 patients with HR+/HER2− mBC reported a relatively higher mPFS of 11.8 months among patients rechallenged with a subsequent CDK4/6i-based regimen (palbociclib combined with ET or abemaciclib combined with ET) [45]. Despite differences in subsequent therapy type, similar mPFS was observed for most studies in the 2L and later setting. In 3L, mPFS ranged from 4.7 months on chemotherapy [51] to 10.3 months on eribulin [54]. In the 3L and later line setting, mPFS ranged from 1.9 months on TTC-352, a selective human ER partial agonist [55] to 6.2 months on chemotherapy [56]. Overall trends in mPFS by treatment type in the 3L or later lines subgroups were unclear because of the limited data. In two studies that reported mPFS in fourth line and later lines, mPFS was very similar between three treatments, with a range from 4 months on eribulin to 4.2 months on ET alone or in combination with targeted agents [51, 54].
Median progression-free survival [PFS, months] (A) and median overall survival [OS, months] (B) of post-cyclin-dependent kinase 4/6 inhibitor (CDK4/6i) treatments grouped by the therapy line in which subsequent therapy was received. Each subsequent treatment is presented along the X-axis with a unique reference number. The size of each bubble represents the sample size. Data were further grouped and color coded by the subsequent treatment received. Complete lists of included studies and reported PFS and OS are presented in Tables 5 and 6, respectively. #L number of lines of therapy, 2L second line, 3L third line, 4L fourth line, CT chemotherapy, ET endocrine therapy, NR not reported, Ref # reference number
Median OS was either not reached or reported from the start of 1L in many studies (Table 6). Therefore, the mOS trend was less conclusive. Available evidence suggests that post-CDK4/6i targeted treatments and mixed treatment regimens had a similar mOS within the 2L and later lines subgroup that ranged from 13.6 months on ET to 37.4 months with everolimus-based therapy [53, 57]. Median OS in 3L was reported by a single study as 11.8 months for chemotherapy, ET, or everolimus combined with exemestane [58]. One study reported post-CDK4/6i mOS in 3L and later lines as 23.9 months for chemotherapy, ET, or targeted therapy [59]. Overall, there are insufficient data to make clear conclusions about optimal post-CDK4/6i treatment sequencing; however, the current evidence available suggests that no major differences in mOS exist across treatment types following progression on CDK4/6i.
3.7 Ongoing Treatment Sequencing and Post-CDK4/6i Clinical Studies
While not formally included in our TLR on CDK4/6i treatment patterns, we identified and summarized ongoing clinical studies investigating CDK4/6i-based treatment sequencing or therapies following progression on CDK4/6i. A total of 48 relevant ongoing clinical trials were identified, including six phase I trials, six phase I/II trials, 15 phase II trials, and 8 phase III trials. Thirteen ongoing observational studies of post-CDK4/6i treatments were also identified. These studies are summarized by order of trial phase in the ESM. The search identified one phase III clinical trial (SONIA; NCT03425838), which is currently recruiting patients to investigate the efficacy of CDK4/6i plus letrozole or anastrozole in 1L followed by fulvestrant in 2L, compared with letrozole or anastrozole in 1L followed by CDK4/6i combined with fulvestrant in 2L [60]. The other 47 ongoing clinical trials are investigating treatments following progression on CDK4/6i, including various targeted therapies (e.g., CDK7 inhibitor SY-5609, AKT inhibitor ipatasertib, and phosphoinositide 3-kinase/mTOR inhibitor gedatolisib), as well as rechallenging on a subsequent CDK4/6i-based regimen. Comparators in ongoing studies include placebo, ET, and treatment of a physician’s choice. The ongoing observational studies are non-interventional and are investigating treatment patterns in various countries around the world in patients who initiated treatment with CDK4/6i (most commonly specified as palbociclib-based therapy) with and without evidence of disease progression (ESM).
4 Discussion
This review sought to provide a comprehensive summary of the current literature on CDK4/6i treatment patterns in breast cancer, including recent reviews and clinical studies. This review represents the most recent data in this rapidly evolving field based on an updated search in October 2022. We identified several reviews that described the preferred treatment strategies for the HR+/HER2− aBC/mBC patient population based on currently available results from clinical trials, and studies that reported current treatment patterns with CDK4/6i and associated clinical outcomes. Overall, these reviews offered conclusions that align with key breast cancer treatment guidelines, with CDK4/6i combined with ET as the preferred regimen in 1L or 2L for patients with HR+/HER2− aBC/mBC if CDK4/6i was not previously received in this setting. However, optimal treatment sequencing and treatment selection following disease progression on a CDK4/6i-based regimen are still unclear.
Several clinical studies reported detailed CDK4/6i treatment patterns by line of therapy. Notably, the study by Basile et al. reported a relatively smaller proportion of patients who received CDK4/6i combined with ET in 1L or 2L compared with the study by Goldschmidt et al. that also reported the proportion of patients who received CDK4/6i treatment patterns across two lines of therapy [36]. This is likely owing to differences in data collection periods, as data were collected in the Basile et al. study between 2008 and 2020, while data were collected in the Goldschmidt et al. study between February and June 2017; given CDK4/6i were approved in Europe between 2016 and 2018, the lower CDK4/6i usage in Basile et al. may be due to inclusion of the earlier period in which CDK4/6i had not yet been approved. Similarly, the relatively lower CDK4/6i usage reported in the study by Davie et al. is likely also related to the study’s data collection period, which occurred in 2017 when CDK4/6i were newly approved in Europe [30].
The clinical evidence suggested that the use of CDK4/6i in earlier lines of therapy for aBC/mBC (i.e., 1L–2L) resulted in relatively higher mPFS, and the limited data suggest a similar trend for mOS. However, a general trend of decreasing mOS with an increasing line of therapy is expected as this may partially be because patients who do not respond to multiple lines of therapy are likely to have more severe disease. The ongoing clinical studies identified in the literature showed there are very few studies specifically investigating CDK4/6i in the context of treatment sequencing (e.g., CDK4/6i in 1L vs in 2L); however, emerging clinical data from ongoing studies of post-CDK4/6i treatments may inform optimal treatment selection following progression on CDK4/6i. Despite the limited evidence, current data suggest survival outcomes are similar between post-CDK4/6i use of chemotherapy, ET, targeted treatments, or mixed treatments across treatment lines. Additionally, there was some variation in mPFS and mOS observed by lines of therapy that may be partially attributed to differences in study design and patient characteristics among the included studies.
The TLR described in this study was conducted in a transparent and thorough manner that followed PRISMA guidelines where possible, such as using a detailed and structured search strategy, a priori PICOS criteria, and a structured two-level screening process. Several limitations were also identified throughout this review. This TLR included studies published since 2015, which encompasses the early marketing period of the CDK4/6i. While treatment usage patterns are expected to change during the early marketing period and stabilize over time, only two studies reported data collection periods ending in 2015–16, and one study reported treatment patterns specifically for patients in an early access program that provides access to innovative drugs in advance of commercial availability [61]. In addition, as specified in the PICOS criteria, the studies included were limited to English-language publications.
Key survival outcomes of mPFS and mOS, as reported in 40 studies and 23 studies, respectively, were compared across CDK4/6i use in different lines of therapy. While outcomes cannot be directly compared across clinical studies given differences in study design and patient selection, naïve comparisons can provide a useful overview of trends across many studies in the absence of comparative studies, or similar studies that can enable more rigorous indirect comparisons. Additionally, the heterogeneity across observational studies in terms of study design, patient population, and reporting of results resulted in some challenges in synthesizing results across studies, which limited the outcomes that could be compared. For example, outcomes such as clinical benefit rate or time to subsequent treatment were only reported in a few studies, and therefore could not be meaningfully compared across studies. Furthermore, variability in patient selection criteria for CDK4/6i and subsequent treatments could also have contributed to the heterogeneity observed.
This review highlighted some key data gaps. Though there is much evidence to support CDK4/6i combined with ET as the preferred regimen in 1L or 2L treatment for patients with HR+/HER2− aBC/mBC without previous use of CDK4/6i therapy, there are very limited comparative data from studies that have investigated optimal sequencing for CDK4/6i. Only one ongoing clinical trial (SONIA) was identified that is investigating CDK4/6i usage in 1L versus 2L [60]. More research is needed to inform optimal treatment sequencing of CDK4/6i use in aBC/mBC. Results from SONIA, as well as ongoing trials and real-world studies investigating the efficacy, effectiveness, and safety of treatments following progression on CDK4/6-based regimens will provide important insights. Furthermore, there is also limited evidence on biomarkers or prognostic factors to guide the selection of post-CDK4/6i treatment. Biomarkers can guide the treatment strategy for individual patients, such as by using next-generation sequencing to detect PIK3CA mutations or gBRCA1/2 mutations to determine suitability for targeted therapies [6, 16, 17]. Machine-learning approaches leveraging real-world data could be considered for predicting optimal treatment for individual patients based on demographic and clinical characteristics. A study that applied machine learning algorithms to real-world data found that less than 50% of patients in the cohort received optimal treatment with CDK4/6i in 1L and 2L as predicted by the model [32]. Studies focusing on or reporting subgroup outcomes in specific patient populations (e.g., high Eastern Cooperative Oncology Group performance status) would also be helpful given the heterogeneity within mBC. Last, few studies reported safety, patient-reported, or quality-of-life outcomes associated with different treatment sequences, which are important treatment selection criteria to consider in conjunction with the clinical benefit.
5 Conclusions
This TLR was conducted to examine the current evidence on CDK4/6i treatment patterns in aBC/mBC. Reviews commonly discussed treatment with ET monotherapy, followed by CDK4/6i combined with ET, followed by a targeted therapy combined with ET, or ET or chemotherapy monotherapy. Clinical studies reported or investigated similar treatment sequences: patients with aBC/mBC generally received ET monotherapy, chemotherapy monotherapy, or everolimus (monotherapy or combined with ET), followed by CDK4/6i or vice versa. While optimal treatment sequencing with CDK4/6i remains unclear owing to a lack of available comparative clinical evidence, our findings suggest that CDK4/6i are an effective treatment option for HR+/HER2− aBC/mBC at any treatment line, while being most effective in earlier lines of treatment. In accordance with recommendations from multiple breast cancer treatment guidelines, overall, this study supports that use of CDK4/6i in earlier lines of therapy are clinically beneficial. Data also suggest survival outcomes are similar between post-CDK4/6i use of chemotherapy, ET, targeted treatments, or mixed treatments across treatment lines. Additional research is needed to investigate optimal sequencing of treatments following progression on CDK4/6i-based therapy, and to identify patient populations that may benefit most from CDK4/6i therapy. As abemaciclib was also recently FDA and European Medicines Agency approved for the adjuvant treatment of eBC among patients with a high risk for recurrence [20, 62], it will be interesting to consider how CDK4/6i treatment sequencing or CDK4/6i rechallenging may shift across stages of eBC, aBC, and mBC.
References
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–49.
NIH SEER Program. Cancer stat facts: female breast cancer subtypes. 2022. https://seer.cancer.gov/statfacts/html/breast-subtypes.html. Accessed 21 Mar 2023.
Seung S, Traore A, Pourmirza B, Fathers K, Coombes M, Jerzak K. A population-based analysis of breast cancer incidence and survival by subtype in Ontario women. Curr Oncol. 2020;27(2): e191.
Blows FM, Driver KE, Schmidt MK, Broeks A, Van Leeuwen FE, Wesseling J, et al. Subtyping of breast cancer by immunohistochemistry to investigate a relationship between subtype and short and long term survival: a collaborative analysis of data for 10,159 cases from 12 studies. PLoS Med. 2010;7(5): e1000279.
Breastcancer.org. Breast cancer stages. 2022. https://www.breastcancer.org/pathology-report/breast-cancer-stages. Accessed 21 Mar 2023.
National Comprehensive Cancer Network, Inc. NCCN clinical practice guidelines in oncology (NCCN Guidelines®) for breast cancer Version 2. 2022. https://www.nccn.org/professionals/physician_gls/pdf/breast.pdf. Accessed 27 June 2022.
US Food and Drug Administration. Highlights of prescribing information: Ibrance (palbociclib) capsules. 2019. https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/207103s008lbl.pdf. Accessed 28 July 2022.
Li J, Fu F, Yu L, Huang M, Lin Y, Mei Q, et al. Cyclin-dependent kinase 4 and 6 inhibitors in hormone receptor-positive, human epidermal growth factor receptor-2 negative advanced breast cancer: a meta-analysis of randomized clinical trials. Breast Cancer Res Treat. 2020;180(1):21–32.
Hortobagyi GN, Stemmer SM, Burris HA, Yap Y-S, Sonke GS, Paluch-Shimon S, et al. Ribociclib as first-line therapy for HR-positive, advanced breast cancer. N Engl J Med. 2016;375(18):1738–48.
Finn RS, Martin M, Rugo HS, Jones S, Im S-A, Gelmon K, et al. Palbociclib and letrozole in advanced breast cancer. N Engl J Med. 2016;375(20):1925–36.
Cristofanilli M, Turner NC, Bondarenko I, Ro J, Im S-A, Masuda N, et al. Fulvestrant plus palbociclib versus fulvestrant plus placebo for treatment of hormone-receptor-positive, HER2-negative metastatic breast cancer that progressed on previous endocrine therapy (PALOMA-3): final analysis of the multicentre, double-blind, phase 3 randomised controlled trial. Lancet Oncol. 2016;17(4):425–39.
Goetz MP, Toi M, Campone M, Sohn J, Paluch-Shimon S, Huober J, et al. MONARCH 3: abemaciclib as initial therapy for advanced breast cancer. J Clin Oncol. 2017;35(32):3638–46.
Tripathy D, Im S-A, Colleoni M, Franke F, Bardia A, Harbeck N, et al. Ribociclib plus endocrine therapy for premenopausal women with hormone-receptor-positive, advanced breast cancer (MONALEESA-7): a randomised phase 3 trial. Lancet Oncol. 2018;19(7):904–15.
Im S-A, Lu Y-S, Bardia A, Harbeck N, Colleoni M, Franke F, et al. Overall survival with ribociclib plus endocrine therapy in breast cancer. N Engl J Med. 2019;381(4):307–16.
Sledge GW, Toi M, Neven P, Sohn J, Inoue K, Pivot X, et al. The effect of abemaciclib plus fulvestrant on overall survival in hormone receptor-positive, ERBB2-negative breast cancer that progressed on endocrine therapy: MONARCH 2: a randomized clinical trial. JAMA Oncol. 2020;6(1):116–24.
Gennari A, André F, Barrios C, Cortés J, De Azambuja E, DeMichele A, et al. ESMO clinical practice guideline for the diagnosis, staging and treatment of patients with metastatic breast cancer. Ann Oncol. 2021;32(12):1475–95.
Burstein HJ, Somerfield MR, Barton DL, Dorris A, Fallowfield LJ, Jain D, et al. Endocrine treatment and targeted therapy for hormone receptor-positive, human epidermal growth factor receptor 2-negative metastatic breast cancer: ASCO guideline update. J Clin Oncol. 2021;39(35):3959–77.
Gil-Gil M, Alba E, Gavilá J, de la Haba-Rodríguez J, Ciruelos E, Tolosa P, et al. The role of CDK4/6 inhibitors in early breast cancer. Breast. 2021;58:160–9.
Agostinetto E, Caparica R, de Azambuja E. CDK4/6 inhibition in HR-positive early breast cancer: are we putting all eggs in one basket? ESMO Open. 2020;5(6):e001132.
US Food and Drug Administration. Highlights of prescribing information: Verzenio (abemaciclib) tablets. 2021. https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/208716s006s007s008lbl.pdf. Accessed 28 July 2022.
Nicolini A, Ferrari P, Duffy MJ. Prognostic and predictive biomarkers in breast cancer: past, present and future. Semin Cancer Biol. 2018;52(Pt 1):56–73.
Thill M, Lüftner D, Kolberg-Liedtke C, Albert US, Banys-Paluchowski M, Bauerfeind I, et al. AGO recommendations for the diagnosis and treatment of patients with locally advanced and metastatic breast cancer: update 2022. Breast Care. 2022;17(4):421–9.
Loibl S, Poortmans P, Morrow M, Denkert M, Curigliano G. Breast cancer. Lancet. 2021;397(10286):1750–69.
Loibl S, Marme F, Martin M, Untch M, Bonnefoi H, Kim SB, et al. Palbociclib for residual high-risk invasive HR-positive and HER2-negative early breast cancer: the Penelope-B Trial. J Clin Oncol. 2021;39(14):1518–30.
Mayer EL, Dueck AC, Martin M, Rubovszky G, Burstein HJ, Bellet-Ezquerra M, et al. Palbociclib with adjuvant endocrine therapy in early breast cancer (PALLAS): interim analysis of a multicentre, open-label, randomised, phase 3 study. Lancet Oncol. 2021;22(2):212–22.
Johnston SRD, Harbeck N, Hegg R, Toi M, Martin M, Shao ZM, et al. Abemaciclib combined with endocrine therapy for the adjuvant treatment of HR+, HER2−, node-positive, high-risk, early breast cancer (monarchE). J Clin Oncol. 2020;38(34):3987–98.
Slamon DJ, Fasching PA, Patel R, Verma S, Hurvitz SA, Chia SK, et al. NATALEE: phase III study of ribociclib (RIBO)+ endocrine therapy (ET) as adjuvant treatment in hormone receptor-positive (HR+), human epidermal growth factor receptor 2-negative (HER2–) early breast cancer (EBC). J Clin Oncol. 2019;37(15_suppl):TPS597.
US Food and Drug Administration (FDA). Highlights of prescribing information: Kisqali (ribociclib) tablets. 2017. https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/209092s013,209935s021lbl.pdf. Accessed 28 July 2022.
Zhang J, Meng Y, Wang B, Wang L, Cao J, Tao Z, et al. Dalpiciclib combined with pyrotinib and letrozole in women with HER2-positive, hormone receptor-positive metastatic breast cancer (LORDSHIPS): a phase Ib study. Front Oncol. 2022;12: 775081.
Davie A, Cuyun Carter G, Rider A, Bailey A, Lewis K, Price G, et al. Real-world clinical profile, treatment patterns and patient-reported outcomes in a subset of HR+/HER2− advanced breast cancer patients with poor prognostic factors: data from an international study. ESMO Open. 2021;6(4): 100226.
Meegdes M, Geurts SME, Erdkamp FLG, Dercksen MW, Vriens B, Aaldering KNA, et al. The implementation of CDK 4/6 inhibitors and its impact on treatment choices in HR+/HER2− advanced breast cancer patients: a study of the Dutch SONABRE Registry. Int J Cancer. 2021;30:30.
Cui ZL, Kadziola Z, Lipkovich I, Faries DE, Sheffield KM, Carter GC. Predicting optimal treatment regimens for patients with HR+/HER2- breast cancer using machine learning based on electronic health records. J Comp Eff Res. 2021;10(9):777–95.
Sawaki M, Muramatsu Y, Togo K, Laurent T, Iwata H. Real-world treatment patterns of palbociclib and blood count monitoring in patients with advanced breast cancer in Japan. Future Oncol. 2022;18(17):2101–11.
Engler T, Fasching PA, Luftner D, Hartkopf AD, Muller V, Kolberg H-C, et al. Implementation of CDK4/6 inhibitors and its influence on the treatment landscape of advanced breast cancer patients: data from the real-world registry PRAEGNANT. Geburtshilfe Frauenheilkunde. 2022;82(10):1055–67.
Goldschmidt D, Dalal AA, Romdhani H, Kelkar S, Guerin A, Gauthier G, et al. Current treatment patterns among postmenopausal women with HR+/HER2− metastatic breast cancer in US community oncology practices: an observational study. Adv Ther. 2018;35(4):482–93.
Basile D, Gerratana L, Corvaja C, Pelizzari G, Franceschin G, Bertoli E, et al. First- and second-line treatment strategies for hormone-receptor (HR)-positive HER2-negative metastatic breast cancer: a real-world study. Breast. 2021;57:104–12.
Jeong H, Jeong JH, Kim JE, Ahn JH, Jung KH, Kim SB. Comparison of the effectiveness and clinical outcome of everolimus followed by CDK4/6 inhibitors with the opposite treatment sequence in hormone receptor-positive, HER2-negative metastatic breast cancer. Cancer Res Treat. 2021;23:23.
Princic N, Aizer A, Tang DH, Smith DM, Johnson W, Bardia A. Predictors of systemic therapy sequences following a CDK 4/6 inhibitor-based regimen in post-menopausal women with hormone receptor positive, HEGFR-2 negative metastatic breast cancer. Curr Med Res Opin. 2019;35(1):73–80.
Zhong B, Zhang J, Wu J, Sun L, Li S, Zeng X, et al. Efficacy and safety of palbociclib plus endocrine therapy for patients with HR+/HER2− advanced breast cancer in real-world clinical practice. Ann Transl Med. 2022;10(6):362.
Whitaker KD, Wang X, Ascha M, Showalter TN, Lewin HG, Calip GS, et al. Racial inequities in second-line treatment and overall survival among patients with metastatic breast cancer. Breast Cancer Res Treat. 2022;196(1):163–73.
Bardia A, Hurvitz SA, DeMichele A, Clark AS, Zelnak A, Yardley DA, et al. Phase I/II trial of exemestane, ribociclib, and everolimus in women with HR+/HER2 advanced breast cancer after progression on CDK4/6 inhibitors (TRINITI-1). Clin Cancer Res. 2021;27(15):4177–85.
Wander SA, Han HS, Zangardi ML, Niemierko A, Mariotti V, Kim LSL, et al. Clinical outcomes with abemaciclib after prior CDK4/6 inhibitor progression in breast cancer: a multicenter experience. J Natl Compr Cancer Netw. 2021;1–8. https://doi.org/10.6004/jnccn.2020.7662
Wander SA, Zangardi M, Niemierko A, Kambadakone A, Kim LS, Xi J, et al. A multicenter analysis of abemaciclib after progression on palbociclib in patients (pts) with hormone receptor-positive (HR+)/HER2-metastatic breast cancer (MBC). J Clin Oncol. 2019;37(15_suppl):1057.
Tamragouri K, Cobleigh MA, Rao RD. Abemaciclib with or without fulvestrant for the treatment of hormone receptor-positive and HER2-negative metastatic breast cancer with disease progression following prior treatment with palbociclib. J Clin Oncol. 2019;37(15_suppl):e12533.
Eziokwu AS, Varella L, Kruse ML, Jia X, Moore HC, Budd GT, et al. Real-world evidence evaluating continuation of CDK4/6 inhibitors beyond first progression in hormone receptor-positive (HR+) metastatic breast cancer. J Clin Oncol. 2019;37(15_suppl):e12538
dos Anjos CH, Razavi P, Herbert J, Colon J, Gill K, Modi S, et al. A large retrospective analysis of CDK 4/6 inhibitor retreatment in ER+ metastatic breast cancer (MBC). J Clin Oncol. 2019;37(15_suppl):1053.
Fountzilas E, Koliou GA, Vozikis A, Rapti V, Nikolakopoulos A, Boutis A, et al. Real-world clinical outcome and toxicity data and economic aspects in patients with advanced breast cancer treated with cyclin-dependent kinase 4/6 (CDK4/6) inhibitors combined with endocrine therapy: the experience of the Hellenic Cooperative Oncology Group. ESMO Open. 2020;5(4):8.
Petracci F, Abuin GG, Pini A, Chacon M. RENATA study-Latin American prospective experience: clinical outcome of patients treated with palbociclib in hormone receptor-positive metastatic breast cancer-real-world use. Ecancermedicalscience. 2020;14:1058.
Masuda N, Mukai H, Inoue K, Rai Y, Ohno S, Ohtani S, et al. Analysis of subsequent therapy in Japanese patients with hormone receptor-positive/human epidermal growth factor receptor 2-negative advanced breast cancer who received palbociclib plus endocrine therapy in PALOMA-2 and -3. Breast Cancer. 2021;28(2):335–45.
Martin JM, Handorf EA, Montero AJ, Goldstein LJ. Systemic therapies following progression on first-line CDK4/6-inhibitor treatment: analysis of real-world data. Oncologist. 2022;27(6):441–6.
Xi J, Oza A, Thomas S, Ademuyiwa F, Weilbaecher K, Suresh R, et al. Retrospective analysis of treatment patterns and effectiveness of palbociclib and subsequent regimens in metastatic breast cancer. J Natl Compr Cancer Netw. 2019;17(2):141–7.
Bidard F-C, Kaklamani VG, Neven P, Streich G, Montero J, Forget F, et al. Elacestrant (oral selective estrogen receptor degrader) versus standard endocrine therapy for estrogen receptor-positive, human epidermal growth factor receptor 2-negative advanced breast cancer: results from the randomized phase III EMERALD Trial. J Clin Oncol. 2022;40(28):3246–56.
Kitano S, Honda A, Itoi N, Lee T. Everolimus for treating hormone receptor-positive metastatic breast cancer previously treated with cyclin-dependent kinase 4/6 inhibitors. Anticancer Res. 2022;42(8):3913–9.
Mougalian SS, Feinberg BA, Wang E, Alexis K, Chatterjee D, Knoth Rl, et al. Observational study of clinical outcomes of eribulin mesylate in metastatic breast cancer after cyclin-dependent kinase 4/6 inhibitor therapy. Future Oncol. 2019;15(34):3935–44.
Dudek AZ, Liu LC, Fischer JH, Wiley El, Sachdev JC, Bleeker J, et al. Phase 1 study of TTC-352 in patients with metastatic breast cancer progressing on endocrine and CDK4/6 inhibitor therapy. Breast Cancer Res Treat. 2020;183(3):617–27.
Seki H, Sakurai T, Sakurada A, Kinoshita T, Shimizu K. Subsequent-abemaciclib treatment after disease progression on palbociclib in patients with ER-positive HER2-negative metastatic breast cancer. Anticancer Res. 2022;42(2):1099–106.
Hayama S, Nakamura R, Miyaki T, Itami M, Yamamoto N. Treatment strategy for patients with HR-positive HER2-negative metastatic breast cancer that progressed on CDK4/6 inhibitors. Breast Care. 2022;17(1):16–23.
Giridhar K, Choong G, Leon-Ferre R, O'Sullivan C, Ruddy K, Haddad T, et al. Abstract P6-18-09: clinical management of metastatic breast cancer (MBC) after CDK 4/6 inhibitors: a retrospective single-institution study. Cancer Res. 2019;79(4_Supplement):P6-18-09.
Rossi L, Biagioni C, McCartney A, Migliaccio I, Curigliano G, Sanna G, et al. Clinical outcomes after palbociclib with or without endocrine therapy in postmenopausal women with hormone receptor positive and HER2-negative metastatic breast cancer enrolled in the TREnd trial. Breast Cancer Res. 2019;21(1):71.
van Ommen-Nijhof A, Konings IR, van Zeijl CJJ, Uyl-de Groot CA, van der Noort V, Jager A, et al. Selecting the optimal position of CDK4/6 inhibitors in hormone receptor-positive advanced breast cancer: the SONIA study: study protocol for a randomized controlled trial. BMC Cancer. 2018;18(1):1146.
Boer K, Rubovszky G, Rokszin G, Abonyi-Toth Z, Foldesi C, Dank M. Demographic characteristics and treatment patterns among patients receiving palbociclib for HR+/HER2− advanced breast cancer: a nationwide real-world experience. OncoTargets Ther. 2021;14:3971–81.
European Medicines Agency (EMA). Summary of product characteristics: Verzenios. 2022. https://www.ema.europa.eu/en/documents/product-information/verzenios-epar-product-information_en.pdf. Accessed 28 July 2022.
Agrawal C, Goyal P, Agarwal A, Tripathi R, Dodagoudar C, Baghmar S, et al. Multicentric real world evidence with palbociclib in hormone positive HER2 negative metastatic breast cancer in Indian population. Sci Rep. 2021;11(1):16236.
Liu C, Li T, Tao Z, Cao J, Wang L, Zhang J, et al. Clinical outcomes of 130 patients with hormone receptor-positive and human epidermal growth factor receptor 2-negative metastatic breast cancer treated with palbociclib plus endocrine therapy and subsequent therapy: a real-world single-center retrospective study in China. Med Sci Monit. 2020;26: e927187.
Rath S, Elamarthi P, Parab P, Gulia S, Nandhana R, Mokal S, et al. Efficacy and safety of palbociclib and ribociclib in patients with estrogen and/or progesterone receptor positive, HER2 receptor negative metastatic breast cancer in routine clinical practice. PLoS ONE. 2021;16(7): e0253722.
Zhang L, Song G, Shao B, Xu L, Xiao Y, Wang M, et al. The efficacy and safety of palbociclib combined with endocrine therapy in patients with hormone receptor-positive HER2-negative advanced breast cancer: a multi-center retrospective analysis. Anti Cancer Drugs. 2021;6:6.
Schneeweiss A, Ettl J, Luftner D, Beckmann, Belleville E, Fasching PA, et al. Initial experience with CDK4/6 inhibitor-based therapies compared to antihormone monotherapies in routine clinical use in patients with hormone receptor positive, HER2 negative breast cancer: data from the PRAEGNANT research network for the first 2 years of drug availability in Germany. Breast. 2020;54:88–95.
Neven P, Johnston SRD, Toi M, Sohn J, Inoue K, Pivot X, et al. MONARCH 2: subgroup analysis of patients receiving abemaciclib plus fulvestrant as first-line and second-line therapy for HR+, HER2− advanced breast cancer. Clin Cancer Res. 2021;10:10.
Palumbo R, Torrisi R, Sottotetti F, Presti D, Rita Gambaro A, Collova E, et al. Patterns of treatment and outcome of palbociclib plus endocrine therapy in hormone receptor-positive/HER2 receptor-negative metastatic breast cancer: a real-world multicentre Italian study. Ther Adv Med Oncol. 2021;13:1758835920987651.
Brufsky A, Mitra D, Davis KL, Nagar SP, McRoy L, Cotter MJ, et al. Treatment patterns and outcomes associated with palbociclib plus letrozole for postmenopausal women with HR+/HER2− advanced breast cancer enrolled in an expanded access program. Clin Breast Cancer. 2019;19(5):317-25.e4.
Lin J, McRoy L, Fisher MD, Hu N, Davis C, Mitra D, et al. Treatment patterns and clinical outcomes of palbociclib-based therapy received in US community oncology practices. Future Oncol. 2021;17(9):1001–11.
Varella L, Eziokwu AS, Jia X, Kruse M, Moore HCF, Budd GT, et al. Real-world clinical outcomes and toxicity in metastatic breast cancer patients treated with palbociclib and endocrine therapy. Breast Cancer Res Treat. 2019;176(2):429–34.
Choong GM, Liddell S, Ferre RAL, O’Sullivan CC, Ruddy KJ, Haddad TC, et al. Clinical management of metastatic hormone receptor-positive, HER2-negative breast cancer (MBC) after CDK 4/6 inhibitors: a retrospective single-institution study. Breast Cancer Res Treat. 2022;196(1):229–37.
Endo Y, Yoshimura A, Sawaki M, Hattori M, Kotani H, Kataoka A, et al. Time to chemotherapy for patients with estrogen receptor-positive breast cancer and cyclin-dependent kinase 4 and 6 inhibitor use. J Breast Cancer. 2022;25(4):296–306.
Fernandez-Cuerva C, Valencia JCDR, Bermejo RT. Effectiveness and safety of palbociclib plus endocrine therapy in hormone receptor-positive, HER2-negative metastatic breast cancer: real-world results. Can J Hosp Pharm. 2022;75(1):26–33.
Gharib KE, Macaron W, Kattan J, Salloum MA, Farhat F, Smith M, et al. Palbociclib and letrozole in hormone-receptor positive advanced breast cancer: predictive response and prognostic factors. Curr Probl Cancer. 2022;46(3): 100859.
Gousis C, Michoglou K, Lowe H, Kapiris M, Angelis V. Beyond first line CDK4/6 inhibitors (CDK4/6i) and aromatase inhibitors (AI) in patients with oestrogen receptor positive metastatic breast cancer (ER+ MBC): the Guy’s Cancer Centre experience. Clin Oncol. 2022;34(4): e178.
Ha MJ, Singareeka Raghavendra A, Kettner NM, Qiao W, Damodaran S, Layman RM, et al. Palbociclib plus endocrine therapy significantly enhances overall survival of HR+/HER2− metastatic breast cancer patients compared to endocrine therapy alone in the second-line setting: a large institutional study. Int J Cancer. 2022;150(12):2025–37.
Mo H, Ma F, Li Q, Zhang P, Yuan P, Wang J, et al. Treatment patterns and clinical outcomes in patients with metastatic breast cancer treated with palbociclib-based therapies: real-world data in the Han population. Chin Med J. 2022;135(14):1734–41.
Li J, Zhang X, Yang C, Lv Y, Yang H, Kong X, et al. Real-world effectiveness and sensitivity of palbociclib plus endocrine therapy in HR+/HER2− patients with metastatic breast cancer. Medicine. 2021;100(44): e27710.
Marschner N, Harbeck N, Thill M, Stickeler E, Zaiss M, Nusch A, et al. 232P Second-line therapies of patients with early progression under CDK4/6-inhibitor in first-line data from the registry platform OPAL. Ann Oncol. 2022;33:S643–4.
Sampedro-Gimeno T, Pampin-Sanchez R, Barbazan-Vazquez FJ, Reguero-Cuervo V, Galeazzi-Martinez V, Pelaez-Fernandez I. Observational real world data with palbociclib associated to hormone therapy for advanced breast carcinoma. Farm Hosp. 2021;45(6):329–34.
Shen L, Zhou J, Chen Y, Ding J, Wei H, Liu J, et al., Treatment patterns, effectiveness, and patient-reported outcomes of palbociclib therapy in Chinese patients with advanced breast cancer: a multicenter ambispective real-world study. Cancer Med. 2022;11:4157–68.
Yildirim HC, Mutlu E, Chalabiyev E, Ozen M, Keskinkilic M, On S, et al. Clinical outcomes of cyclin-dependent kinase 4–6 (CDK 4–6) inhibitors in patients with male breast cancer: a multicenter study. Breast. 2022;66:85–8.
Mycock K, Zhan L, Hart K, Taylor-Stokes G, Milligan G, Atkinson C, et al. Real-world treatment patterns and clinical outcomes in patients receiving palbociclib combinations for HR+/HER2− advanced/metastatic breast cancer in Japan: results from the IRIS study. Cancer Treat Res Commun. 2022;32: 100573.
Battisti NML, Kingston B, King J, Denton A, Waters S, Sita-Lumsden A, et al. Palbociclib and endocrine therapy in heavily pretreated hormone receptor-positive HER2-negative advanced breast cancer: the UK Compassionate Access Programme experience. Breast Cancer Res Treat. 2019;174(3):731–40.
Martin JM, Handorf EA, Montero AJ, Goldstein LJ. Systemic therapies following progression on first-line CDK4/6-inhibitor treatment: analysis of real-world data. Oncologist. 2022;27(6):41–6.
Albanell J, Perez-Garcia JM, Gil-Gil M, Curigliano G, Ruiz Borrego M, Comerma L, et al. Palbociclib rechallenge for hormone receptor-positive/human epidermal growth factor receptor-negative advanced breast cancer: findings from the phase II BioPER Trial. Clin Cancer Res. 2023;29(1):67–80.
Edman Kessler L, Wiklander O, Hamberg E, Bergh J, Foukakis T, Matikas A. Efficacy and safety of cyclin dependent kinases 4/6 inhibitors in the treatment of metastatic breast cancer: a real-world experience. Acta Oncol. 2020;59(11):1382–7.
Martin M, Zielinski C, Ruiz-Borrego M, Carrasco E, Ciruelos EM, Munoz M, et al. Overall survival with palbociclib plus endocrine therapy versus capecitabine in postmenopausal patients with hormone receptor-positive, HER2-negative metastatic breast cancer in the PEARL study. Eur J Cancer. 2022;168:12–24.
Collins JM, Nordstrom BL, McLaurin KK, Dalvi TB, McCutcheon SC, Bennett JC, et al. A real-world evidence study of CDK4/6 inhibitor treatment patterns and outcomes in metastatic breast cancer by germline BRCA mutation status. Oncol Ther. 2021;25:25.
Gao JJ, Cheng J, Prowell TM, Bloomquist E, Tang S, Wedam SB, et al. Overall survival in patients with hormone receptor-positive, HER2-negative, advanced or metastatic breast cancer treated with a cyclin-dependent kinase 4/6 inhibitor plus fulvestrant: a US Food and Drug Administration pooled analysis. Lancet Oncol. 2021;22(11):1573–81.
Bardia A, Kaklamani V, Wilks S, Weise A, Richards D, Harb W, et al. Phase I study of elacestrant (RAD1901), a novel selective estrogen receptor degrader, in ER-positive, HER2-negative advanced breast cancer. J Clin Oncol. 2021;39(12):1360–70.
Cook MM, Al Rabadi L, Kaempf AJ, Saraceni MM, Savin MA, Mitri ZI. Everolimus plus exemestane treatment in patients with metastatic hormone receptor-positive breast cancer previously treated with CDK4/6 inhibitor therapy. Oncologist. 2021;26(2):101–6.
Dhakal A, Antony Thomas R, Levine EG, Brufsky A, Takabe K, Hanna MG, et al. Outcome of everolimus-based therapy in hormone-receptor-positive metastatic breast cancer patients after progression on palbociclib. Breast Cancer. 2020;14:1178223420944864.
Zhou J, Wu X, Zhang H, Wang X, Yuan Y, Zhang S, et al. Clinical outcomes of tucidinostat-based therapy after prior CDK4/6 inhibitor progression in hormone receptor-positive heavily pretreated metastatic breast cancer. Breast. 2022;66:255–61.
Lim JSJ, Wong ALA, Ow SGW, Ngoi NYL, Chan GHJ, Ang YLE, et al. Phase Ib/II dose expansion study of lenvatinib combined with letrozole in postmenopausal women with hormone receptor-positive breast cancer. Clin Cancer Res. 2022;28(11):2248–56.
Mo H, Renna CE, Moore HCF, Abraham J, Kruse ML, Montero AJ, et al. Real-world outcomes of everolimus and exemestane for the treatment of metastatic hormone receptor-positive breast cancer in patients previously treated with CDK4/6 inhibitors. Clin Breast Cancer. 2022;22(2):143–8.
Li Y, Li W, Gong C, Zheng Y, Ouyang Q, Xie N, et al. A multicenter analysis of treatment patterns and clinical outcomes of subsequent therapies after progression on palbociclib in HR+/HER2− metastatic breast cancer. Ther Adv Med Oncol. 2021;13:17588359211022890.
Rozenblit M, Mun S, Soulos P, Adelson K, Pusztai L, Mougalian S. Patterns of treatment with everolimus exemestane in hormone receptor-positive HER2-negative metastatic breast cancer in the era of targeted therapy. Breast Cancer Res. 2021;23(1):14.
Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Int J Surg. 2021;88: 105906.
Hui R, de Boer R, Lim E, Yeo B, Lynch J. CDK4/6 inhibitor plus endocrine therapy for hormone receptor-positive, HER2-negative metastatic breast cancer: the new standard of care. Asia Pac J Clin Oncol. 2021;17(Suppl. 1):3–14.
Kearney MR, McGuinees JE, Kalinsky K. Clinical trial data and emerging immunotherapeutic strategies: hormone receptor-positive, HER2− negative breast cancer. Breast Cancer Res Treat. 2021;189(1):1–13.
Li CLX. Advances in therapy for hormone receptor (HR)-positive, human epidermal growth factor receptor 2 (HER2)-negative advanced breast cancer patients who have experienced progression after treatment with CDK4/6 inhibitors. OncoTargets Ther. 2021;14:2929–39.
Mavratzas AM, Marme F. Treatment of luminal metastatic breast cancer beyond CDK4/6 inhibition: is there a standard of care in clinical practice? Breast Care. 2021;16(2):115–28.
Migliaccio IB, McCartney M, Guarducci C, Benelli M, Biganzoli L, Di Leo A, Malorni L. CDK4/6 inhibitors: a focus on biomarkers of response and post-treatment therapeutic strategies in hormone receptor-positive HER2-negative breast cancer. Cancer Treat Rev. 2021;93: 102136.
Nagaraj GM, Ma CX. Clinical challenges in the management of hormone receptor-positive, human epidermal growth factor receptor 2-negative metastatic breast cancer: a literature review. Adv Ther. 2021;38(1):109–36.
Xu XQ, Pan XH, Wang TT, Wang J, Yang B, He QJ, Ding L. Intrinsic and acquired resistance to CDK4/6 inhibitors and potential overcoming strategies. Acta Pharmacol Sin. 2021;42(2):171–8.
Cogliati V, Capici S, Pepe FF, di Mauro P, Riva F, Cicchiello F, et al. How to treat HR+/HER2− metastatic breast cancer patients after CDK4/6 inhibitors: an unfinished story. Life. 2022;12(3):378.
Munzone E, Pagan E, Bagnardi V, Montagna E, Cancello G, Dellapasqua S, et al. Systematic review and meta-analysis of post-progression outcomes in ER+/HER2- metastatic breast cancer after CDK4/6 inhibitors within randomized clinical trials. ESMO Open. 2021;6(6): 100332.
Garcia-Saenz JA, Martinez-Janez N, Cubedo R, Jerez Y, Lahuerta A, Gonzalez-Santiago S, et al. Sapanisertib plus fulvestrant in postmenopausal women with estrogen receptor-positive/HER2-negative advanced breast cancer after progression on aromatase inhibitor. Clin Cancer Res. 2022;28(6):1107–16.
Cuyun Carter G, Sheffield KM, Gossai A, Huang YJ, Zhu YW, Bowman L, et al. Real-world treatment patterns and outcomes of abemaciclib for the treatment of HR+, HER2− metastatic breast cancer. Curr Med Res Opin. 2021;37(7):1179–87.
Gong J, Cho M, Yu KW, Waisman J, Yuan Y, Mortimer J. A single institution experience with palbociclib toxicity requiring dose modifications. Breast Cancer Res Treat. 2018;168(2):381–7.
Norman H, Lee KT, Stearns V, Alcorn SR, Mangini NS. Incidence and severity of myelosuppression with palbociclib after palliative bone radiation in advanced breast cancer: a single center experience and review of literature. Clin Breast Cancer. 2021;27:27.
Pizzuti L, Giordano A, Michelotti A, Mazzotta M, Natoli C, Gamucci T, et al. Palbociclib plus endocrine therapy in HER2 negative, hormonal receptor-positive, advanced breast cancer: a real-world experience. J Cell Physiol. 2019;234(6):7708–17.
Taylor-Stokes G, Mitra D, Waller J, Gibson K, Milligan G, Iyer S. Treatment patterns and clinical outcomes among patients receiving palbociclib in combination with an aromatase inhibitor or fulvestrant for HR+/HER2-negative advanced/metastatic breast cancer in real-world settings in the US: results from the IRIS study. Breast. 2019;43:22–7.
Waller J, Mitra D, Mycock K, Taylor-Stokes G, Milligan G, Zhan L, et al. Real-world treatment patterns and clinical outcomes in patients receiving palbociclib for hormone receptor-positive, human epidermal growth factor receptor 2-negative advanced or metastatic breast cancer in Argentina: the IRIS Study. J Glob Oncol. 2019;5:JGO1800239.
Marineau A, St-Pierre C, Lessard-Hurtubise R, David M-E, Adam J-P, Chabot I. Cyclin-dependent kinase 4/6 inhibitor treatment use in women treated for advanced breast cancer: integrating ASCO/NCODA patient-centered standards in a community pharmacy. J Oncol Pharm Pract. 2022;0(0):10781552221102884. https://doi.org/10.1177/107815522211028.
Novick D, Lee SY, Koo DH, Szende A, Colman S. Real world evidence study on treatment patterns and health resource utilization in patients with HR+/HER2− locally advanced or metastatic breast cancer in Korea. J Drug Assess. 2022;11(1):12–9.
Smyth EN, Beyrer J, Saverno KR, Hadden E, Abedtash H, DeLuca A, et al. Real-world patient characteristics, utilization patterns, and outcomes of US patients with HR+, HER2− metastatic breast cancer treated with abemaciclib. Drugs-Real World Outcomes. 2022;9:681–93.
ClinicalTrials.gov. Description of treatment patterns and description and comparison of healthcare resource utilization and costs of women with metastatic HR+/HER2- Breast Cancer Treated With CDK4/6 Inhibitors. 2022.https://clinicaltrials.gov/ct2/show/NCT05153135. Accessed 21 Mar 2023.
ClinicalTrials.gov. Treatment patterns and clinical outcomes among patients in Latin America receiving first line palbociclib combinations For HR+/HER2− advanced/metastatic breast cancer in real world settings. 2021.https://clinicaltrials.gov/ct2/show/NCT05155566. Accessed 21 Mar 2023.
ClinicalTrials.gov.A study to describe the breast cancer patient population, treatment, and results in indian patients receiving combinations of the medicines called palbociclib for advanced breast cancer. 2022. https://clinicaltrials.gov/ct2/show/NCT05584644. Accessed 21 Mar 2023.
Bardia A, Hurvitz SA, DeMichele A, Clark AS, Zelnak AB, Yardley DA, et al. Triplet therapy (continuous ribociclib, everolimus, exemestane) in HR+/HER2− advanced breast cancer postprogression on a CDK4/6 inhibitor (TRINITI-1): efficacy, safety, and biomarker results. J Clin Oncol. 2019;37(15_suppl):1016.
Agrawal C, Doval D, Agarwal A, Goyal P, Baghmar S, Talwar V, et al. Real world evidence of palbociclib use in metastatic hormone positive HER negative metastatic breast cancer in Indian population. Eur J Cancer. 2020;138:S103.
McLaurin K, Dalvi T, Collins JM, Nordstrom BL, McCutcheon S, Bennett C, et al., A real-world evidence study of CDK4/6 inhibitor treatment patterns and outcomes in metastatic breast cancer by gBRCA mutation status. J Clin Oncol. 2019;37(15_suppl):1563.
Check DK, Jackson BE, Spees L, Dinan MA, Kwong J, Mehta S, et al. Treatment patterns and health care resource use of patients with metastatic breast cancer with HER2-low expression: a cancer registry-linked insurance claims study. J Clin Oncol. 2022;40(28_suppl):399.
Parola S, Di Lauro V, De Placido P, Forestieri V, Buono G, von Arx C, et al. 105P CDK4/6 inhibitors in hormone receptor-positive, HER2-negative, locally advanced breast cancer (LABC): biological and clinical activity, and post-surgical approaches. Ann Oncol. 2022;33:S172–3.
Acknowledgements
The authors acknowledge Alexis Jenkins, Rhett Figliuzzi, Amanda Griffin, Michaela Spence, and Manvir Rai for their support in the targeted literature review, as well as Becky Skidmore who developed, conducted, and documented the database searches.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This literature review and manuscript was funded by Pfizer Inc.
Conflict of interest
Melody Zhao, Yixie Zhang, and Anna Zhou are employees of EVERSANA, which was a paid consultant to Pfizer in connection with the development of this manuscript. Kent A. Hanson is an employee of University of Illinois at Chicago, which was a paid consultant to Pfizer in connection with the development of this manuscript. Ashley S. Cha-Silva is an employee of and stockholder in Pfizer Inc. Alexis Jenkins, Rhett Figliuzzi, Amanda Griffin, Michaela Spence, and Manvir Rai are employees of EVERSANA. Becky Skidmore is a contractor of EVERSANA.
Ethics approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Availability of data and material
The datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.
Code availability
Not applicable.
Authors’ contributions
All authors contributed to the conception and design of the research and provided critical revisions and intellectual content to the manuscript. All authors provided substantial intellectual content and revisions to the manuscript. MZ and YZ conducted analyses and drafted the manuscript, and all authors discussed the results and contributed to the final version of the manuscript. All authors provided critical and intellectual feedback of the research, analysis, and manuscript.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Zhao, M., Hanson, K.A., Zhang, Y. et al. Place in Therapy of Cyclin-Dependent Kinase 4/6 Inhibitors in Breast Cancer: A Targeted Literature Review. Targ Oncol 18, 327–358 (2023). https://doi.org/10.1007/s11523-023-00957-7
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11523-023-00957-7